Introduction
Cytogenetic changes are diagnostically and
prognostically significant in the case of acute myeloid leukaemia
(AML). However, for certain rare cytogenetic changes, including
t(11; 12) (p15; q13), t(16; 21) (p11; q22) (1) and t(8; 17; 15; 21) (q22; q15; q23; q22)
(2), the pathogenesis, clinical
features and impact of the cytogenetic changes on the biological
characteristics of the leukaemic cells of these patients remain
unclear due to the low incidence of these changes. In the present
study, we report the case of a leukaemia patient, recently
diagnosed at the Zhongshan Hospital of Xiamen University, China,
whose morphological and immunological manifestations were
consistent with acute monocytic leukaemia (AML-M5b), and who
demonstrated a rare chromosomal change of t(11; 12) (p15; q13)
along with a positive FLT3-ITD mutation. The clinical features and
therapeutic responses are presented in the case report. Written
informed consent was obtained from the patient and ethical approval
was obtained from the Medical Ethics Committee of Zhongshan
Hospital of Xiamen University (Xiamen, China).
Case report
Patient presentation
A 43-year-old Han Chinese female experiencing fever
and cough for two days was admitted to the Zhongshan Hospital of
Xiamen University. The results of her physical examination were as
follows: body temperature, 37.0°C; pulse, 82 beats/min;
respiration, 21 times/min; and blood pressure, 102/60 mmHg. The
patient appeared mildly anaemic; no skin mucocutaneous petechiae or
ecchymoses, no scleral jaundice, no tenderness in the sternum, and
no gingival hyperplasia were observed. No superficial enlarged
lymph nodes were palpable, pharyngeal congestion and enlarged
tonsils at degree III were observed, and no enlargement of the
liver and spleen was detected. The results of the routine blood
laboratory test were as follows: white blood cells (WBC),
76.41×109/l; neutrophils, 33.62×109/l;
monocytes, 15.28×109/l; haemoglobin, 92 g/l; platelets,
296×109/l; and primitive cells, 27%. Bone marrow
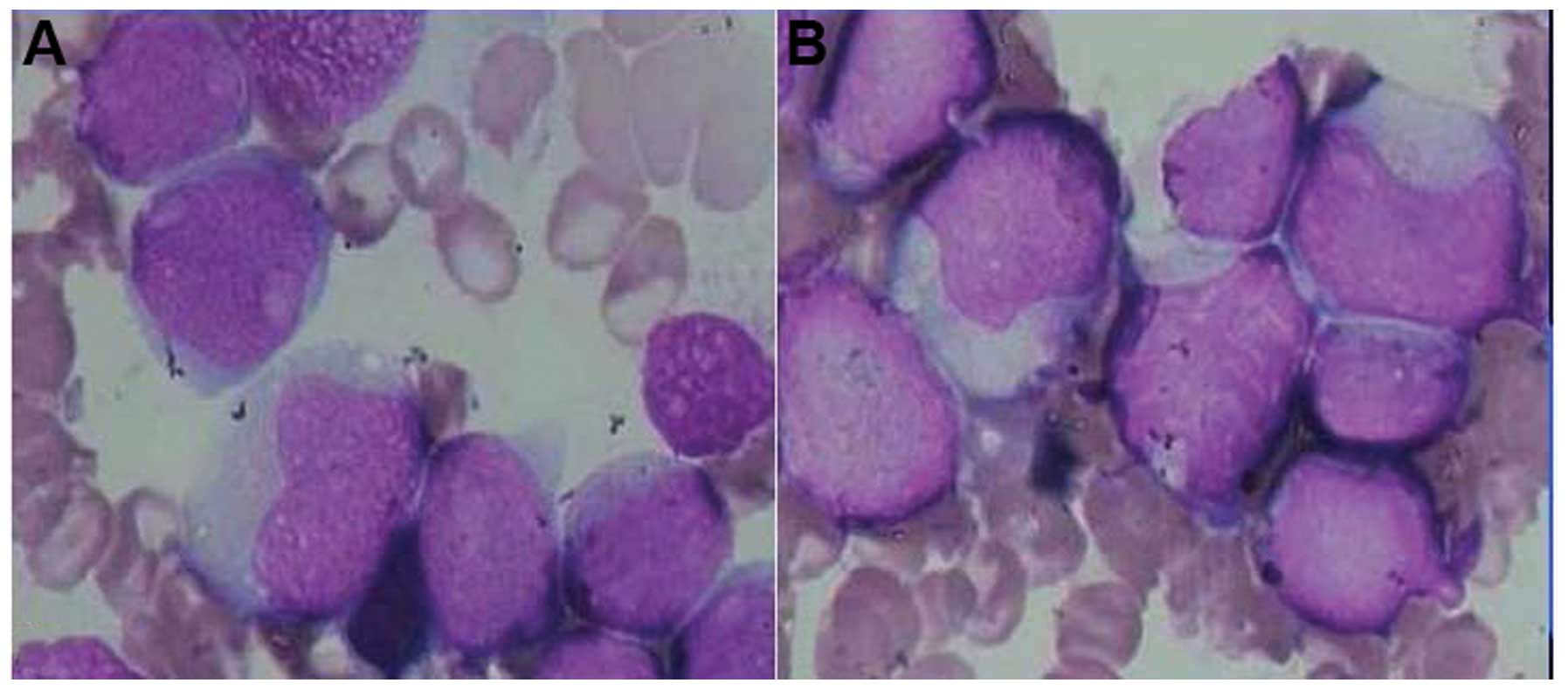
morphology revealed that the proliferation of nucleated cells was
extremely active, whereas myeloid and erythroid proliferation was
inhibited. Monoblasts accounted for 25.5% of cells, and
premonocytes accounted for 49.0% (Fig.
1A). Peroxidase detection was positive, accompanied by periodic
acid-Schiff-positive granules. The hot-saline solubility test was
negative and α-naphthyl butyrate esterase was strongly positive
(Fig. 1B). The immunophenotyping
results were as follows: CD69, CD14(+) cells accounted for 82.1%,
CD33(+) cells accounted for 68.7%, and CD34(+) cells accounted for
64.2%. The screening of the 18 AML-related fusion genes (BCR-ABL,
AML1-ETO, CBFβ-MYH11, PML-RARα, MLL-AF9, AML1-MDS1, NPM-MLF1,
AML1-ETO, NPM-RARα, PLZF-RARα, DEK-CAN, MLL-ELL, AML1-EAP,
MLL-AF10, SET-CAN, TEL-ABL1, TLS-ERG and NPM-ALK) yielded negative
results.
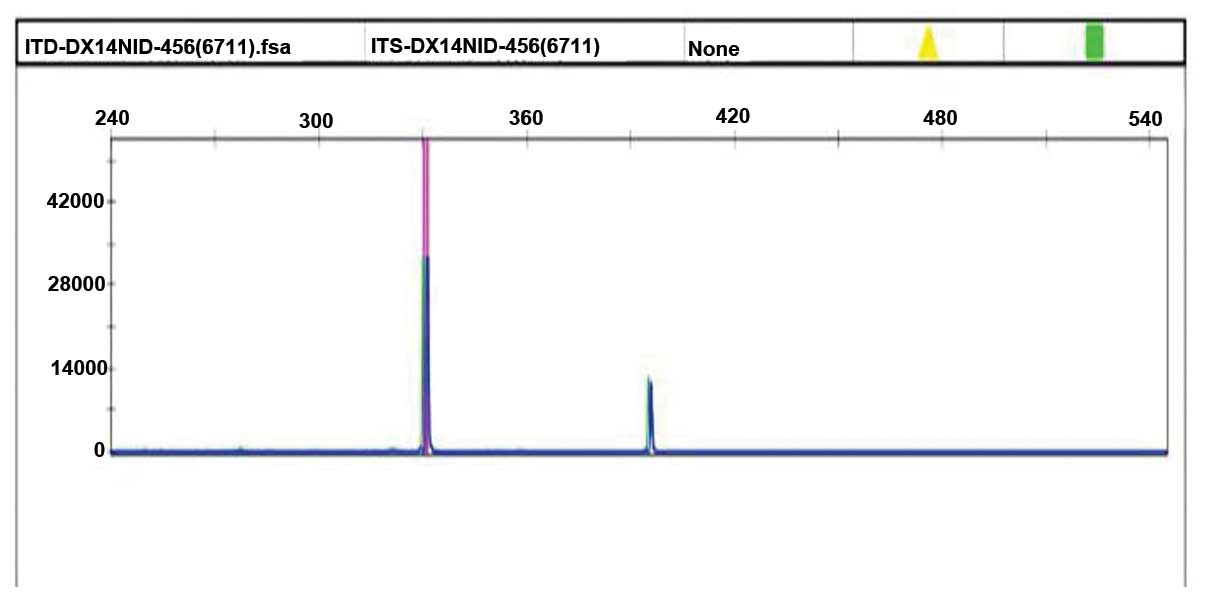
The leukaemia gene mutation screening results
revealed that the CEBPA gene, WT1 gene exon 7, exon 9, DNMT3A R882,
KIT gene, IDH1 gene exons, IDH2 exon 4, RUNX1 gene, FLT3-TKD (D835)
gene and NPM1 gene were negative. An FLT3-ITD gene mutation was
detected (exon 14, 15) (Fig. 2). The
karyotype was 46, XX, t(11; 12) (p15; q13) (4) (the chromosome image is not available due
to malfunction of the image acquisition instrument). In accordance
with the combined morphologic, immunologic, cytogenetic and
molecular biologic classification, the patient was diagnosed with
AML-M5b.
Treatment process
Following the initial diagnosis, the patient
received chemotherapy, using an idarubicin and Ara-c (cytarabine)
(IA) regimen. After one week of chemotherapy, the proliferation of
the bone marrow cells was at a low level, with primitive cells
accounting for 1.5%. After two weeks, bone marrow cell
proliferation became active, with primitive cells accounting for
35%. Subsequently, the patient received another IA chemotherapy
treatment course. After one week, the proliferation of the bone
marrow cells was at an extremely low level, with primitive cells
accounting for 3%, and after 2 weeks, bone marrow cell
proliferation became active, with primitive cells accounting for
40%. No remission was observed.
Discussion
In 1998, Wong et al (3) reported the first case of AML that
developed following radiotherapy for breast cancer, demonstrating a
karyotype of t(11; 12) (p15; q13), following administration of a
dose-adjusted daunorubicin and Ara-c (cytarabin) (DA) regimen
chemotherapy. Complete remission (CR) was achieved and
re-examination revealed that the chromosomes exhibited a normal
karyotype. In 2002, Taketani (4)
et al reported a case of primary AML-M1 with t(11; 12) (p15;
q13). CR was observed one month after the administration of the
ANLL-91 regimen. This patient received haematopoietic stem cell
transplantation 9 months later and succumbed due to a relapse after
six months of sustained remission. Taketani et al identified
an NUP98-HOXC11 gene fusion caused by the t(11; 12) translocation
for the first time. In 2003, La Starza et al (5) also reported a case of primary AML-M2
with t(11; 12) (p15; q13) chromosome translocation, and confirmed
the formation of the NUP98-HOXC13 fusion gene (6). Subsequently, a number of cases of such
chromosomal abnormalities in leukaemia were reported, including
AML-M2a, M2, M4 and M5b (6–8). To date, four cases of positive
NUP98-HOXC13 and NUP98-HOXC11 fusion genes have been reported
(3,6–8). In 2009,
La Starza et al (9) again
identified a case of AML-M2 with t(11; 12) (p15; q13). However, the
fluorescence in situ hybridisation method failed to detect
the NUP98-HOXC13 and NUP98-HOXC11 fusion genes.
In 2011, Such et al (10) published a study on a 35-year-old male
diagnosed with acute promyelocytic leukaemia (APL) in accordance
with the French-American-British (FAB) criteria, but lacking the
PML-RARα fusion gene. The patient's karyotype was also t(11; 12)
(p15; q13), which formed the NUP98-RARr fusion gene. In this fusion
gene, the NUP98 breakpoint was located on exon 12, and the RARr
breakpoint was located on exon 4. There was a complete absence of
HOXC11, HOXC13 and PML-RARα fusion genes. This patient had a WBC
count of 12×109/l and achieved CR after receiving the
standard DA chemotherapy without tretinoin treatment. Later on, the
patient underwent autologous stem cell transplantation and remained
in CR at the 8-month follow-up point. In 2013, Gong et al
(11) also reported two cases of APL
with karyotype t(11; 12) (p15; q13). Neither of the patients
underwent tests to detect HOXC11 and HOXC13 fusion genes, and the
tretinoin and arsenic trioxide therapies were ineffective. One of
the patients had a WBC count of 14.6×109/l at the time
of diagnosis. No remission was achieved following the DA
chemotherapy and subsequent myeloablative chemotherapy, and the
patient succumbed to infection. The patient had a WBC count of
3.7×109/l and positive FLT3-TKD; CR was achieved
following high-dose asparaginase (HDA) chemotherapy. Subsequently,
the patient remained in CR following two courses of consolidation
chemotherapy.
A study by Gu et al (7) revealed that the NUP98 gene on chromosome
11p15 encodes the protein associated with a nuclear pore complex,
which regulates the nucleocytoplasmic transport of proteins and
mRNA. Mouse bone marrow transplantation experiments revealed that
the NUP98 fusion protein causes leukaemia, and that the
translocation of the NUP98-HOXA9 fusion protein of the bone marrow
cells causes AML in mice. The NUP98-HOXC11 fusion protein is not
involved in HOXC11 transcriptional regulation, but promotes the
expression of the upstream reporter gene as a reverse activator. In
addition, t(11; 12) (p15; q13) unbalanced translocation may lead to
a loss of significant tumour suppressor genes on the telomere of
chromosome 11p, which may also be one of the key mechanisms that
lead to leukaemia.
AML patients with t(11; 12) (p15; q13) undergo
primary or secondary clonal changes, which may return to normal
following treatment. La Starza et al (5) reported a new t(1; 21) (p32; q22)
karyotype that occurred in a patient with relapsed AML. Masuya
et al (12) published a study
on a 55-year-old female patient with therapy-related
myelodysplastic syndrome who had normal karyotype chromosomes at
diagnosis, but demonstrated t(1; 2) (p36; p21) and secondary t(11;
12) (p15; q13) when the relapse occurred. This patient survived for
more than 6 years. In the cases of secondary AML reported by Wong
et al (3) and APL reported by
Gong et al (11), the
chromosomes returned to the normal state following DA
chemotherapy.
Thus, the t(11; 12) (p15; q13) chromosomal
abnormality is a rare recurrent genetic event in AML, and may occur
in primary AML or in chemotherapy-related secondary AML. Various
subtypes of AML with t(11; 12) (p15; q13) have been reported, with
the exception of M6 and M7. To date, 13 AML patients with t(11; 12)
(p15; q13) between the ages of 2 and 59 years have been reported.
Males account for 40% and females for 60% of patients. Among these
patients, the WBC count was generally in the range
3.5–25×109/l, with the exception of one patient, in whom
it reached 211×109/l. Taken together, the t(11; 12)
(p15; q13) chromosomal abnormality exhibits strong heterogeneity,
and the translocation may involve different genes or may have
different breakpoints even when the same genes are involved.
However, the pathogenic mechanism and the prognosis remain
unclear.
AML with t(11; 12) (p15; q13) combined with FLT3-ITD
mutations has also been reported (8,13). These
patients succumbed in the early stages following treatment,
indicating poor prognosis (13).
However, the prognosis of the FLT3-TKD mutation is relatively
superior. In the APL patient with FLT3-TKD mutations reported by
Gong et al (11), CR was
achieved following one course of HDA remission induction. This
patient's chromosomes returned to normal and the FLT3-TKD was
observed to be negative. The patient remained in CR following two
subsequent courses of consolidation treatment.
In the patient reported in the present study, one of
the molecular biological characteristics of leukaemia was the
combination of the FLT3-ITD mutation and a negative NPM1 gene. The
FLT3-ITD mutation-positive AML cases often have a poor outcome, and
demonstrate a poor response to chemotherapy, leading to a low
remission rate and high early mortality (14–16). The
positive expression rate of the NPM1 gene is 20–50% in newly
diagnosed AML patients, and AML patients expressing the NPM1 gene
have a relatively superior prognosis (17). The clinical efficacy (including CR,
event-free survival and overall survival rate) of the AML patients
with FLT3-ITD mutations and positive NPM1 gene expression is
superior to that of patients with only positive FLT3-ITD mutations
(18–20). Most of the literature suggests that
clinical features of FLT3-ITD mutation-positive patients include a
high number of peripheral blood leukocytes and a high percentage of
leukaemia cells in the bone marrow (16,21). At
the time of diagnosis, the WBC count of our patient was
76.41×109/l, and the monoblasts and premonocytes
accounted for 75% of bone marrow cells. This result was consistent
with the previous reports in literature. However, this patient did
not exhibit any symptoms of leukaemia infiltration. Unlike the
patients with FLT3-ITD mutations who succumbed during the early
stages, this patient responded positively to the IA chemotherapy,
which quickly eliminated the leukaemia cell clones. However, the
proliferation rate of the leukaemia cells was high during the
intermission of chemotherapy. Four weeks after chemotherapy, the
proportion of immature bone marrow cells was 38%, which remained
unchanged after another course of IA chemotherapy. Subsequently,
two courses of chemotherapy could still not achieve a complete
haematological remission, as indicated by the fact that the
proportion of bone marrow immature cells remaining were only 40% of
the expected total. It was speculated that the patient's rapid
proliferation of leukaemia cells may be related to the FLT3-ITD
gene mutation. Under normal circumstances, the juxtamembrane region
and the ‘activation loop’ of tyrosine kinase receptors exhibit a
self-inhibiting function to maintain an inactive conformation of
the kinase. An FLT3-ITD mutation leads to the continuous activation
of the tyrosine kinases, thus causing a spontaneous and
non-dependent excessive proliferation of cells.
Currently, studies on the immunology and cytogenetic
features of FLT3-ITD mutation-positive AML are scarce (22,23). Among
the 14 cases of AML with t(11; 12) (p15; q13) reported, including
the present case, 21% (3 cases) demonstrated an FLT3-ITD mutation.
Further cases need to be studied to determine whether a correlation
exists between the FLT3-ITD mutation and t(11; 12) (p15; q13).
In conclusion, AML patients with t(11; 12) (p15;
q13) combined with FLT3-ITD mutations are expected to have a short
life expectancy; however, an early haematopoietic stem cell
transplantation therapy may improve the treatment outcome for these
patients (10,24).
Acknowledgements
This study was supported by the National Nature
Science Fund (no. 81172246).
References
|
1
|
Kamada Y, Suzukawa K, Taoka K, Okoshi Y,
Hasegawa Y and Chiba S: Relapse of acute myeloid leukemia with t
(16; 21)(p11; q22) mimicking autoimmune pancreatitis after second
allogeneic bone marrow transplantation. ISRN Hematol.
2011:2854872011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vieira L, Oliveira V, Ambrósio AP, Marques
B, Pereira AM, Hagemeijer A and Boavida MG: Translocation
(8;17;15;21)(q22;q23;q15;q22) in acute myeloid leukemia (M2). a
four-way variant of t(8;21). Cancer Genet Cytogenet. 128:104–107.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong KF, Kwong YL and So CC: De novo AML
with trilineage myelodysplasia and a novel t(11;12)(p15;q13).
Cancer Genet Cytogenet. 100:49–51. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taketani T, Taki T, Shibuya N, Kikuchi A,
Hanada R and Hayashi Y: Novel NUP98-HOXC11 fusion gene resulted
from a chromosomal break within exon 1 of HOXC11 in acute myeloid
leukemia with t(11;12)(p15;q13). Cancer Res. 62:4571–4574.
2002.PubMed/NCBI
|
|
5
|
LaStarza R, Trubia M, Crescenzi B,
Matteucci C, Negrini M, Martelli MF, Pelicci PG and Mecucci C:
Human homeobox gene HOXC13 is the partner of NUP98 in adult acute
myeloid leukemia with t(11;12)(p15;q13). Genes Chromosomes Cancer.
36:420–423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panagopoulos I, Isaksson M, Billström R,
Strömbeck B, Mitelman F and Johansson B: Fusion of the NUP98 gene
and the homeobox gene HOXC13 in acute myeloid leukemia with
t(11;12)(p15;q13). Genes Chromosomes Cancer. 36:107–112. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu BW, Wang Q, Wang JM, Xue YQ, Fang J,
Wong KF, Chen B, Shi ZZ, Shi JY, Bai XT, et al: Major form of
NUP98/HOXC11 fusion in adult AML with t(11;12)(p15;q13)
translocation exhibits aberrant trans-regulatory activity.
Leukemia. 17:1858–1864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tosić N, Stojiljković M, Colović N,
Colović M and Pavlović S: Acute myeloid leukemia with NUP98-HOXC13
fusion and FLT3 internal tandem duplication mutation: Case report
and literature review. Cancer Genet Cytogenet. 193:98–103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaStarza R, Brandimarte L, Pierini V,
Nofrini V, Gorello P, Crescenzi B, Berchicci L, Matteucci C, Romoli
S, Beacci D, et al: A NUP98-positive acute myeloid leukemia with a
t(11;12)(p15;q13) without HOXC cluster gene involvement. Cancer
Genet Cytogenet. 193:109–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Such E, Cervera J, Valencia A, Barragán E,
Ibañez M, Luna I, Fuster O, PerezSirvent ML, Senent L, Sempere A,
et al: A novel NUP98/RARG gene fusion in acute myeloid leukemia
resembling acute promyelocytic leukemia. Blood. 117:242–245. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong BF, Li QH, Li W, Wang Y, Wei H, Wang
JY, Zhao XL, Lin D, Li CW, Liu XP, et al: Acute myeloid leukemia
with t(11;12)(p15;q13) translocation: Two cases report and
literature review. Zhonghua Xue Ye Xue Za Zhi. 34:830–833. 2013.(In
Chinese). PubMed/NCBI
|
|
12
|
Masuya M, Katayama N, Inagaki K, Miwa H,
Hoshino N, Miyashita H, Suzuki H, Araki H, Mitani H, Nishii K, et
al: Two independent clones in myelodysplastic syndrome following
treatment of acute myeloid leukemia. Int J Hematol. 75:182–186.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taketani T, Taki T, Nakamura T, Kobayashi
Y, Ito E, Fukuda S, Yamaguchi S and Hayashi Y: High frequencies of
simultaneous FLT3-ITD, WT1 and KIT mutations in hematological
malignancies with NUP98-fusion genes. Leukemia. 24:1975–1977. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pratz KW, Sato T, Murphy KM, Stine A,
Rajkhowa T and Levis M: FLT3-mutant allelic burden and clinical
status are predictive of response to FLT3 inhibitors in AML. Blood.
115:1425–1432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gale RE, Green C, Allen C, Mead AJ,
Burnett AK, Hills RK and Linch DC: Medical Research Council Adult
Leukaemia Working Party: The impact of FLT3 internal tandem
duplication mutant level, number, size, and interaction with NPM1
mutations in a large cohort of young adult patients with acute
myeloid leukemia. Blood. 111:2776–2784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Whitman SP, Ruppert AS, Radmacher MD,
Mrózek K, Paschka P, Langer C, Baldus CD, Wen J, Racke F, Powell
BL, et al: FLT3 D835/I836 mutations are associated with poor
disease-free survival and a distinct gene-expression signature
among younger adults with de novo cytogenetically normal acute
myeloid leukemia lacking FLT3 internal tandem duplications. Blood.
111:1552–1559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martelli MP, Pettirossi V, Thiede C,
Bonifacio E, Mezzasoma F, Cecchini D, Pacini R, Tabarrini A,
Ciurnelli R, Gionfriddo I, et al: CD34+ cells from AML with mutated
NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia
in immunocompromised mice. Blood. 116:3907–3922. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schnittger S, Kern W, Tschulik C, Weiss T,
Dicker F, Falini B, Haferlach C and Haferlach T: Minimal residual
disease levels assessed by NPM1 mutation-specific RQ-PCR provide
important prognostic information in AML. Blood. 114:2220–2231.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Xu WL, Meng HT, Qian WB, Mai WY,
Tong HY, Mao LP, Tong Y, Qian JJ, Lou YJ, et al: FLT3 and NPM1
mutations in Chinese patients with acute myeloid leukemia and
normal cytogenetics. J Zhejiang Univ Sci B. 11:762–770. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Falini B, Martelli MP, Bolli N,
Sportoletti P, Liso A, Tiacci E and Haferlach T: Acute myeloid
leukemia with mutated nucleophosmin (NPM1): is it a distinct
entity? Blood. 117:1109–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haferlach T, Bacher U, Alpermann T,
Haferlach C, Kern W and Schnittger S: Amount of bone marrow blasts
is strongly correlated to NPM1 and FLT3-ITD mutation rate in AML
with normal karyotype. Leuk Res. 36:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunlap J, Beadling C, Warrick A, Neff T,
Fleming WH, Loriaux M, Heinrich MC, Kovacsovics T, Kelemen K,
Leeborg N, et al: Multiplex high-throughput gene mutation analysis
in acute myeloid leukemia. Hum Pathol. 43:2167–2176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dang H, Jiang A, KamelReid S, Brandwein J
and Chang H: Prognostic value of immunophenotyping and gene
mutations in elderly patients with acute myeloid leukemia with
normal karyotype. Hum Pathol. 44:55–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishiyama M, Arai Y, Tsunematsu Y,
Kobayashi H, Asami K, Yabe M, Kato S, Oda M, Eguchi H, Ohki M, et
al: 11p15 translocations involving the NUP98 gene in childhood
therapy-related acute myeloid leukemia/myelodysplastic syndrome.
Genes Chromosomes Cancer. 26:215–220. 1999. View Article : Google Scholar : PubMed/NCBI
|