Introduction
Craniopharyngiomas (CPs) are rare benign suprasellar
tumors that have an incidence of between 1.2 and 4.6% (1–5) with no
differences between males and females as well Caucasians and
African Americans and with a bimodal age distribution, one peak
occurring in children and the other in adults between the 4th and
6th decades of life (1–5). The survival rate at 5 years is ~80%
overall, with survival much better among children than the elderly
(1–5).
CPs arise from epithelial remnants of the embryonic Rathke's pouch,
and is characterized by slow growth (2–5). CPs are
divided into two histological subtypes: (i) Adamantinomatous CP,
typically occurring in children and adolescents; and (ii) papillary
CP, observed almost exclusively in adults (2). Cystic and solid components with frequent
calcifications characterize the adamantinomatous subtype, while the
papillary subtype is commonly solid with low incidence of
calcifications (2). In the
adamantinomatous variant, the solid component frequently extends
into the sellar region of the sphenoid bone, while the associated
cystic component is situated outside and above the sella turcica
(1).
The standard treatment for CP is complete surgical
resection, or partial resection followed by adjuvant radiotherapy
(RT) (3). The latter option has the
advantage of reducing the risk of neurological, visual and
endocrine (panhypopituitarism) complications (3). Adjuvant RT is typically administered at
a total dose of 54 Gy with 1.8 Gy/fraction, although doses up to
59.4 Gy have previously been administered by fractionated proton RT
(2). In order to improve the
therapeutic outcomes, novel RT techniques have been proposed,
including stereotactic radiosurgery (SRS), stereotactic RT (SRT)
and intensity-modulated radiation therapy (IMRT) (3).
The treatment of relapse following surgery is
complex and, in such cases, it is difficult to treat the disease
without producing significant side effects, including optic
neuropathy, neuropsychological sequel and delayed
hypothalamic-pituitary damage (5).
The aim of the current report was to present a case of CP treated
with high-dose IMRT and subsequently monitored by clinical and
instrumental evaluation, including magnetic resonance imaging
(MRI).
Case report
A 7-year-old girl was diagnosed with cystic CP in
March 1992, following presentation of symptoms including growth
arrest, headache and panhypopituitarism, in addition to bitemporal
hemianopia. Partial resection of the tumor and drainage into the
cystic lesion were performed by craniotomy, followed by intracystic
bleomycin administration (6). After 7
years, in September 1999, an MRI examination documented local
progression of the residual lesion. A further partial resection was
performed by craniotomy. In March 2008, at 22 years of age, a local
progression of the disease with bitemporal hemianopia was observed.
A third partial resection was performed by trans-sphenoidal access.
However, 2 months later (May 2008), the patient was referred to our
RT unit reporting headache and fatigue. The patient was receiving
regular replacement therapy with levothyroxine (100 mg/day),
desmopressin acetate, estradiol, medroxyprogesterone acetate and
somatropin. A neurosurgeon clinically diagnosed a fourth
recurrence. Given the limited prospects of further salvage surgery,
a novel potentially curative treatment with high-dose
step-and-shoot intensity-modulated radiotherapy (IMRT) was
prescribed. IMRT by which the intensity or radiant fluence of each
beam is purposely altered by the summation of hundreds of beamlets,
may represent a valid strategy in order to satisfy clinical goals
of target and normal tissue doses: IMRT has been demonstrated to
reduce the incidence of acute and late cerebral toxicities by
assisting the conformality of dose distribution, confining the
high-dose portions of radiation fields and reducing the absorbed
dose and volume in critical organs (7). A total of 63 Gy in 30 fractions, 5 days
per week were prescribed. Written informed consent was obtained
from the patient and her family.
A brain MRI scan was performed utilizing a Signa
Excite MR 1.5T system (GE Medical Systems, Milwaukee, WI, USA).
Study of the whole brain was conducted with 5-mm slices using the
following sequences: Fluid-attenuated inversion recovery (FLAIR),
gradient echo (GRE) sequence, diffusion weighted imaging (DWI) and
fast spin-echo (FSE) T1- and T2-weighted imaging. Following
infusion of a paramagnetic contrast agent (Dotarem, 0.5 mmol/ml;
Guerbet BP, Paris, France), dynamic coronal sequences of the sellar
region were acquired, followed by the acquisition of FSE
T1-weighted images at a high resolution on the sagittal and coronal
planes, both with a small field of view, targeted to the sellar
region. For all high-resolution sequences, the slice thickness was
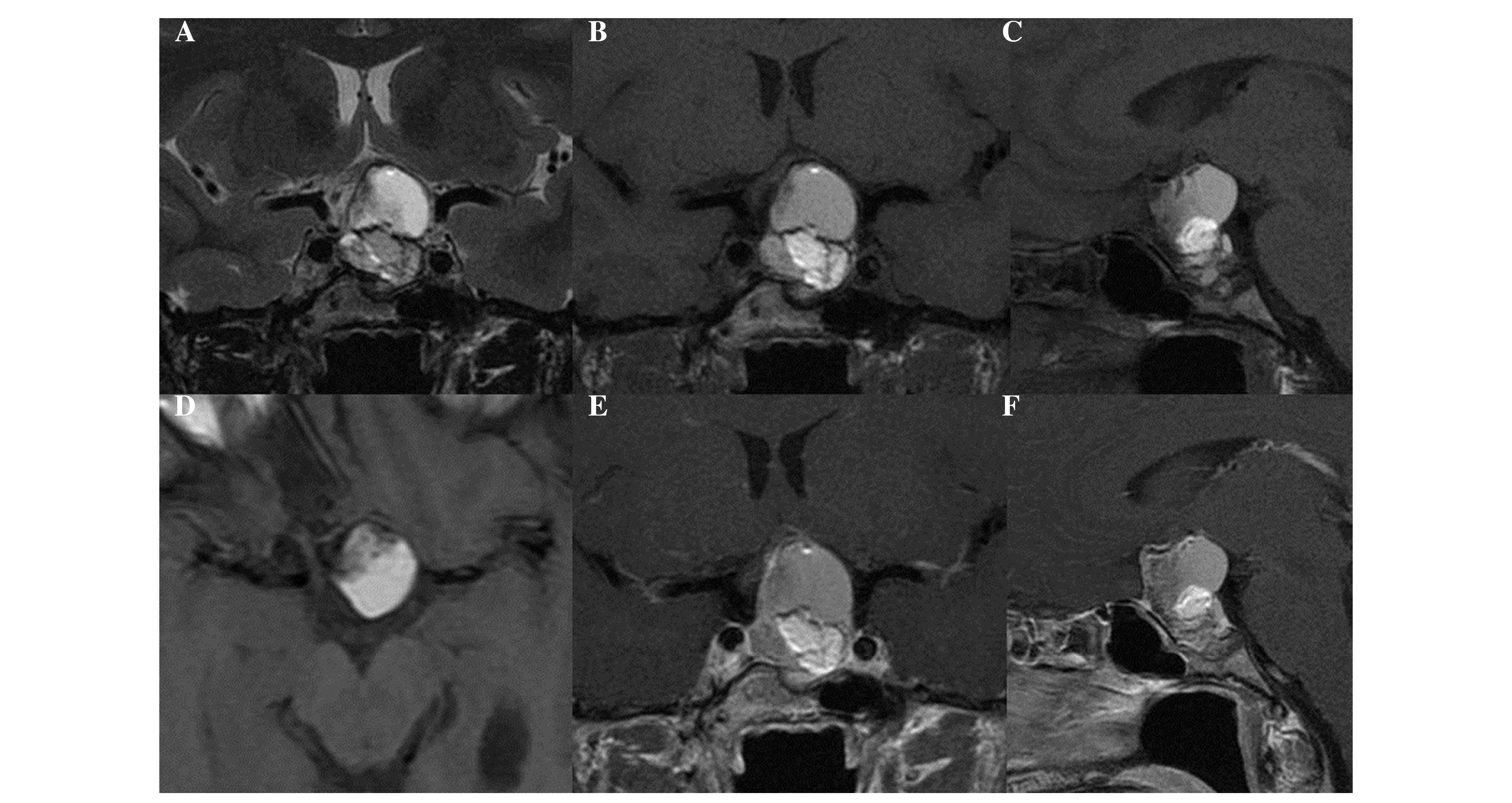
3 mm. The MRI examination demonstrated an inhomogeneous mass
(maximum diameter, 3.3 cm) located in the intra- and suprasellar
regions (Fig. 1). The solid and
cystic components of the expansive mass cranially displaced the
optic chiasm and partially obliterated the chiasmatic cistern.
Thereafter, the patient, immobilized by a
thermoplastic mask, underwent computed tomography (CT) simulation
(2 mm slice spacing) preliminary to radiotherapy. Image fusion
between CT and T1-weighted MRI scans with contrast was performed on
the basis of an anatomy surface-matching approach, a strategy aimed
to match brain surfaces merging anatomical information, or landmark
positions to establish the best correspondence. All clinical
structures were delineated individually on the CT/MR axial slices
by a radiologist (Dr Antonio Pierro) and a radiation oncologist (Dr
Marica Ferro) subsequent to fusion approval by a senior RT
physician (Professor Alessio G Morganti). The gross tumor volume
was defined as the clinical target volume (CTV); to account for
set-up uncertainties, a planning target volume (PTV) was generated
from the CTV by a geometrical expansion of 5 mm. Critical organs at
risk (OAR), including the brainstem, chiasm, optical nerves and
eyes, were contoured. A total dose of 63 Gy with 2.1 Gy/fraction
was prescribed. The following maximum dose (Dmax)
constraints were used: Lens, <5 Gy; eyeballs, <40 Gy; optic
chiasm, <54 Gy; and brainstem, <54 Gy. A treatment plan was
generated using the IMRT dose optimization engine in the Oncentra
MasterPlan treatment planning system (Elekta, Crawley, UK). A class
solution based on 7 fields (5 coplanar and 2 non-coplanar) was
adopted, using an X-ray beam energy of 6-MV. Dose-volume histograms
of the PTVs and OARs were analyzed. Target coverage was assessed by
D5% and D95% metrics, which are the minimum doses delivered to 5
and 95% of the PTV volume, respectively. A uniformity index (UI)
was used and defined as: UI=D5/D95. In addition, the mean dose and
the percentage target volume receiving at least 90% of the
prescribed dose (V90%) were calculated, and the sparing of the OARs
was evaluated by recording their maximum doses. Beams and fields
set-up were iteratively optimized in order to maintain OAR doses
below the critical values and obtain coverage of at least 90% of
the prescribed dose up to 95% of the PTV volume. For the PTV, D95%
and D5% were 90.5 and 103.5% of the prescribed dose, respectively.
The UI was 1.14, PTV V95% was 86.1% and the PTV mean dose was 62.1
Gy. Maximum doses to brainstem and optic chiasma were limited to
55.8 Gy (D1%, 51.2 Gy) and 54.1 Gy (D1%, 53.5 Gy), respectively.
Maximum doses to eyes, lens and optic nerves were limited to 18.4,
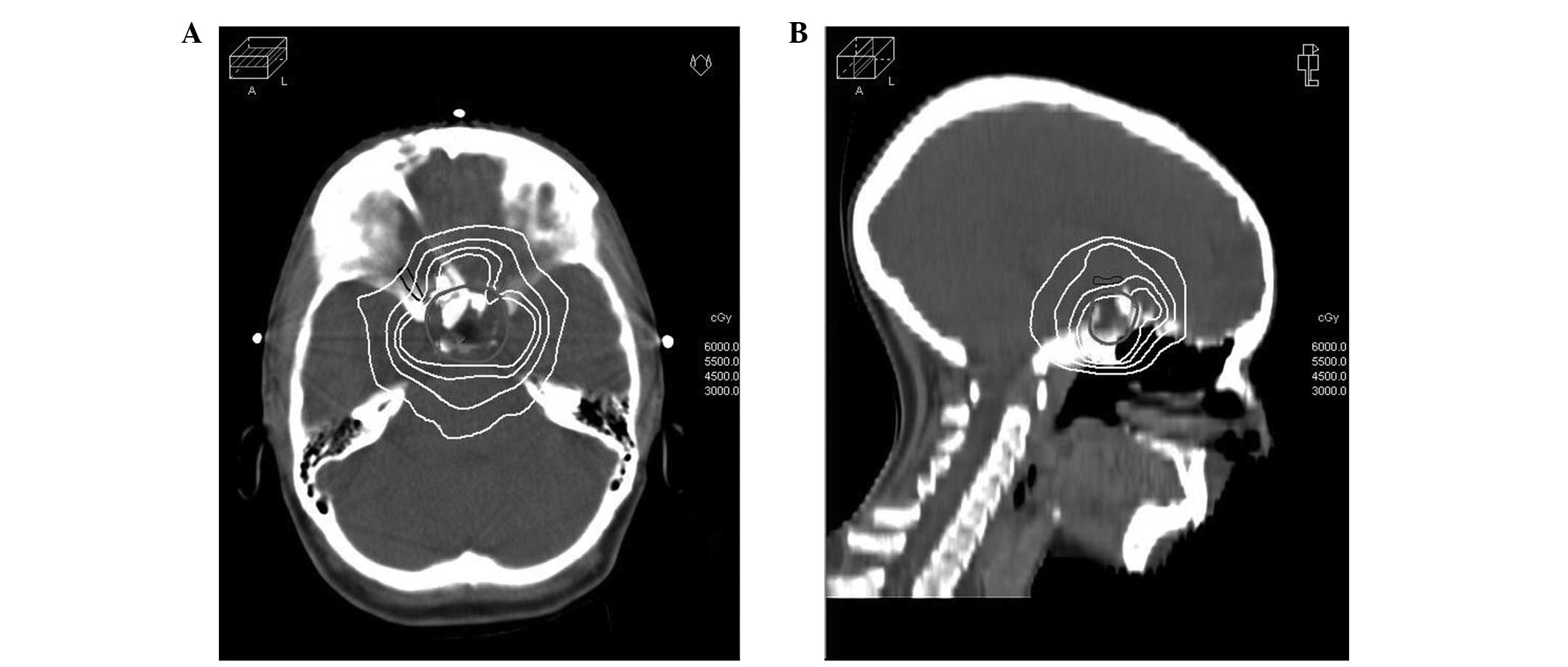
2.8 and 53.9 Gy, respectively. Fig. 2
illustrates the dose distribution in representative transverse and
sagittal planes obtained with IMRT-based treatment planning,
highlighting a highly shaped dose distribution and rapid periphery
fall-off. The treatment was delivered using a Precise Treatment
System linear accelerator, equipped with a standard 40-leaf dynamic
multileaf collimator (Elekta AB, Stockholm, Sweden). The treatment
time for each fraction was ~14 min and the number of monitor units
was 580/fraction. A daily online correction protocol of isocenter
position was applied using portal imaging (EPID I-VIEW; Elekta,
Crawley, UK), with set-up correction in case of deviations >0.3
cm in any direction (8). The
treatment was conducted without interruption and without
significant acute toxicity. At the subsequent follow-up
evaluations, the patient presented patchy alopecia and reported
occasional episodes of tinnitus and mild nausea. Neurological
examinations were normal and clinical controls confirmed the
absence of sequelae (such as headache, drowsiness, visual
disturbances or hearing impairment) associated with the radiation
treatment (3). In addition, the
hormonal situation remained stable and the replacement therapy was
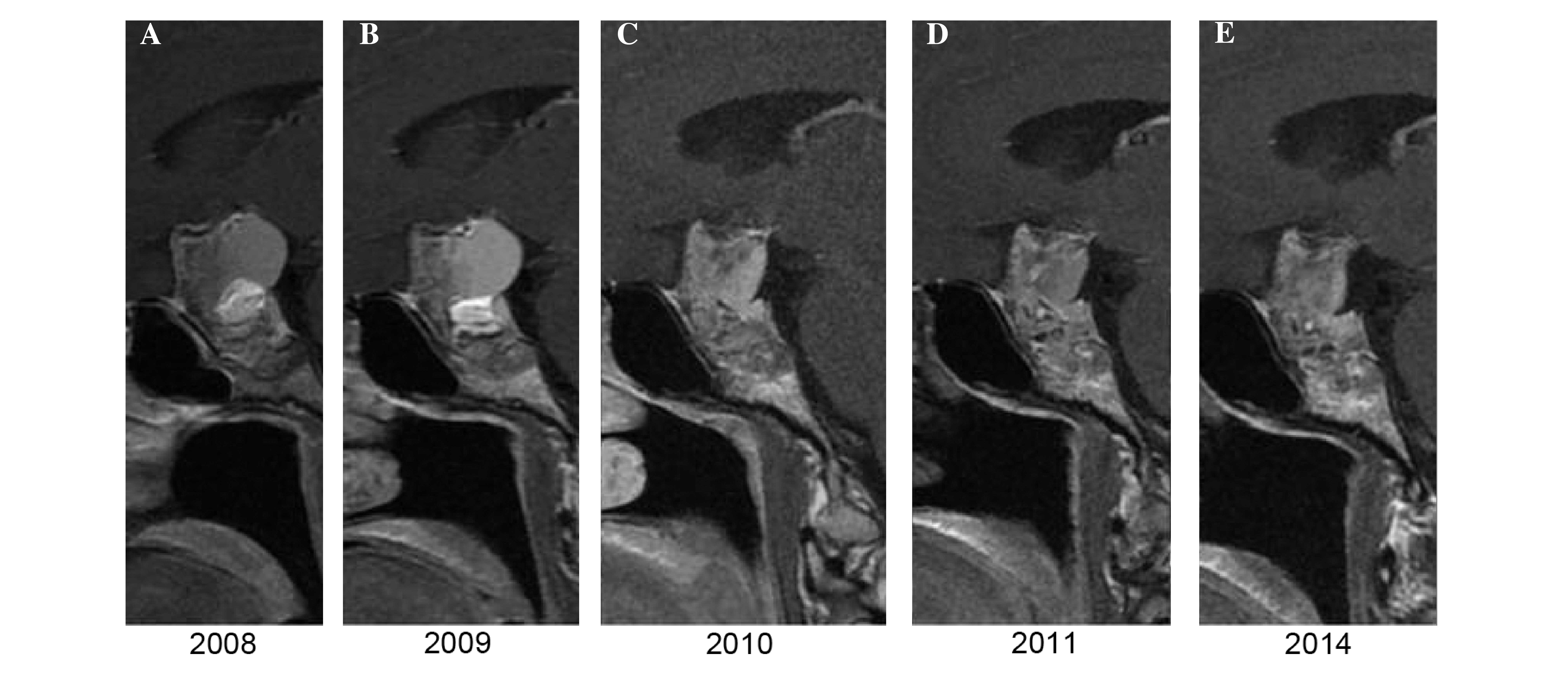
unchanged. Following RT, the patient was checked by MRI once per
year, according to the previously described protocol above. At 1
year subsequent to RT, no dimensional or morphological variations
of the residual disease were observed, while 2 years later, a
millimetric reduction in the size of the largest cystic component
was noted. In all subsequent MRI scans, until 2014, the morphology
and size of the mass remained unchanged, as shown in Fig. 3.
Discussion
In the present study, a patient suffering from a
large CP residue following a third surgical resection was treated
with a high IMRT dosage. Given the benign nature of the tumor and
the requirement to reduce the risk of side effects, particularly
due to the multiple surgical manipulations, a treatment plan
providing the prescribed dose (63 Gy) to a vast majority of the
residual tumor was used, maintaining the lowest possible dose to
the surrounding critical organs. At the 6-year follow-up, the
patient presented no visual or neurological sequelae and did not
require an increase in hormonal therapy. The CP remnants, following
a small dimensional reduction, had remained stable.
The treatment of CP with radiation is particularly
complex due to its proximity to other radiosensitive organs, which
has stimulated the experimentation of advanced irradiation
techniques. Boehling et al (9)
evaluated the use of three-dimensional conformal proton RT (3D-PRT)
and intensity-modulated proton therapy (IMPT) in pediatric patients
with CP, and compared these techniques with IMRT. The authors
observed that the PTV target coverage was adequate for all
modalities. IMRT and IMPT yielded the most conformal plans in
comparison with 3D-PRT; however, the proton therapies were able to
avoid excess integral radiation dose to a variety of normal
structures at all dose levels, while maintaining equal target
coverage (9). Gupta et al
(7) treated 27 patients with
residual, recurrent and/or progressive low-grade intracranial and
skull base tumors with a 54-Gy total dose of tomotherapy. The early
results (median follow-up, 19 months) of their study demonstrated
the 2-year clinicoradiological progression-free survival and
overall survival rates to be 93.3 and 100%, respectively.
MRI is the examination of choice in the diagnosis of
CP and the evaluation of spatial associations between tumors and
adjacent structures (2,10–12). In
the present case, the combination of MRI and CT images allowed
higher precision definition of the RT target and organs at risk. A
further optimization may be based on the use of an adaptive
strategy to take account of changes in PTV, as a result of cyst
expansion, during treatment that might adversely affect tumor
control rates and normal tissue. In fact, these variations can not
be ignored, as documented by a study based on MRI monitoring
throughout the course of RT that showed a target growth in the
majority (64.3%) of cases (13).
In conclusion, to the best of our knowledge, the
current case is the first report of a high-dose (>60 Gy) IMRT CP
treatment with long active surveillance. The use of modern RT
techniques, including IMRT and MRI image fusion, permitted the
achievement of tumor stabilization without late radiation-induced
sequelae.
References
|
1
|
Bunin GR, Surawicz TS, Witman PA,
PrestonMartin S, Davis F and Bruner JM: The descriptive
epidemiology of craniopharyngioma. J Neurosurg. 89:547–551. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Curran JG and O'Connor E: Imaging of
craniopharyngioma. Childs Nerv Syst. 21:635–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winkfield KM, Bazan JG, Gibbs IC, Eng TY
and Thomas CR: Nonmalignant diseasePerez and Brady's Principles and
Practice of Radiation Oncology. Halperin EC, Perez CA and Brady LW:
6th. Lippincott Williams & Wilkins; Philadelphia, PA, USA: pp.
1729–1752. 2013
|
|
4
|
Kalapurakal JA: Radiation therapy in the
management of pediatric craniopharyngiomas - a review. Childs Nerv
Syst. 21:808–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahmathulla G and Barnett GH: Minimally
invasive management of adult craniopharyngiomas: An analysis of our
series and review of literature. Surg Neurol Int. 4 (Suppl
6):S411–S421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hader WJ, Steinbok P, Hukin J and Fryer C:
Intratumoral therapy with bleomycin for cystic craniopharyngiomas
in children. Pediatr Neurosurg. 33:211–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta T, Wadasadawala T, Master Z,
Phurailatpam R, PaiShetty R and Jalali R: Encouraging early
clinical outcomes with helical tomotherapy-based image-guided
intensity-modulated radiation therapy for residual, recurrent,
and/or progressive benign/low-grade intracranial tumors: A
comprehensive evaluation. Int J Radiat Oncol Biol Phys. 82:756–764.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deodato F, Cilla S, Massaccesi M, Macchia
G, Ippolito E, Caravatta L, Picardi V, Romanella M, Di Falco C,
Bartollino A, et al: Daily on-line set-up correction in
3D-conformal radiotherapy: Is it feasible? Tumori. 98:441–444.
2012.PubMed/NCBI
|
|
9
|
Boehling NS, Grosshans DR, Bluett JB,
Palmer MT, Song X, Amos RA, Sahoo N, Meyer JJ, Mahajan A and Woo
SY: Dosimetric comparison of three-dimensional conformal proton
radiotherapy, intensity-modulated proton therapy, and
intensity-modulated radiotherapy for treatment of pediatric
craniopharyngiomas. Int J Radiat Oncol Biol Phys. 82:643–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsuda M, Takahashi S, Higano S, Kurihara
N, Ikeda H and Sakamoto K: CT and MR imaging of craniopharyngioma.
Eur Radiol. 7:464–469. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karavitaki N, Cudlip S, Adams CB and Wass
JA: Craniopharyngiomas. Endocr Rev. 27:371–397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SH, Kwon BJ, Na DG, Kim JH, Han MH
and Chang KH: Pituitary adenoma, craniopharyngioma, and Rathke
cleft cyst involving both intrasellar and suprasellar regions:
Differentiation using MRI. Clin Radiol. 62:453–462. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beltran C, Roca M and Merchant TE: On the
benefits and risks of proton therapy in pediatric
craniopharyngioma. Int J Radiat Oncol Biol Phys. 82:e281–e287.
2012. View Article : Google Scholar : PubMed/NCBI
|