Introduction
Treatment of complex head and neck cancers with
intensity-modulated radiation therapy (IMRT) may facilitate
improved survival and local disease control (1–3). IMRT
conforms to the target and administers a limited radiation dose to
the organs at risk (OAR). However, IMRT also results in increased
whole-body exposure to low-dose radiation, an extended treatment
time (proportional to the total number of segments required) and
increased monitor units (MUs); therefore, treatment efficiency is
reduced. Larger MUs also increase the incidence of radiation
leakage, resulting in a higher risk of development of secondary
cancers. An IMRT plan with fewer segments and MUs may overcome
these treatment complications; however, it is difficult to optimize
the IMRT plan whilst maintaining high quality treatment with good
target conformance, improving the therapeutic ratio and reducing
the risk of secondary cancer.
Hall and Wuu (4)
reported that IMRT with a larger irradiation volume and a lower
dose increased radiation-induced secondary cancer compared with
conventional radiotherapy from ~1 to ~1.75% for patients surviving
10 years. From the forward IMRT plan initially advocated, to the
inverse IMRT plan proposed by Bortfeld (5), planning quality and IMRT methods have
markedly improved. Inverse IMRT is able to significantly improve
the coverage of the planned target with a more uniform dose, while
also decreasing the number of segments and reducing the MUs.
Therefore, inverse IMRT continues to be widely used. There are two
optimization methods for inverse IMRT: Intensity modulation (IM)
fluence and direct step-and-shoot (DSS). The IM fluence
optimization method, also known as the two-step optimization
method, involves fluence optimization, followed by generation of
the segment by combining the multi-leaf collimator (MLC) and other
physical constraints. The segment generated sacrifices the quality
of fluence optimization, and consequently, plan quality is not
improved. Therefore, studies have proposed improved fluence
optimization methods (6–8), including intensity limitation and
segment smoothing; however, these changes are insufficient. For
example, intensity limitation does not prevent increases in the
small MU fluctuation during processing, and segment smoothing
deteriorates plan quality when there is a steep dose gradient
distribution. Matuszak et al (8) proposed the addition of the penalty
function to the segment smoothing method in intensive modulation,
in order to obtain a larger MU reduction. The second optimization
method, DSS, is also known as the one-step optimization method.
With the DSS method, the MLC and other physical constraint
parameters are directly included in the optimization process, and
no subsequent leaf sequencing is required (9–12).
Furthermore, there is no detriment to treatment quality, and the
plan is able to be directly executed. Based on the methods used,
DSS may be further divided into direct machine parameter
optimization (DMPO), in the case of a gradient descent algorithm;
or direct aperture optimization (DAO), in the case of a simulated
annealing algorithm. Since DSS results in improved plan quality and
fewer segments than IM, it has become the preferred optimization
method for inverse IMRT (5,9,10,13). Bedford and Webb (6) reported that the segment shape constraint
function was able to reduce the calculating parameters of
optimization, and combined with the utilization of a smooth and
regular shape to the limit segments, reduced jaggedness was
achievable. They also proposed a feedback optimization method to
generate subsequent segment parameters based on the first segments,
so that a simple and efficient plan may be created. Carlsson
(12) reported a method of generating
fewer, less jagged segments with a large area. This method was
based on the shortest path of fluence gradient downwards; during
the optimization process, irregular or small areas of segments were
avoided, and when it was necessary to alter the dose, the segments
exhibited improved conformal change. Pinnacle's white paper on DMPO
mentioned the gradient descent method, but not the feedback method
(13). In the present study, the
method of DMPO with feedback constraints was applied. The feedback
constraints reflected the dose distribution expected for the
planned target and the mutual association between the geometric and
topological structure between the target and OAR.
Studies by Dobler et al (9,10) revealed
that, in the treatment of head and neck cancer, DMPO exhibited
greater advantages and a wider application than IM. Nasopharyngeal
carcinoma (NPC) is one of the most common types of head and neck
cancer in South China, and the treatment of NPC requires complex
planning as the dose distribution of the target and OAR contain and
compete with each other. To improve the clinical outcome of NPC, an
improved IMRT approach is required. The present study designed 2
types of IMRT plan for the treatment of NPC: The DMPO radiotherapy
(DMPO-RT) method and the feedback constraint DMPO radiotherapy
(fc_DMPO-RT) method. The plan quality, segment number and MU output
of these 2 types of IMRT plan were generated using various methods
and compared, in order to establish which strategy was
superior.
Materials and methods
Patients
Twenty-three patients with NPC were treated at the
Sir Run Run Shaw Hospital (Hangzhou, China) during the latter half
of 2012. The median age of the patients was 57 years (range, 36–70
years), and additional patient characteristics are outlined in
Table I. The present study was
approved by the ethics committee of the Sir Run Run Shaw Hospital
(Hangzhou, China) and all participating patients provided informed
consent.
 | Table I.Patient characteristics (n=23) and
planning target volumes in the present study. |
Table I.
Patient characteristics (n=23) and
planning target volumes in the present study.
| No. | Gender | Age, years | Pathology | Stage | PTV70,
cm3 | mPTV60,
cm3 | mPTV50,
cm3 |
|---|
| 1 | M | 68 | Nk carcinoma | T4N1M0 | 196.2 | 552.8 | 244.0 |
| 2 | M | 65 | Udf carcinoma | T1N2M0 | 154.5 | 637.4 | 399.6 |
| 3 | F | 57 | Nk carcinoma | T2N2M0 | 123.1 | 355.0 | 426.7 |
| 4 | M | 56 | Pdf squamous
carcinoma | T4N1M0 | 206.6 | 83.5 | 612.0 |
| 5 | M | 58 | Nk carcinoma | T4N2M0 | 192.5 | 290.7 | 689.7 |
| 6 | M | 64 | Nk carcinoma | T2N0M0 | 120.1 | 507.6 | 467.5 |
| 7 | M | 52 | Nk carcinoma | T2N2M0 | 216.0 | 342.1 | 117.3 |
| 8 | F | 61 | Udf carcinoma | T2N1M0 |
91.4 | 285.7 | 549.2 |
| 9 | M | 77 | Udf carcinoma | T2N2M0 | 119.5 | 128.6 | 538.3 |
| 10 | M | 49 | Udf carcinoma | T2N2M0 | 123.6 | 583.9 | 311.1 |
| 11 | M | 67 | Udf carcinoma | T2N1M0 | 248.1 | 467.2 | 830.3 |
| 12 | F | 56 | Nk carcinoma | T2N2M0 | 196.5 | 243.3 | 411.3 |
| 13 | M | 54 | Nk carcinoma | T3N0M0 | 149.8 | 492.1 | 295.3 |
| 14 | M | 64 | Nk carcinoma | T3N1M0 | 141.8 | 662.4 | 407.2 |
| 15 | M | 48 | Pdf squamous
carcinoma | T4N1M0 | 135.5 | 438.4 | 363.4 |
| 16 | M | 59 | Nk carcinoma | T4N1M0 | 280.5 | 348.9 | 349.2 |
| 17 | M | 63 | Nk carcinoma | T4N1M0 | 140.7 | 621.3 | 346.1 |
| 18 | M | 36 | Pdf squamous
carcinoma | T1N1M0 |
57.3 | 477.6 | 362.4 |
| 19 | F | 59 | Udf carcinoma | T2N0M0 |
62.6 | 290.0 | 263.9 |
| 20 | M | 52 | Udf carcinoma | T2N1M0 | 196.4 | 679.2 | 183.9 |
| 21 | M | 36 | Pdf squamous
carcinoma | T2N3M0 | 173.1 | 478.9 | 886.9 |
| 22 | M | 46 | Udf carcinoma | T3N1M0 | 153.5 | 484.7 | 199.6 |
| 23 | M | 41 | Udf carcinoma | T3N2M0 | 382.8 | 269.2 | 423.6 |
Immobilization and computed tomography
(CT) scanning
Utilizing head thermoplastic immobilization (Q Fix
Systems, Avondale, PA, USA), patients in the supine position
underwent CT analysis, with a slice thickness of 3 mm using a
SOMATOM Definition AS CT scanner (Siemens AG, Erlangen, Germany).
The scan range extended from the orbital cavity to the
sternoclavicular joint below.
Target and OAR delineation
The primary tumor plus grossly enlarged lymph nodes
were defined as the gross tumor volume (GTV), any microscopic
extensions of the GTV together with the regional lymphatics were
defined as clinical target volume 1 (CTV1), and
potential sites of microscopic extension were defined as clinical
target volume 2 (CTV2). Based on the hospital data
regarding the set-up position error, the appropriative margins of
6.0, 3.0 and 3.0 mm extensions of GTV, CTV1, and
CTV2 were defined as planning target volume (PTV)70,
PTV60 and PTV50, with prescription doses 70, 60 and 50.4 Gy,
respectively. The modified PTV (mPTV) parameter was proposed to
contain only 1 dose level by truncating at the point of overlap
with higher-dose PTVs. Therefore, mPTV50 indicated PTV50 truncated
at where PTV60 and PTV70 overlapped with PTV50, and mPTV60
indicated PTV60 truncated at where PTV70 overlapped with PTV60.
OARs were defined and delineated according to the International
Commission on Radiation Units and Measurements (ICRU) report 83
(14).
Design of treatment plan
Pinnacle v7.6 (Philips Medical Systems, Madison, WI,
USA) treatment planning software (TPS) was used for IMRT planning.
The Pinnacle DMPO optimizer is a RayOptimizer with the Nonlinear
Programming Systems Optimization Laboratory at Stanford core, based
on the gradient function. In every iteration, the DMPO optimizer
uses the gradient of the objective function with respect to the
optimization parameters (leaf positions and weights) to find an
update of the parameters that improves the objective function; the
result includes the machine parameter, allowing it to be directly
delivered without additional conversion. It is important with the
DMPO method to define a control point as iterative initials. In the
present study, when a stage of optimization was completed, this
stage was defined as the initial control point. Subsequently, the
feedback constraints, which compensated for a hot or cold dose
region on the planned target, were added. The feedback constraints
also included areas that presented the mutual association between
the geometric and topological structure of the target and organ
that may compete with the dose. Optimization was recycled until the
optimization outcome met the plan requirements.
For the present planning study, the Siemens Primus
linear accelerator with a double-focused MLC (1 cm resolution at
the isocenter; 27 inner leaf pairs; 6.5 cm for the 2 outer leaf
pairs; 6 MV photon energy) was used. Treatment plans utilized
synchronous boost technology, comprising 33 fractions with a total
dose of 70, 60 and 59.4 Gy for PTV70, mPTV60 and mPTV50,
respectively. In these 33 fractions, the sample with a total dose
of 59.4 Gy for mPTV50 was virtual and convenient for planning,
while 28 fractions with a total dose of 50.4 Gy for mPTV50 was
actual. Thus, following irradiation of 28 fractions, treatment
plans for the remaining 5 fractions were recalculated by discarding
PTV50. The planning study presented here was conducted using the
33-fraction plan. This was considered sufficient for assessment of
the quality of the optimization strategy. The dose constraints of
the target and OAR in the plan were V95% of PTV≥98% and
V110%≤10%, (Vx%, volume percentage of
planning target with X% indicating prescription dose received);
D1cm3 of the spinal cord (1 cm3
volume maximum dose) <42 Gy; D1cm3 of the
brainstem <52 Gy; D50% of the parotid gland (dose on
50% volume) <26 Gy; D1% (dose on 1% volume) of the
optic nerve and optic chiasm <52 Gy; and D1% of the
eye lens as low as possible (≤5 Gy). Two plans were developed for
each patient using the two methods outlined, resulting in a total
of 46 plans for the 23 NPC patients. The 2 types of plan were
designed as follows: i) For the DMPO-RT, 7 coplanar fields with
angles of 280°, 240°, 210°, 180°, 150°, 120° and 80° at the lower
section of the head were chosen in order to locate the mouth and
eyes, which are low tolerance organs, at the end of the exposure
path. Based on the current situation at the Sir Run Run Shaw
Hospital, the parameter in the TPS defining the maximum segment
number for optimization was set to 80, the smallest segment area
was set to 8 cm2 and the minimum MU was set to 8
(segments with less jaggedness, larger area and larger minimum MUs
had better therapeutic accuracy). The DMPO-RT plan was obtained
following optimization based on the above constraint conditions,
and the iteration number was set to 80. The segment and iteration
numbers were set as high as possible, in order to promote the
program optimization process and thus improve plan quality. ii) For
the fc_DMPO-RT, all the conditions for the optimization design
method were identical to those for DMPO-RT, except the segment
number was set to 40–45, and the iteration number was set to 40.
Following the above optimization, the iteration number was reduced
to 20 and the dose distribution deviation (hot or cold regions) was
delineated. Furthermore, certain key areas known as competitive
belts (for example, the joint areas between the brainstem and
target, where the brainstem is in proximity to the target) are in
dose competition with each other; therefore, they have a specific
mutual geometric and topological structure, which must be
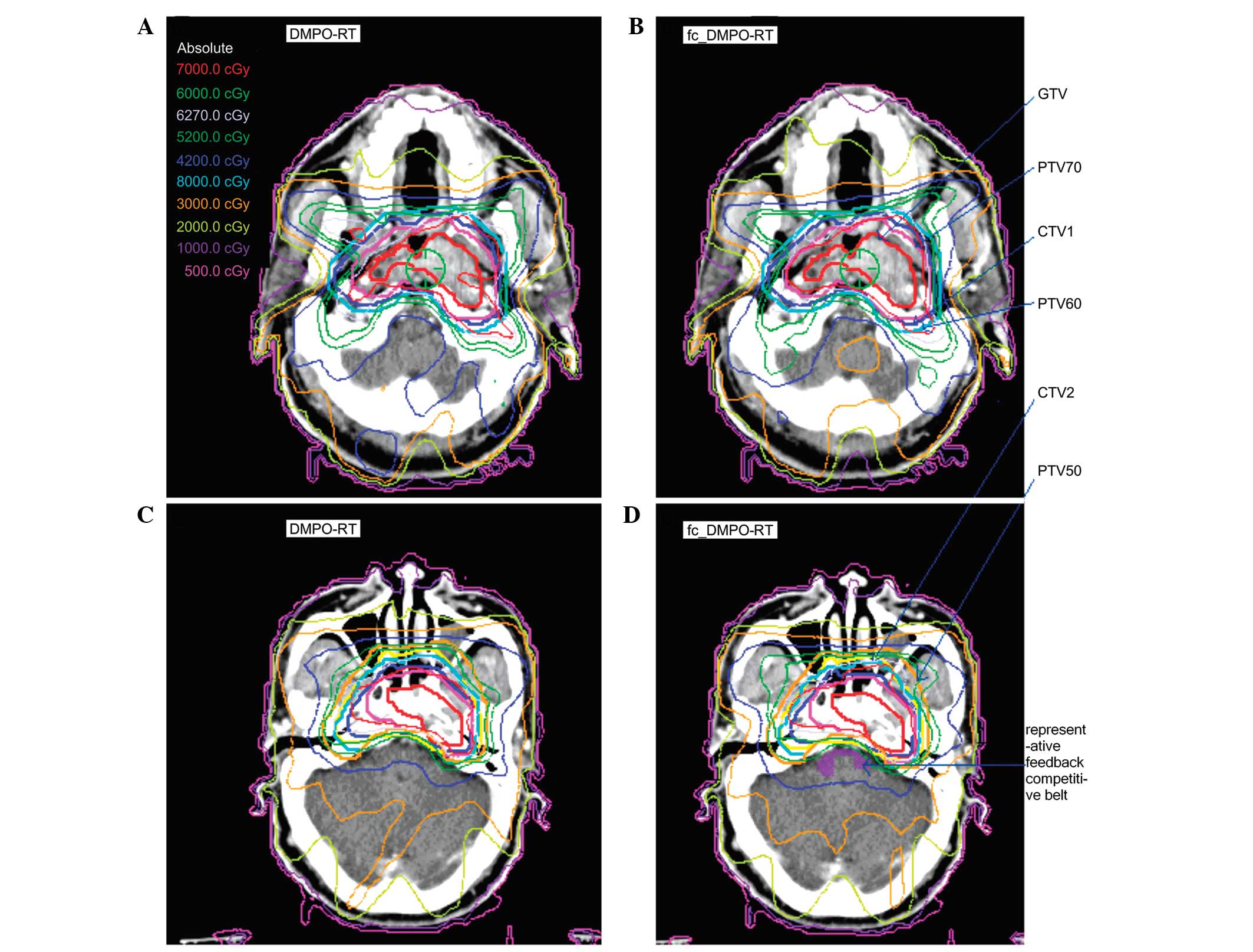
identified and characterized (Fig.
1). Additional competitive belts exist, including those between
the parotid gland and mPTV60 and between the optic chiasm and PTV.
These competitive belts were also delineated during treatment
planning, defined as constraint conditions and fed back into the
original constraint condition. These delineated parameters were
given varying weights, based on priority. The following
optimization was operated by the iteration set to 20. This kind of
optimization by feedback constraints was able to be operated 2–4
times, depending on planning complexity. The iteration number could
also be reduced following a decrease in the complexity of the
feedback condition.
Plan evaluation and comparison
The comparison of dose quality for each plan was
based on the dose distribution and dose-volume histogram (DVH).
Based on the approximate maximum dose of D2%, the
approximate minimum dose of D98% and the median dose of
D50% outlined in the ICRU report 83, as well as the
homogeneity index (HI) and conformity index (CI), the dose
distribution on the target was assessed, where
HI=D5%/D95% (D5% and
D95% are the doses covering 5% and 95% of the PTV,
respectively). The higher the value of the HI, the less homogeneous
the plan. CI was calculated as CI=VTref/VT ×
VTref/Vref (VT, target volume;
VTref, target volume covered by the reference isodose
and Vref, total volume covered by the reference
isodose); the higher the CI value, the better the conformance of
the plan. D1cm3 and Dmean were
computed to evaluate the spinal cord and brainstem; D1%
and Dmean were used to evaluate the optic nerve, optic
chiasm and eye lens; and D50% was employed to evaluate
the parotid gland, where D1cm3 and D1% were
the doses for 1 cm3 and 1% of OAR volume, respectively
and Dmean was the average dose.
Comparison of plan execution
efficiency and dose accuracy
The two kinds of plans were compared in terms of
segment number, MUs and delivery time. Delivery time was calculated
using a tested formula (11)
according to the number of gantry angles, the number of segments
and the number of MUs for a Siemens machine with static IMRT:
Delivery time (sec)=(segment number-1)×9+(total MUs/dose
rate)×60+(number of gantry angles-1)×13. The 2-dimensional diode
array detector verification system (MapCheck; Sun Nuclear Corp.,
Melbourne, FL, USA) was used to test the dose accuracy of the 2
types of plan.
Statistics
Normally distributed data are presented as the mean
± standard deviation. A paired t-test, using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA), was performed to analyze
the two plans. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of dose distribution to the
target
Compared with DMPO-RT, fc_DMPO-RT resulted in a more
rational PTV70 dose distribution. Furthermore, the dose and minimum
dose (D98%) were increased with fc_DMPO-RT, resulting in
a significant improvement in local tumor control. Thus, it was
hypothesized that feedback constraint may supplement the cold point
in PTV70 and improve the conformity of PTV70. With feedback
constraint, the distribution of the total dose volume was enhanced
by slightly increasing the value of the MUs, which were in the
normal fluctuation range of the prescribed PTV70 dose. Areas around
the competitive belt, close to the PTVs, had a tighter dose
gradient and isodose contour distribution (Fig. 1). The dose of mPTV60 also increased,
but this was not as marked a change as that observed in the PTV70,
and there was no statistically significant increase in mPTV50. In
addition, there were no statistically significant differences in HI
and CI (Table II and Fig. 1).
 | Table II.Dose distribution parameters and
comparisons of PTV70, mPTV60 and mPTV50 for the two plans (n=23 per
group). |
Table II.
Dose distribution parameters and
comparisons of PTV70, mPTV60 and mPTV50 for the two plans (n=23 per
group).
| Target | Plan | D50%,
cGy | D2%,
cGy | D98%,
cGy | HI | CI |
|---|
| PTV70 | fc_RT |
7340.1±107.0a |
7903.4±168.8b |
6767.5±197.1c |
1.1±0.0 |
0.5±0.1 |
|
| RT |
7139.0±58.2 |
7695.5±141.5 |
6417.3±298.8 |
1.2±0.0 |
0.4±0.1 |
| mPTV60 | fc_RT |
6212.6±91.5d |
7026.3±150.7 |
4767.6±531.8 |
1.3±0.1 |
0.4±0.1 |
|
| RT |
6127.2±68.1 |
6959.7±92.6 |
4700.8±538.8 |
1.3±0.1 |
0.4±0.1 |
| mPTV50 | fc_RT |
5102.6±209.5 |
5692.7±88.4 |
3596.2±1272.3 |
1.5±0.4 |
0.5±0.1 |
|
| RT |
5041.8±172.4 |
5639.9±121.3 |
3540.2±1262.7 |
1.5±0.5 |
0.5±0.1 |
Comparison of dose distribution to the
OAR
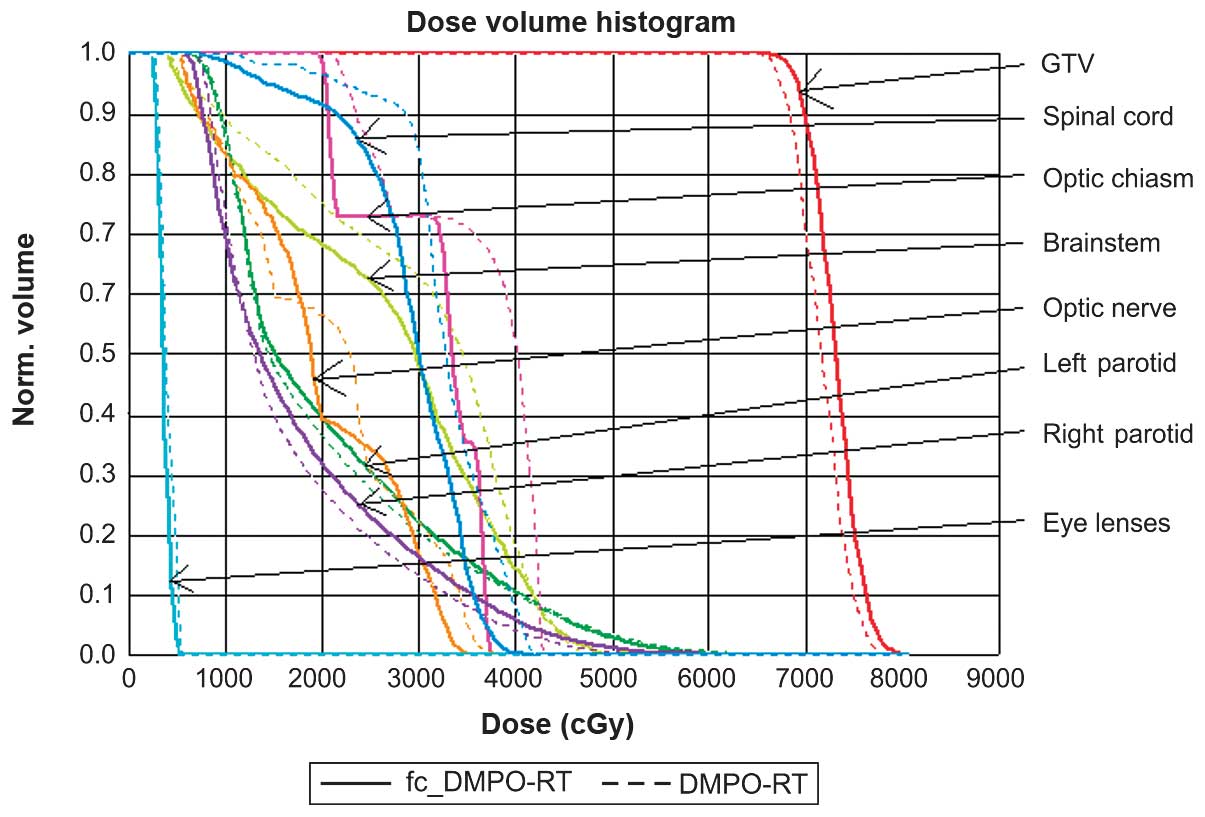
fc_DMPO-RT resulted in a significant decrease
(P<0.05) in the maximum dose to the spinal cord as compared with
that of DMPO-RT. There were no statistically significant
differences in doses to the brainstem, optic chiasm, optic nerve,
parotid gland or eye lenses between the two methods. The dose
distribution to the OAR for both methods met the clinical
requirements well (Table III). A
representative DVH for the 23 patients is depicted in Fig. 2.
 | Table III.Dose distribution parameters and
comparisons of doses to the spinal cord, brainstem, parotid gland,
optic chiasm, optic nerve and eye lenses for the 2 plans (n=23 per
group). |
Table III.
Dose distribution parameters and
comparisons of doses to the spinal cord, brainstem, parotid gland,
optic chiasm, optic nerve and eye lenses for the 2 plans (n=23 per
group).
| A, Spinal cord,
brainstem and parotid gland |
|---|
|
|---|
|
| Spinal cord | Brainstem | Parotid gland
D50%, cGy |
|---|
|
|
|
|
|
|---|
| Plan |
D1cm3, cGy | Dmean,
cGy |
D1cm3, cGy | Dmean,
cGy | Left | Right |
|---|
|
| fc_RT |
3907.7±91.3a |
3178.8±235.0 |
4790.4±238.7 |
3475.2±345.0 |
2304.8±913.1 |
2006.5±436.4 |
| RT |
3982.6±97.1 |
3241.7±217.2 |
4698.8±278.1 |
3463.5±243.5 |
2248.4±928.7 |
1890.7±440.8 |
|
| B, Optic chiasm,
optic nerve and eye lenses |
|
|
| Optic chiasm | Optic nerve | Eye lenses |
|
|
|
|
|
| Plan | D1%,
cGy | Dmean,
cGy | D1%,
cGy | Dmean,
cGy | D1%,
cGy | Dmean,
cGy |
|
| fc_RT |
4993.5±1189.8 |
4168.0±1357.6 |
4662.7±1511.6 |
3061.6±1266.6 |
489.5±46.7 |
401.6±50.5 |
| RT |
4946.0±1128.1 |
4112.4±1384.2 |
4453.8±1421.9 |
3052.5±1329.5 |
498.0±59.0 |
409.5±57.4 |
Comparison of treatment efficiency and
dose verification
There was a significant difference in the MUs, total
numbers of segments and delivery time between the two methods
(t=6.2, 393.4 and 244.3, respectively; P<0.05). Delivery
time was reduced by 26.8% with fc_DMPO-RT, compared with that of
DMPO-RT. However, the MapCheck verification pass ratio indicated no
statistical difference between the 2 plans (Table IV).
 | Table IV.Comparison of MUs, segment numbers,
delivery time and pass ratio of the 2 plans (n=23). |
Table IV.
Comparison of MUs, segment numbers,
delivery time and pass ratio of the 2 plans (n=23).
| Plan | MUs, Mu | Segment
numbers | Delivery time,
sec | Pass ratio, % |
|---|
| fc_RT |
992.4±99.1a |
39.9±1.7b |
725.6±36.6c |
94.8±0.7 |
| RT |
1054.3±67.0 |
67.3±6.4 |
990.6±72.7 |
94.8±0.8 |
Discussion
In addition to DVH and dose assessment, the
efficiency of planning execution should be carefully considered in
the evaluation of the complex IMRT plan. Although the use of a
greater number of segments improves the plan quality, there is a
threshold for the maximum number of segments (12,13,15). When
the segment number reaches a certain threshold, the plan is unable
to be further improved as a larger number of segments will increase
the plan complexity and sacrifice the efficiency of plan execution.
In addition, a larger number of segments will result in a longer
delivery time, thereby decreasing the accuracy of radiotherapy due
to the variable changes in patient location and fractional
position. In addition, the excessive radiation leakage may increase
the risk of secondary cancer, and the biological effects of the
treatment would be poor (4,16).
Advancements in complex IMRT planning have continued
to improve. A particular method aimed to reduce the total number of
segments and MUs with equal plan quality. Bratengeier et al
(5,17–19)
reported that segments should reflect the geometry and topology of
the PTV and OAR in the research of plan optimization by segment
reduction. Therefore, segments should fit well with the PTV and OAR
structures, and consequently, the plan should be more efficient.
Accordingly, they proposed to construct the segments prior to
optimization. Three types of segment were created: 0 order
segments, comprising conformal PTV including the OAR; 1 order
segment, to shape PTV with the OAR blocked; 2 order segment,
compensation of block-out-losses in the PTV with multi-directions
and narrowed to direct towards PTV areas adjacent to the OAR
(5,17–21). The
use of the 2 order segment may make the dose distribution gradient
between the PTV and OAR steeper and more desirable. Based on these
3 types of segment, it was suggested that this DMPO plan required
fewer segments than Pinnacle's DMPO plan, while maintaining
equivalent or better plan quality. However, the MUs in their DMPO
plan were slightly greater than those in Pinnacle's DMPO plan. The
IM plan is not comparable to these DMPOs in terms of quality
(18); however, it was also suggested
that quantitative definition and introduction of the 2 order
segment has a key role in determining plan quality (5,17). The
DMPO with the initial optimal point of 2 order segments may have a
more rapid and improved convergence (5). In the present study, a large segment
with less jaggedness was utilized by limiting the smallest segments
and MUs, prior to the introduction of the feedback constraint
optimization method. This feedback constraint included intensive
modulation for the anticipated dose distribution, as well as
adaptive modulation for the anatomy and geometry of the PTV and
OAR. For example, the feedback intensive modulation on the space
between the GTV and brainstem resulted in a steeper dose gradient.
The methods demonstrated in the present study may be similar to the
aforementioned 2 order segment proposed by Bratengeier et al
(5,17–21). The
present study highlighted that the timely introduction of feedback
based on the anticipated dose distribution and the interactive
position between the PTV and OAR may have adaptive and intelligent
processing, resulting in an improved IMRT plan. In the fc_DMPO-RT
method, the segment created to optimally fit with the geometric
shape of PTVs and the anatomical structure of OARs was able to
better reflect the relative position of each object (including PTVs
and OARs) and the dose gradient of competitive belts. Thus, fewer
segments may be sufficient for the construction of good conformity
between the fc_DMPO-RT plan with the DMPO-RT plan, while achieving
similar conformity of the DMPO-RT plan requires a greater number of
assembled segments.
A good IMRT plan should have few segments and MUs.
However, there is a trade-off between the quality of the plan and
the number of segments. Considering the distribution of the OAR and
PTV structure and the degree of complexity, a plan requires varying
segment numbers. Based on DAO, Jiang et al (15) reported that the number of segments
should be ≥60 for complex head and neck cancers, while Ludlum and
Xia (11) reported that using the
Pinnacle DMPO, treatment of NPC requires >50 segments. In the
present study, the treatment plan for NPC required ~40 segments to
optimize the plan and meet clinical requirements.
In the DMPO-RT plan, the TPS parameter of the
maximum number of segments was set to 80, which was considered
sufficient for optimization. The optimizer had no restriction on
the maximum number of segments and automatically consumed
sufficient segments in every plan to build the perfect plan.
Nevertheless, the number of segments in the DMPO-RT plan did not
exceed 80. If identical maximum numbers of segments were set in the
DMPO-RT plan as in the fc_DMPO-RT plan, the optimized DMPO-RT plan
quality would not be comparable with that when the plan was set to
80 maximum segments. The fc_DMPO-RT plan, which incorporated
feedback constraint optimization, may obtain improved quality with
40 segments than that of the DMPO-RT plan with 80 segments, thereby
meeting the clinical treatment requirements.
In planning studies for head and neck cancer, a
primary concern has been regarding how to reduce MUs as much as
possible. Table V lists several
studies using the DMPO method with DSS radiation technology. The
MUs in the present study were similar to those reported in studies
by Dobler et al (10) and
Ludlum and Xia (11). However, the
MUs reported in the present study were greater than those reported
by Oliver et al (22).
Therefore, MUs may be relative to the TPS version, cancer site or
beam setup; and a more detailed discussion of this topic is outside
the scope of this article.
 | Table V.Comparison of data and MUs resulting
from various plans. |
Table V.
Comparison of data and MUs resulting
from various plans.
| Study | MUs | TPS | Beams | Cancer site |
|---|
| Present study | 992.4±99.0 | Pinnacle, V7.6 | 7, UES | Nasopharyngeal |
| Ludlum and Xia,
2008 | 1050 | Pinnacle, V7.6 | 7, UES | Nasopharyngeal |
| Dobler et
al, 2009 | 944±160 | Oncentra
masterplan, V1.5 | 9, ES | Oropharyngeal |
| Oliver et
al, 2012 | 711.6±68.1 | Pinnacle, V9.0 | 7, ES | Larynx; tonsil;
base of tongue; oropharynx; hypopharynx |
The plan delivery time is not only proportional to
the number of segments and MUs of the plan, but is also dependent
on the accelerator machine performance, including gantry angling
and segment formation times; if the former is larger than the
latter, gantry angling will consume more time during treatment and
the delivery time will be increased.
There were certain limitations to the present study.
Bratengeier et al (5,17) reported that the number of segments may
be decreased with an increase in beam angles and that plan quality
is able to be maintained or even improved. Therefore, for the
static IMRT methods of treatment of NPC proposed in this paper, 7
angles may be insufficient, and the application of ≥9 beam angles
in IMRT may be evaluated by further study. Furthermore, there is no
strict mathematical proof or logical reasoning behind the methods
of optimization presented in the current study. In addition, the
present method only addressed optimization of the IMRT plan for NPC
patients, but may also apply to complex IMRT planning for other
carcinomas, including prostate and breast cancer. Finally, rather
than developing a novel in-house planning system, the method used
in the current study employed Pinnacle, a commercial TPS, to
illustrate the methods of feedback constraint and its
effectiveness.
Although intensity-modulated arc therapy and
volumetric-modulated arc therapy have advantages over IMRT in terms
of exposure time, IMRT remains the gold standard strategy for a
complex treatment plan, for example that required for the treatment
of NPC. It is important to optimize the IMRT plan with fewer
segments and MUs while maintaining or even increasing the plan
quality. An optimized IMRT plan incorporating these criteria may
improve the therapeutic ratio and reduce treatment time, resulting
in an improved machine utilization rate with more patients treated
per hour. Therefore, we propose that the fc_DMPO-RT method is
promising and should be considered for IMRT planning.
Acknowledgements
The authors would like to thank Dr Li Englobe
(Medkey Med-Tek Development Co., Ltd., Hangzhou, China) for his
English language assistance.
References
|
1
|
Chao KS, Majhail N, Huang CJ, Simpson JR,
Perez CA, Haughey B and Spector G: Intensity-modulated radiation
therapy reduces late salivary toxicity without compromising tumor
control in patients with oropharyngeal carcinoma: A comparison with
conventional techniques. Radiother Oncol. 61:275–280. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rothschild S, Studer G, Seifert B,
Huguenin P, Glanzmann C, Davis JB, Lütolf UM, Hany TF and Ciernik
IF: PET/CT staging followed Intensity-Modulated Radiotherapy (IMRT)
improves treatment outcome of locally advanced pharyngeal
carcinoma: A matched-pair comparison. Radiat Oncol. 2:222007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Studer G, Zwahlen RA, Graetz KW, Davis BJ
and Glanzmann C: IMRT in oral cavity cancer. Radiat Oncol.
2:162007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hall EJ and Wuu CS: Radiation-induced
second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bortfeld T, Burkelbach J, Boesecke R and
Schlegel W: Methods of image reconstruction from projections
applied to conformation radiotherapy. Phys Med Biol. 35:1423–34.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bedford JL and Webb S: Constrained segment
shapes in direct-aperture optimization for step-and-shoot IMRT. Med
Phys. 33:944–958. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coselmon MM, Moran JM, Radawski JD and
Fraass BA: Improving IMRT delivery efficiency using intensity
limits during inverse planning. Med Phys. 32:1234–1245. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matuszak MM, Larsen EW and Fraass BA:
Reduction of IMRT beam complexity through the use of beam
modulation penalties in the objective function. Med Phys.
34:507–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dobler B, Pohl F, Bogner L and Koelbl O:
Comparison of direct machine parameter optimization versus fluence
optimization with sequential sequencing in IMRT of hypopharyngeal
carcinoma. Radiat Oncol. 2:332007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dobler B, Koelbl O, Bogner L and Pohl F:
Direct machine parameter optimization for intensity modulated
radiation therapy (IMRT) of oropharyngeal cancer - a planning
study. J Appl Clin Med Phys. 10:30662009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carlsson F: Combining segment generation
with direct step-and-shoot optimization in intensity-modulated
radiation therapy. Med Phys. 35:3828–3838. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hårdemark B, Liander A, Rehbinder H and
Löf J: P3IMRT®: Direct machine parameter optimization.
Pinnacle3® White Paper No. 4535_983_02483. 2003.
|
|
13
|
Ludlum E and Xia P: Comparison of IMRT
planning with two-step and one-step optimization: A way to simplify
IMRT. Phys Med Biol. 53:807–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
ICRU: Report 83: Prescribing, recording
and reporting photon-beam intensity-modulated radiation therapy
(IMRT). J ICRU. 10:NP. 2010.
|
|
15
|
Jiang Z, Earl MA, Zhang GW, Yu CX and
Shepard DM: An examination of the number of required apertures for
step- and-shoot. IMRT. 50:5653–5663. 2005.
|
|
16
|
Hall EJ: Intensity-modulated radiation
therapy, protons and the risk of second cancers. Int J Radiat Oncol
Biol Phys. 65:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bratengeier K, Gainey MB and Flentje M:
Fast IMRT by increasing the beam number and reducing the number of
segments. Radiat Oncol. 6:1702011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bratengeier K, Meyer J and Flentje M:
Pre-segmented 2-Step IMRT with subsequent direct machine parameter
optimisation - a planning study. Radiat Oncol. 3:382008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bratengeier K, Polat B, Gainey M, Grewenig
P, Meyer J and Flentje M: Is ad-hoc plan adaptation based on 2-Step
IMRT feasible? Radiother Oncol. 93:266–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bratengeier K: 2-Step IMAT and 2-Step
IMRT: A geometrical approach. Med Phys. 32:777–785. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bratengeier K: 2-Step IMAT and 2-Step IMRT
in three dimensions. Med Phys. 32:3849–3861. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oliver M, McConnell D, Romani M,
McAllister A, Pearce A, Andronowski A, Wang X and Leszczynski K:
Evaluation of the trade-offs encountered in planning and treating
locally advanced head and neck cancer: Intensity-modulated
radiation therapy vs dual-arc volumetric-modulated arc therapy. Br
J Radiol. 85:1539–1545. 2012. View Article : Google Scholar : PubMed/NCBI
|