Introduction
As a novel member of the inhibitor of apoptosis
protein, Livin has been demonstrated to promote cellular
proliferation and result in the resistance of tumors to
chemotherapeutic medications under overexpression conditions
(1). Livin was also revealed to be
involved in the apoptosis of human bladder cancer cells through
caspase-3 (2) and closely associated
with recurrent bladder cancer, indicating the significance of Livin
in the treatment of bladder cancer (1,3).
Mitomycin-C (MMC) is a widely used antitumor chemical and is
suggested to be useful in treating non-invasive bladder tumors
(4), while the suppression of Livin
may increase the apoptosis of bladder cancer T24 cells induced by
MMC (5). This suggests a potential
correlation of Livin inhibition with antitumor medications in tumor
therapy.
RNA interference (RNAi), including short interfering
RNA (siRNA) and short hairpin RNA, is one newly developed
technology to silence post-transcriptional genes and is widely used
in biopharmaceutical studies (6).
Through siRNA and lipofection techniques, the present study
delivered specific siRNA targeting Livin into EJ human bladder
carcinoma cells to measure the expression of Livin in transfected
cells, the effect of siRNA Livin on the apoptosis of EJ cells and
the sensitivity of EJ cells to chemotherapeutic medication. This
study is expected to provide a further theoretical and experimental
basis for the use of chemotherapy in bladder carcinoma.
Materials and methods
Design of specific siRNA targeting
Livin
In accordance with GenBank, the common sequences of
isomers (α, NM_139317 and β, NM_022161) of human Livin (AF311388)
designed and synthesized by Shanghai GenPharma Company (Shanghai,
China) were sense, 5′-GAGAGAGGUCCAGUCUGAATT-3′ and antisense,
5′-UUCAGACUGGACCUCUCUCTT-3′. Non-targeting homologous genes were
excluded through comparison with BLAST. Non-human complementary
siRNA sequences (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) were used as negative controls and
labeled with FAM fluorescence (negative control FAM).
Cell culture and transfection
EJ human bladder carcinoma cells (Nanjing KeyGen
BioTech. Co. Ltd., Nanjing, China) were cultured in a 5%
CO2 incubator at 37°C with RPMI-1640 medium (HyClone,
Logan, UT, USA) supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin. EJ cells from the logarithmic phase were
inoculated in 6-well or 96-well plates containing RPMI-1640 medium.
When the confluence reached 40–60%, the cells were assigned into
different groups according to treatment: blank control group
(without treatment), intervention group [with the same volume of
siRNA and Lipofectamine® 2000 reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA)], chemotherapy group [24 h
treatment with 0.1 mg/ml MMC (Sigma-Aldrich, St. Louis, MO, USA)],
or combination group (24 h siRNA transfection and 24 h treatment
with 0.1 mg/ml MMC). Cells were transfected with negative siRNA or
with Lipofectamine 2000 reagent alone. The transfection rate of
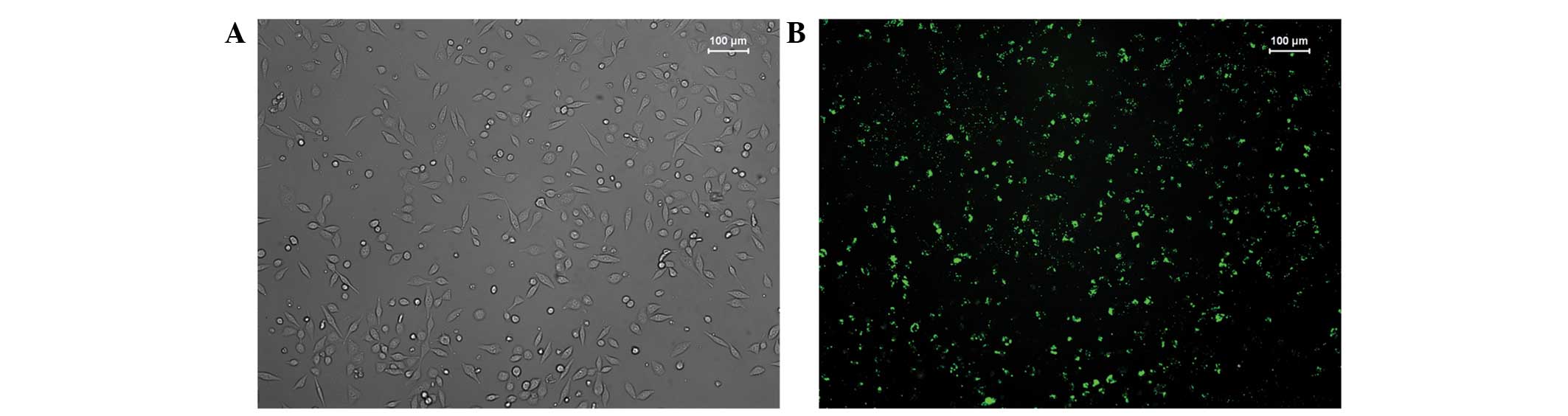
cells was observed under inverted fluorescence microscopy after
24–48 h (Fig. 1). Our preliminary
experiments revealed that the transfection rate in the 6-well plate
reached 70% under conditions of 3×105 cells/well, 100
pmol/well siRNA and 5 µl/well Lipofectamine 2000 reagent.
Similarly, the transfection rate in the 96-well plate reached 70%
under conditions of 4×103 cells/well, 5 pmol/well siRNA
and 0.25 µl/well Lipofectamine 2000 reagent.
Measurement of Livin mRNA with
semi-quantitative reverse transcription-polymerase chain reaction
(RT-PCR)
After 24 h of transfection, the cells were rinsed
with phosphate-buffered saline (PBS) twice, then 1 ml TRIzol was
added to the lysate cells. The total RNA was extracted according to
the manual and its quantity was measured using a UV
spectrophotometer (Beijing ComWin Biotech Co., Ltd., Beijing,
China). cDNA was synthesized under conditions of 42°C for 40 min
and 85°C for 5 min, and used as a template for the amplification of
the Livin gene with pre-denaturation at 94°C for 2 min, 30 cycles
of denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec
and extension at 72°C for 30 sec, then final extension at 72°C for
2 min. The sense and antisense primers of Livin and β-actin genes
were 5′-GTGGATGGGCAGATCCTG-3′ and 5′-CCTTGTCCTGATGGCCTG-3′
(resulting in the production of 214 bp), and
5′-AGGTGACAGCAGTCGGTTGG-3′ and 5′-CGAAGGCTCATCATTCAAAA-3′
(resulting in the production of 300 bp), respectively, synthesized
by Beijing AuGCT DNA-SYN Biotechnology Co. Ltd. (Beijing, China).
Following the reaction, the PCR production was identified with
electrophoresis in 1.5% sucrose and analyzed with a SensiAnsys
gel-imaging system (LI-COR Biosciences, Lincoln, NE, USA). The
expression of Livin mRNA was semi-quantitatively calculated using
the optical density ratio of Livin and β-actin. The experiment was
repeated three times.
Measurement of Livin protein with
western blot analysis
Following 48 h of transfection, the EJ cells were
removed from the medium, rinsed twice with pre-cooled PBS, mixed
with 100 µl RIPA lysis buffer and 10 µl PMSF in an ice-bath for 10
min, and centrifuged at 12,000 rpm at 4°C for 10 min. The total
cellular protein was collected and quantified with the
bicinchoninic acid assay. Then 30 µg protein was loaded onto 12%
polyacrylamide gel for electrophoresis to isolate the protein,
which was transferred to a polyvinylidene fluoride membrane using
the semi-dry method and blocked with Tris-buffered saline and
Tween-20 (TBST) solution containing 5% skimmed milk at room
temperature for 2 h. Primary Livin antibody (1:500) and β-actin
antibody (1:1000; both from Beijing ComWin Biotech Co., Ltd.) were
added for incubation at 4°C overnight. The membrane was rinsed with
TBST three times and horseradish peroxidase-conjugated secondary
antibodies (goat anti-rabbit 1:2000; Beijing ComWin Biotech Co.,
Ltd.) were added for incubation at room temperature for 1 h. After
washing, the membrane was developed with the enhanced
chemiluminescence method and exposed under a photographic system.
The optical density of Livin protein was compared with that of
β-actin as an internal reference. The experiment was repeated three
times.
Cellular proliferation measurement by
Cell Counting Kit-8 (CCK-8) assay
The cells were inoculated in 96-well plates at 100
µl/well with five repetition wells for each group, and cultured in
a 5% CO2 incubator at 37°C. Then the medium was replaced
by 10 µl CCK-8 (Shanghai Yeasen Biotech Company, Shanghai, China)
for 2 h incubation and the absorption (D) was measured at 450 nm to
calculate the relative inhibition rate of cell proliferation
according to the formula: inhibition rate (%) = [(D in control
group - D in experimental groups) / D in control group] ×100%. The
experiment was repeated three times.
Measurement of cell apoptosis by flow
cytometry
EJ cells were inoculated in six-well plates as
above. Following treatment, the cells were digested with
ethylenediaminetetraacetic acid-free trypsin and centrifuged at
2,000 rpm at room temperature for 5 min. Then the cells were
re-suspended with 1X PBS (4°C) and centrifuged. After removal of
the supernatant, the cells were mixed with 300 µl 1X binding buffer
and 5 µl Annexin V-FITC (Beijing Jiamay Biotech, Beijing, China),
gently agitated and incubated at room temperature in the dark for
45 min. Then the cell suspension was filtered with a 300-mesh nylon
net into the flow tube. A total of 5 µl propidium iodide and 200 µl
1X binding buffer was added 5 min prior to and immediately before
loading. The apoptosis was measured by BD FACSAria-III (Beijing
Jiamay Biotech) and analyzed with Modfit LT3.3 (Verity Software
House, Topsham, ME, USA). The experiment was repeated three
times.
Statistic analysis
All data were expressed as the means ±
standard deviation. SPSS 19.0 (IBM SPSS, Armonk, NY, USA) was used
for analysis with one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
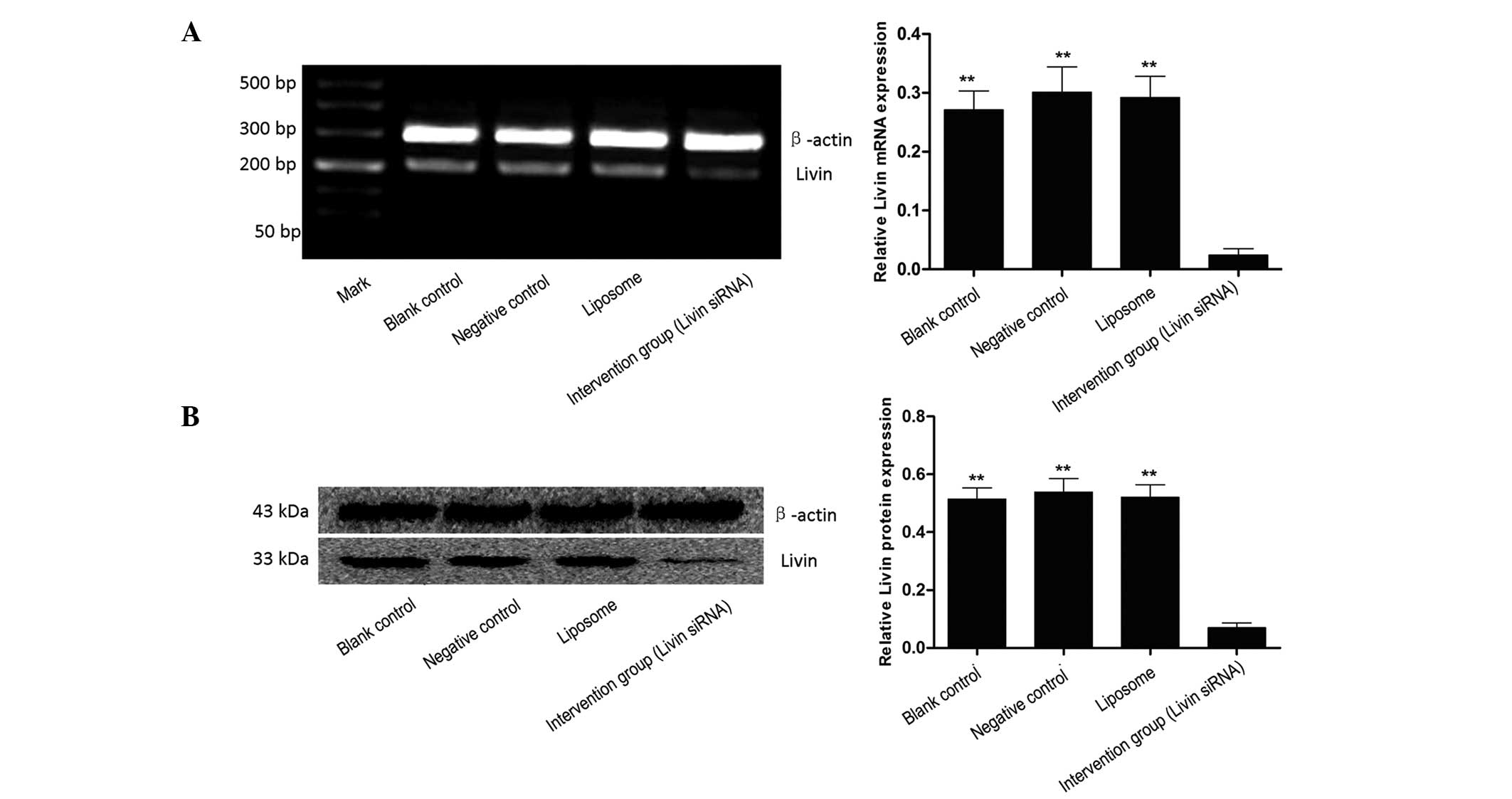
Inhibition of Livin mRNA by siRNA
After 24 h of transfection with siRNA Livin, the
expression intensity of Livin mRNA in the blank control, negative
control, liposome and intervention group (siRNA Livin) was
0.271±0.032, 0.301±0.043, 0.292±0.036 and 0.024±0.011,
respectively, indicating that siRNA Livin significantly inhibited
the expression of Livin mRNA (P<0.01). There was no significant
difference between the blank control, negative control and liposome
group (P>0.05, Fig. 2A).
Inhibition of Livin protein by siRNA
Livin
Western blot analysis further indicated that the
Livin protein in EJ cells was significantly decreased following the
decrease of gene transcription. After 48 h of transfection, the
relative expression of Livin protein in the blank control, negative
control, liposome and intervention group (siRNA Livin) was
0.515±0.038, 0.539±0.047, 0.521±0.043 and 0.070±0.016,
respectively. The statistic analysis indicated that Livin protein
in EJ cells was significantly decreased following transfection of
siRNA Livin (P<0.01). There was no significant difference
between the blank control, negative control and liposome group
(P>0.05, Fig. 2B).
Inhibition of EJ cell proliferation by
siRNA Livin
Measurement of CCK-8 indicated that the
proliferation of EJ cells was inhibited by siRNA Livin. The
inhibition in the combination group was significantly higher than
in the single intervention and single chemotherapy group
(P<0.01, Table I).
 | Table I.Inhibition of proliferation of EJ
cells in different groups (mean ± standard deviation). |
Table I.
Inhibition of proliferation of EJ
cells in different groups (mean ± standard deviation).
| Groups | Optical density | Inhibition rate
(%) |
|---|
| Blank control
group |
0.862±0.174 |
2.14±0.761 |
| Intervention
group |
0.794±0.163 |
7.787±4.032a,b |
| Chemotherapy
group |
0.660±0.156 |
23.375±7.813a,b |
| Combination
group |
0.471±0.096 |
45.355±5.617b |
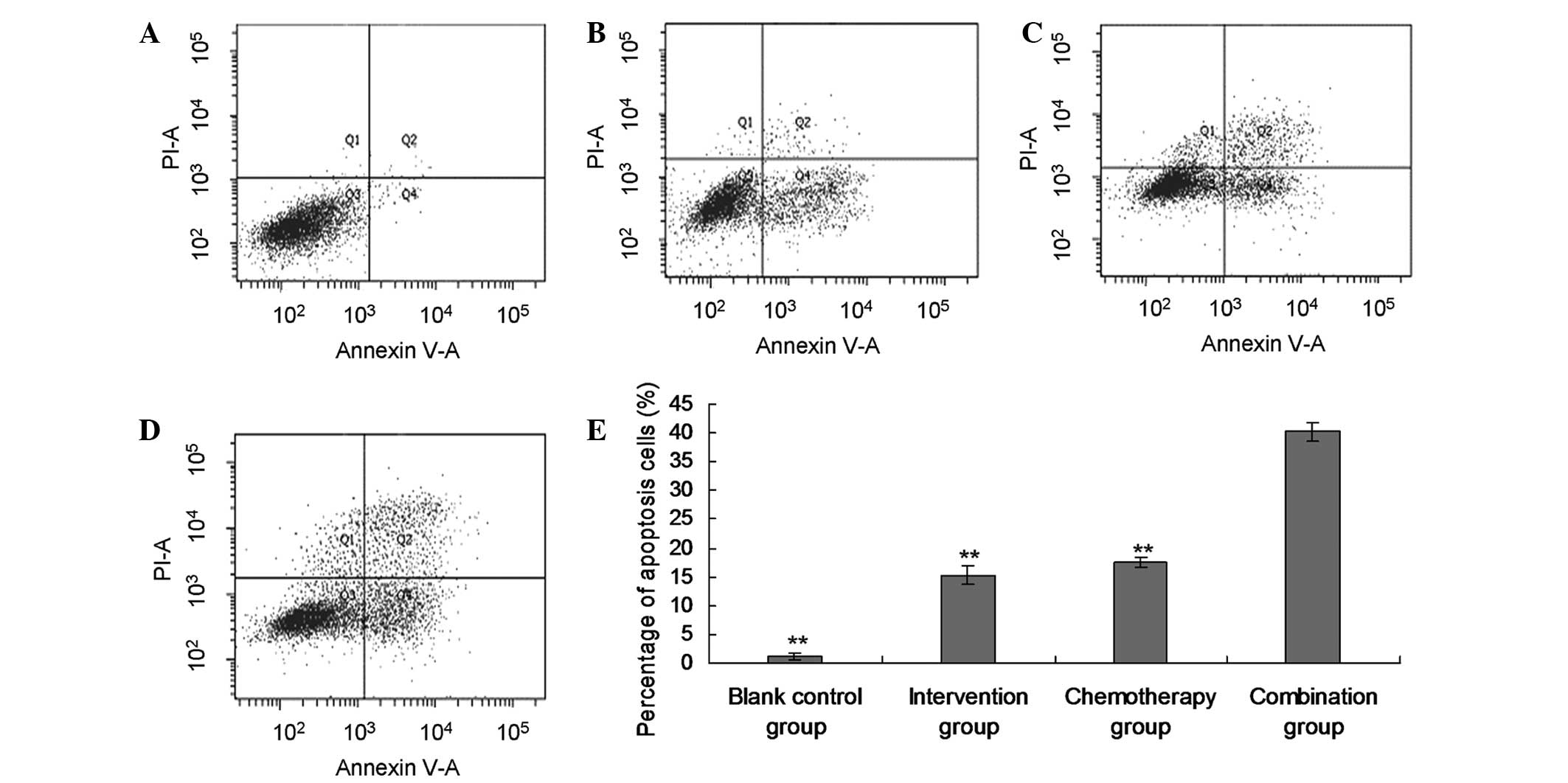
Promotion of apoptosis of EJ cells by
siRNA Livin
The flow cytometry assessment indicated that
apoptosis was promoted in the intervention group (15.29±1.64%),
chemotherapy group (17.57±0.82%) and combination group
(40.19±1.53%), when compared with the blank control group
(1.18±0.47%; P<0.01). Furthermore, the apoptotic rate in the
combination group was significantly higher than in the single
intervention and single chemotherapy group (P<0.01, Fig. 3).
Discussion
Bladder carcinoma is the most common malignant tumor
of the urinary system and one of the 10 most common tumors of the
whole body. Although there are multiple strategies of accessory
intra-bladder perfusion with variable medications following
surgery, the recurrence rate is still high, which is mainly due to
the resistance of tumor cells to chemotherapeutic medication
(7). Therefore, finding new targeting
sites and enhancing chemotherapeutic sensitivity are critical for
the therapy of bladder carcinoma. Previous studies indicated that
Livin is not expressed or expressed at low levels in most
differentiated terminal tissues of adults, but overexpressed in a
number of malignant tumors (8–10).
Overexpression of Livin inhibits the apoptosis of tumor cells and
leads to the resistance of tumors to pro-apoptotic factors
(11,12). In addition, Livin is associated with
the invasiveness of tumors and oncogenic phenotypes (13). These studies suggest that Livin is one
potential targeting site for the therapy of bladder carcinoma.
siRNA is a small segment of RNA with specific length
and structure (~21–23 bp). It binds to certain enzymes to form
RNA-induced silencing complex which specifically binds to and cuts
the homologous regions of mRNA expressed by exogenous genes,
resulting in the degradation of specific mRNA (14,15). siRNA
has previously been used in biological experiments which have led
to the development of new pathways of tumor therapy, due to siRNA's
features of strong specificity, high efficiency and simple
operation (2,16). The present study indicated that siRNA
Livin inhibited the proliferation and enhanced the apoptosis of EJ
human bladder carcinoma cells, suggesting the effectiveness and
practicality of siRNA Livin in treating bladder carcinoma.
Furthermore, CCK-8 measurement and flow cytometry
revealed that growth inhibition and apoptosis of EJ cells was
greatest in the combination group, followed by the chemotherapy
group, then the intervention group. These results suggest that
transfection of EJ cells with siRNA Livin enhances the sensitivity
of EJ cells to chemotherapeutic medications, which is consistent
with a previous study using T24 human bladder cancer cells
(5). The mechanism has been suggested
to involve caspase-3 (2).
In summary of the present study, effective siRNA
Livin was designed, which inhibited the expression and
proliferation of Livin in EJ human bladder carcinoma cells, and
promoted apoptosis. siRNA Livin also enhanced the sensitivity of EJ
cells to MMC. These results provide a theoretical basis and
experimental evidence to support therapy of bladder carcinoma using
siRNA Livin.
Acknowledgements
This study was supported by grants from the
Scientific Research Fund of Guangxi Provincial Education Department
(grant no.'s 200911LX290, 200103YB092) and the Scientific Research
and Technology Development Projects of Guilin City (grant no.
20100315-3).
References
|
1
|
Chen X, Wang T, Yang D, Wang J, Li X, He
Z, Chen F, Che X and Song X: Expression of the IAP protein family
acts cooperatively to predict prognosis in human bladder cancer
patients. Oncol Lett. 5:1278–1284. 2013.PubMed/NCBI
|
|
2
|
Liu C, Wu X, Luo C, Hu Z, Yin Z, He Y, Du
H, Zhang W, Jiang Q and Lin Y: Antisense oligonucleotide targeting
Livin induces apoptosis of human bladder cancer cell via a
mechanism involving caspase 3. J Exp Clin Cancer Res. 29:632010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xi RC, Sheng YR, Chen WH, Sheng L, Gang
JJ, Tong Z, Shan Z, Ying GH and Dong LC: Expression of survivin and
livin predicts early recurrence in non-muscle invasive bladder
cancer. J Surg Oncol. 107:550–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang D, Jiang Y, Ouyang S, Liu B, Zhu T,
Niu H and Tian Y: Inhibitory effect of valproic acid on bladder
cancer in combination with chemotherapeutic agents in vitro and in
vivo. Oncol Lett. 6:1492–1498. 2013.PubMed/NCBI
|
|
5
|
Yang D, Song X, Zhang J, Ye L, Wang S, Che
X, Wang J, Zhang Z and Wang L: Suppression of livin gene expression
by siRNA leads to growth inhibition and apoptosis induction in
human bladder cancer T24 cells. Biosci Biotechnol Biochem.
74:1039–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubowicz P, Żelaszczyk D and Pekala E:
RNAi in clinical studies. Curr Med Chem. 20:1801–1816. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang XL, Geng ZH, Lu XX, Wei C, Wang JG,
Wang SZ, Ma S, Liu HX, Xu GY, Zhang HW and Wang GY: Detecting
multi-drug resistance of bladder cancer for the intravesical
chemotherapy. Zhonghua Wai Ke Za Zhi. 42:285–287. 2004.PubMed/NCBI
|
|
8
|
Guo H, Gao YT, Zhang Q, Jing L, Liu T, Shi
WX, Zhai DK, Jing X and Du Z: Expression and clinical significance
of livin protein in hepatocellular carcinoma. Dis Markers.
35:489–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung CY, Park YL, Kim N, Park HC, Park
HB, Myung DS, Kim JS, Cho SB, Lee WS and Joo YE: Expression and
prognostic significance of Livin in gastric cancer. Oncol Rep.
30:2520–2528. 2013.PubMed/NCBI
|
|
10
|
Xu M, Xia LP, Fan LJ, Xue JL, Shao WW and
Xu D: Livin and caspase-3 expression are negatively correlated in
cervical squamous cell cancer. Eur J Gynaecol Oncol. 34:152–155.
2013.PubMed/NCBI
|
|
11
|
Ding ZY, Liu GH, Olsson B and Sun XF:
Upregulation of the antiapoptotic factor Livin contributes to
cisplatin resistance in colon cancer cells. Tumour Biol.
34:683–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun JG, Liao RX, Zhang SX, Duan YZ, Zhuo
WL, Wang XX, Wang ZX, Li DZ and Chen ZT: Role of inhibitor of
apoptosis protein Livin in radiation resistance in nonsmall cell
lung cancer. Cancer Biother Radiopharm. 26:585–592. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung CY, Park YL, Kim N, Park HC, Park
HB, Myung DS, Kim JS, Cho SB, Lee WS and Joo YE: Expression and
prognostic significance of Livin in gastric cancer. Oncol Rep.
30:2520–2528. 2013.PubMed/NCBI
|
|
14
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou W, Chen H, Hong X, Niu X and Lu Q:
Knockdown of DNA methyltransferase-1 inhibits proliferation and
derepresses tumor suppressor genes in myeloma cells. Oncol Lett.
8:2130–2134. 2014.PubMed/NCBI
|
|
16
|
Płuciennik E, Nowakowska M, Stȩpien A,
Wołkowicz M, Stawiński A, Różański W, Lipiński M and Bednarek AK:
Alterating expression levels of WWOX tumor suppressor and
cancer-related genes in patients with bladder cancer. Oncol Lett.
8:2291–2297. 2014.PubMed/NCBI
|