Introduction
Salivary gland tumors produce a variety of
histological patterns making tumor classification difficult
(1).
Low-grade cribriform cystadenocarcinoma (LGCCC) is a
malignant salivary gland originally reported as a variant of
salivary duct carcinoma (2). However,
LGCCC differs from typical salivary duct carcinomas with respect to
the growth pattern, a lack of evident nuclear atypia, invasiveness
to the surrounding tissues, and regional lymph node metastasis
(2,3).
Thus, the 2005 World Health Organization classification considers
this neoplasm to be a variant of cystadenocarcinoma, mainly due to
its cystic morphology, since no definite association has been found
between salivary duct carcinoma and LGCCC (1). In this classification system, LGCCC is
defined by its histological similarity to breast atypical ductal
hyperplasia or low-grade ductal carcinoma in situ (1).
LGCCC is extremely rare and thus, the incidence rate
of LGCCC remains unknown. The parotid gland is the most commonly
involved site. Few cases arise at other sites, such as the
submandibular gland and palate (2–7). The
present study reports a case of LGCCC that occurred at the palatal
gland of the hard palate. Written informed consent was obtained
from the patient.
Case report
A 56-year-old female was referred to Saitama Medical
University Hospital (Moroyama, Saitama, Japan) from a local dentist
in July 2013 due to the presence of an intraoral mass. The patient
had noticed this mass 1 month previously.
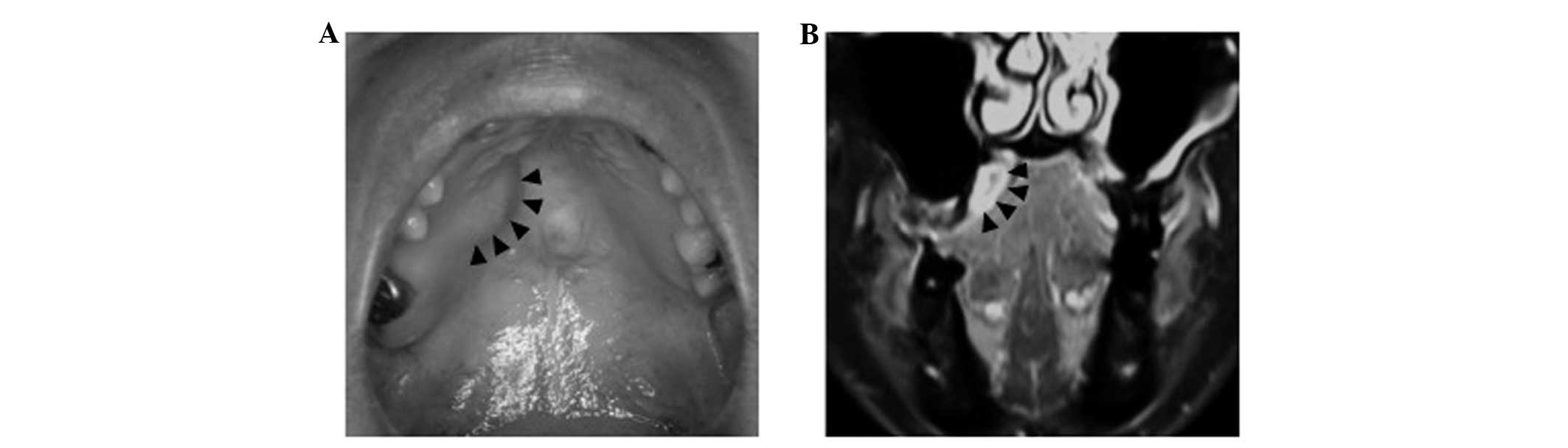
The mass, measuring 20×18 mm in diameter, was
located on the right side of the hard palate (Fig. 1A). The mass was soft and non-tender,
and did not adhere to the oral mucosa. The surface showed normal
mucosa. X-ray examination revealed no abnormality of the bone.
Computed tomography could not detect any abnormalities at the
palatal region, probably as the mass was too small to detect.
Magnetic resonance imaging showed a high-intensity cystic mass with
fluid internally on T1-weighted imaging of the right palatal region
(Fig. 1B). Fine-needle aspiration
(FNA) revealed that this mass contained brown-yellow fluid.
Cytological examination and biopsy led to a diagnosis favoring a
neoplasm, but with uncertain malignant potential. Thus, the tumor
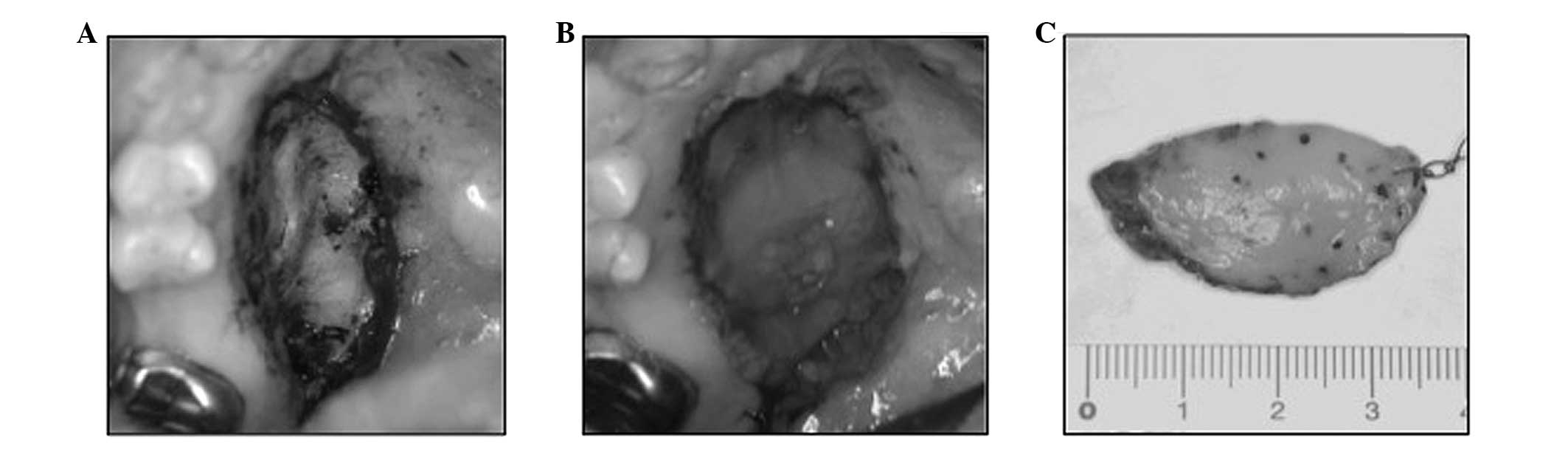
was resected with a safe surgical margin. The tumor was easily
dissected from the adjacent palatal bone (Fig. 2A). Subsequent to resection, the
palatal bone was covered with a poly-glycolic acid sheet and fibrin
(Fig. 2B).
The surgical specimen measured 3.5 cm at its largest
diameter (Fig. 2C). The specimen was
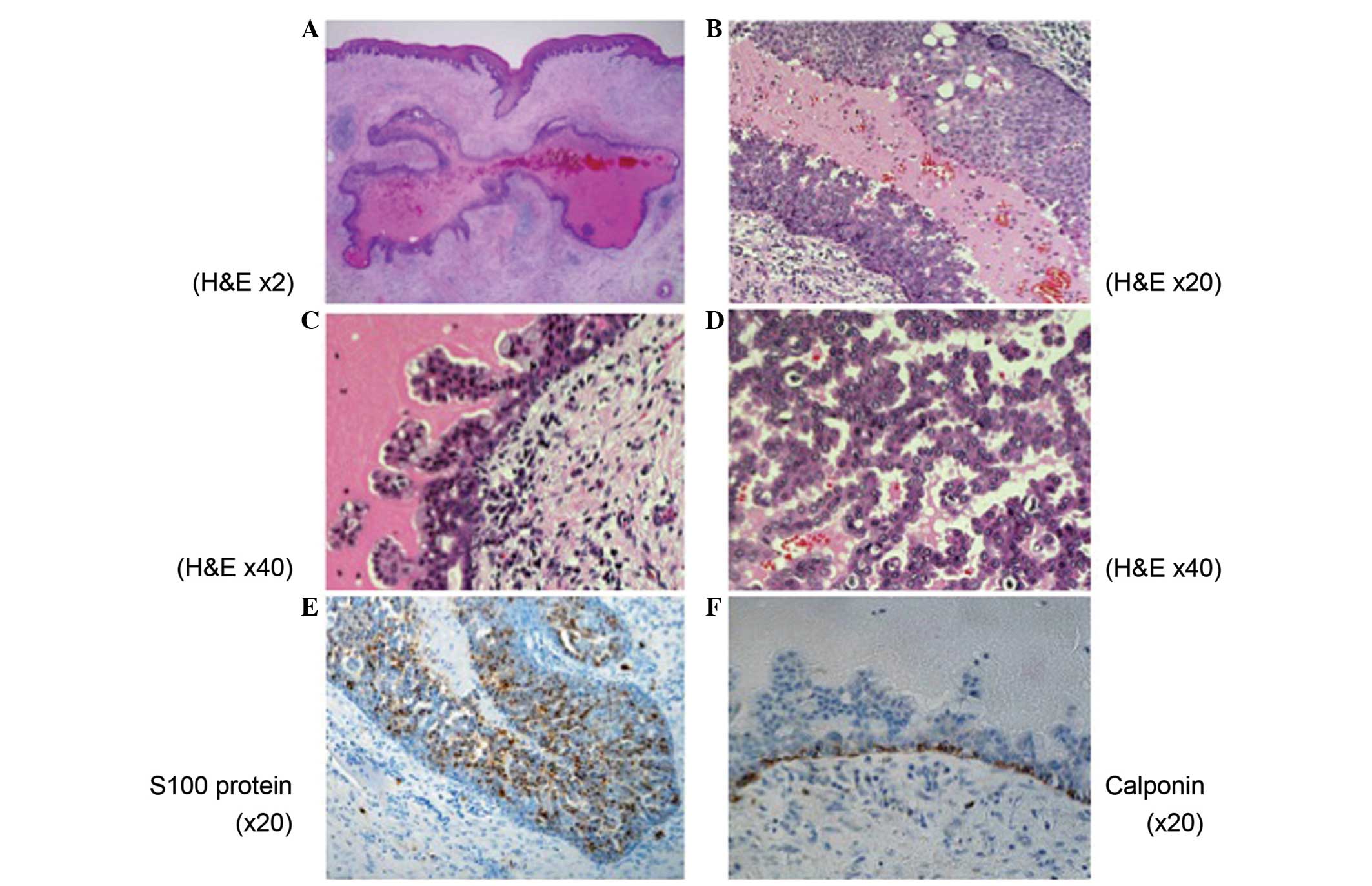
thoroughly examined. Microscopically, there was a well-demarcated,
unilocular cyst with the lumen lined by tumor cells (Fig. 3A). The tumor cells were arranged in
tubular, cribriform and solid structures in the area of the
intracystic mass lesions (Fig. 3B–D).
Nuclear atypia was inconspicuous, although mitotic figures were
observed throughout the tumor. Neither local nor perineural
invasion was present. On immunohistochemistry, the tumor cells were
diffusely positive for S-100 protein (Fig. 3E). Myoepithelial markers, calponin and
p63, highlighted the cells rimming the cystic mass, confirming the
intraductal nature of tumor (Fig.
3F). The final histopathological diagnosis was of LGCCC.
The tumor was completely resected, and 1 year later,
the patient exhibited no recurrence or distant metastasis.
Discussion
To date, there have been 12 published studies
reporting a total of 39 cases of LGCCC (2–5,7–14).
Characteristics of the reported cases together with the present
case are summarized in Table I. From
the literature, it is evident that LGCCC commonly occurs among
older patients (median age, 60.73 years), with a female
predominance (females:males, 23:16; gender was unreported in 1
case). A total of 36 tumors (90%) arose from the parotid glands,
including the intraparotid lymph node and accessory parotids. The
remaining 4 cases arose in the palate and submandibular gland,
suggesting that the present case, which also occurred in the
palate, is an rare event. LGCCC is regarded as a low-grade
malignant tumor with indolent clinical behavior. Due to this
clinical indolence, the majority of cases were treated with tumor
excision without radiotherapy. Among the cases with follow-up data
available, no patient experienced recurrence of the tumor or
succumbed due to the tumor (Table I)
(7).
 | Table I.Characteristics of the reported cases
and the present case of low-grade cribriform
cystadenocarcinoma. |
Table I.
Characteristics of the reported cases
and the present case of low-grade cribriform
cystadenocarcinoma.
| Case | First author, year
(ref.) | Age, years | Gender | Location | Size, cm | Treatment | Follow-up,
months |
|---|
| 1 | Delgado et al,
1996 (2) | 58 | M | Parotid | 1.0 | Parotidectomy | Not mentioned |
| 2 |
| 62 | F | Parotid | 0.7 | Parotidectomy | Not mentioned |
| 3 |
| 32 | F | Parotid | 1.1 | Parotidectomy,
Radiotherapy | NED, 144 |
| 4 |
| 63 | M | Parotid | 1.3 | Parotidectomy | NED, 132 |
| 5 |
| 74 | M | Parotid | 1.8 | Parotidectomy | NED, 72 |
| 6 |
| 56 | F | Parotid | 1.0 | Parotidectomy | NED, 24 |
| 7 |
| 42 | M | Parotid | 1.2 | Parotidectomy | NED, 24 |
| 8 |
| 69 | F | Parotid | 4.0 | Parotidectomy | NED, 24 |
| 9 |
| 69 | M | Parotid | 0.9 | Parotidectomy | Not mentioned |
| 10 |
| 52 | F | Parotid | 0.8 | Parotidectomy,
Radiotherapy | NED, 9 |
| 11 | Tatemoto et
al, 1996 (8) | 58 | F | Palate | 1.0 | Not mention | NED, 30 |
| 12 | Brandwein-Gensler
et al, 2004 (3) | 62 | F | 15 parotid, 1
submandibular gland | N/A | Not mentioned | NED, 12 |
| 13 |
| 82 | M |
|
|
| NED, 44 |
| 14 |
| 78 | F |
|
|
| NED, 17 |
| 15 |
| 72 | F |
|
|
| NED, 108 |
| 16 |
| 93 | F |
|
|
| NED, 24 |
| 17 |
| 64 | F |
|
|
| NED, 30 |
| 18 |
| 66 | U |
|
|
| NED, 62 |
| 19 |
| 57 | F |
|
|
| NED, 33 |
| 20 |
| 63 | M |
|
|
| Not mentioned |
| 21 |
| 57 | F |
|
|
| NED, 30 |
| 22 |
| 63 | F |
|
|
| Not mentioned |
| 23 |
| 64 | M |
|
|
| NED, 6 |
| 24 |
| 62 | M |
|
|
| NED, 132 |
| 25 |
| 72 | M |
|
|
| NED, 40 |
| 26 |
| 76 | M |
|
|
| NED, 24 |
| 27 |
| 54 | M |
|
|
| Not mentioned |
| 28 | Weinreb et
al, 2006 (4) | 50 | F | Parotid | 2.0 | Parotidectomy | NED, 5 |
| 29 |
| 73 | M | Parotid | 1.8 | Parotidectomy and
supraomohyoid neck dissection | NED, 60 |
| 30 |
| 67 | F | Parotid | 2.5 | Parotidectomy and
chemotherapy and radiotion therapy | Not mentioned |
| 31 | Arai et al,
2009 (5) | 32 | F | Parotid | 2.8 | Parotidectomy | NED, 24 |
| 32 | Laco et al,
2010 (9) | 50 | F | Parotid | 1.5 | Enucleation of the
tumor | NED, 24 |
| 33 | Nakazawa et
al, 2010 (10) | 56 | F | Parotid | 3.0 | Parotidectomy | NED, 12 |
| 34 | Kusafuka et
al, 2010 (11) | 38 | F | Parotid | 3.5 | Superficial
lobectomy of parotid gland | NED, 8 |
| 35 | Weinreb et
al, 2011 (12) | 59 | F | Parotid | 3.5 | Not mentioned | Not mentioned |
| 36 | Wang et al,
2013 (7) | 48 | M | Parotid | 2.0 | Parotidectomy | NED, 16 |
| 37 |
| 58 | F | Parotid | 3.5 | Parotidectomy | NED, 7 |
| 38 | Projetti F et
al, 2014 (13) | 57 | M | Parotid | 2.7 | Not mentioned | Not mentioned |
| 39 | Obokata A et
al, 2013 (14) | 65 | M | Submandibular | 4.0 | Not mentioned | Notmentioned |
| 40 | Present case | 56 | F | Palate | 2.0 | resection of
tumor | NED, 12 |
| Mean |
| 59.21 | 23 F, 16 M, 1
U |
|
|
| |
The differential diagnosis of LGCCC includes several
salivary tumors, such as papillary cystic variant of acinic cell
carcinoma (PCVACC) and other variants of cystadenocarcinoma
(1). Indeed, in the present case,
when five specialists of salivary gland pathology were consulted,
they listed PCVACC as a differential diagnosis for LGCCC. PCVACC
contains vacuolated cells similar to the microvacuolated cells of
LGCCC. However, the vacuoles of LGCCC are smaller, refractile and
associated with a yellow-brown pigment, while areas with periodic
acid Schiff-positive diastase-resistant fine cytoplasmic granules
are found in PCVACC (1,15). In contrast to PCVACC (1,16), LGCCC
also strongly expresses S-100 protein, as observed in the present
case (Fig. 3E). Conventional
cystadenocarcinoma differs from LGCCC by the lack of intraductal
proliferation, golden brown pigment, solid cellular foci, and an
overall resemblance to atypical hyperplasia or carcinoma in
situ of the breast. Cystadenocarcinoma tends to be an invasive
tumor, whereas LGCCC is usually confined to the cystic wall
(1,15).
Necrosis of the central region of the proliferating
tumor nests frequently occurs (1,17).
Necrosis is usually uncommon in LGCCC, although in the present
case, the central portion of the tumor exhibited necrosis. Biopsy
or FNA, which was performed in the present study, may affect the
tumor, and necrosis may occur in a tumor region.
The variability and histological complexity of
salivary gland tumors does not allow an easy diagnosis (1,18). The
variations appear to be based on the multi-differential
potentiality of the salivary gland cells and their stem cells
(19–21). Indeed, in the present case, the
formation of a final diagnosis was difficult. Although the
morphological and immunohistochemical features are comparatively
well investigated, little else is known about the genetics or
pathogenesis of these tumors (22).
Recently, the association between the rearrangement of genes such
as PLAG1 and HMGA2 with the prognosis of LGCCC have
been investigated (22). Such studies
may confer novel insights with regard to an understanding of LGCCC
and the establishment of novel methods to diagnose the disease.
In conclusion, the present study reported a LGCCC
that arose in the hard palate. Although LGCCC is regarded as
clinically indolent, there is limited literature on prognosis
prediction, since LGCCCs, and particularly those that arise in the
palatal salivary gland, are rare tumors. Moreover, several cases
have been reported of more aggressive and invasive carcinomas that
are presumed to arise in LGCCC (4,6,22). Thus, greater experience and longer
follow-periods are necessary to find an optimal/curative treatment
for LGCCC and to clarify the pathophysiology.
Acknowledgements
The authors would like to thank the members of the
Department of Oral and Maxillofacial Surgery, Faculty of Medicine,
Saitama Medical University for providing valuable comments and
discussions. The authors are particularly grateful to Dr Shojiro
Morinaga (Kitasato University) for supplying valuable suggestions
and to Dr William N. Addison (Harvard School of Dental Medicine)
for correcting and editing the original manuscript. The authors
also thank Mr. Noriaki Kasai and Mr. Kei Nishikori for providing
encouragement.
Abbreviations:
|
LGCCC
|
low-grade cribriform
cystadenocarcinoma
|
|
PCVACC
|
papillary cystic variant of acinic
cell carcinoma
|
References
|
1
|
BrandweinGensler MS and Gnepp DR:
Low-grade cribriform cystadenocarcinoma. World Health Organization
Classification of TumoursPathology and Genetics Head and Neck
Tumors. Barnes L, Eveson JW, Reichart P and Sidransky D: IARC
Press; Lyon: pp. 2332005
|
|
2
|
Delgado R, Klimstra D and Albores-Saavedra
J: Low grade salivary duct carcinoma. A distinctive variant with a
low grade histology and a predominant intraductal growth pattern.
Cancer. 78:958–967. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
BrandweinGensler M, Hille J, Wang BY,
Urken M, Gordon R, Wang LJ, Simpson JR, Simpson RH and Gnepp DR:
Low-grade salivary duct carcinoma: Description of 16 cases. Am J
Surg Pathol. 28:1040–1044. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weinreb I, TabandaLichauco R, Van der
Kwast T and Perez-Ordoñez B: Low-grade intraductal carcinoma of
salivary gland: Report of 3 cases with marked apocrine
differentiation. Am J Surg Pathol. 30:1014–1021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arai A, Taki M, Mimaki S, Ueda M and Hori
S: Low-grade cribriform cystadenocarcinoma of the parotid gland: A
case report. Auris Nasus Larynx. 36:725–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakatsuka S, Harada H, Fujiyama H, Takeda
K, Kitamura K, Kimura H, Nagano T, Ito M and Asada Y: An invasive
adenocarcinoma of the accessory parotid gland: A rare example
developing from a low-grade cribriform cystadenocarcinoma? Diagn
Pathol. 6:1222011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Liu Y, Lin X, Zhang D, Li Q, Qiu X
and Wang EH: Low-grade cribriform cystadenocarcinoma of salivary
glands: Report of two cases and review of the literature. Diagn
Pathol. 8:282013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tatemoto Y, Ohno A and Osaki T: Low
malignant intraductal carcinoma on the hard palate: A variant of
salivary duct carcinoma? Eur J Cancer B Oral Oncol. 32B:275–277.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laco J, Podhola M and Dolezalova H:
Low-grade cribriform cystadenocarcinoma of the parotid gland: A
neoplasm with favorable prognosis, distinct from salivary duct
carcinoma. Int J Surg Pathol. 18:369–373. 2010.PubMed/NCBI
|
|
10
|
Nakazawa T, Kondo T, Yuminomochi T,
Nakazawa K, Ishii Y, Mochizuki K, Kawasaki T, Yamane T, Miyata M,
Motosugi U and Katoh R: Fine-needle aspiration biopsy of low-grade
cribriform cystadenocarcinoma of the salivary gland. Diagn
Cytopathol. 39:218–222. 2011.PubMed/NCBI
|
|
11
|
Kusafuka K, Itoh H, Sugiyama C and
Nakajima T: Low-grade salivary duct carcinoma of the parotid gland:
Report of a case with immunohistochemical analysis. Med Mol
Morphol. 43:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weinreb I: Intraductal carcinoma of
salivary gland (so-called low-grade cribriform cystadenocarcinoma)
arising in an intraparotid lymph node. Head Neck Pathol. 5:321–325.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Projetti F, LacroixTriki M, Serrano E,
Vergez S, Barres BH, Meilleroux J, Delisle MB and Uro-Coste E: A
comparative immunohistochemistry study of diagnostic tools in
salivary gland tumors: Usefulness of mammaglobin, gross cystic
disease fluid protein 15, and p63 cytoplasmic staining for the
diagnosis of mammary analog secretory carcinoma? J Oral Pathol Med.
44:244–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obokata A, Sakurai S, Hirato J, Sakamoto
K, Takekoshi T and Aoki J: Cytologic features of low-grade
cribriform cystadenocarcinoma of the submandibular gland: A case
report. Acta Cytol. 57:207–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Foss RD, Ellis GL and Auclair PL: Salivary
gland cystadenocarcinomas. A clinicopathologic study of 57 cases =
Am J Surg Pathol. 20:1440–1447. 1996.
|
|
16
|
Takahashi H, Fujita S, Okabe H, Tsuda N
and Tezuka F: Distribution of tissue markers in acinic cell
carcinomas of salivary gland. Pathol Res Pract. 188:692–700. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anderson C, Muller R, Piorkowski R, Knibbs
DR and Vignoti P: Intraductal carcinoma of major salivary gland.
Cancer. 69:609–614. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eversole LR: Histogenic classification of
salivary tumors. Arch Pathol. 92:433–443. 1971.PubMed/NCBI
|
|
19
|
Regezi JA and Batsakis JG: Histogenesis of
salivary gland neoplasms. Otolaryngol Clin North Am. 10:297–307.
1977.PubMed/NCBI
|
|
20
|
Sato M, Hayashi Y, Yoshida H, Yanagawa T,
Yura Y and Nitta T: Search for specific markers of neoplastic
epithelial duct and myoepithelial cell lines established from human
salivary gland and characterization of their growth in vitro.
Cancer. 54:2959–2967. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayashi Y, Yanagawa T, Yoshida H, Yura Y,
Nitta T and Sato M: Induction of other differentiation stages in
neoplastic epithelial duct and myoepithelial cells from the human
salivary gland grown in athymic nude mice. Cancer. 55:2575–2583.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bahrami A, PerezOrdonez B, Dalton JD and
Weinreb I: An analysis of PLAG1 and HMGA2 rearrangements in
salivary duct carcinoma and examination of the role of precursor
lesions. Histopathology. 63:250–262. 2013. View Article : Google Scholar : PubMed/NCBI
|