Introduction
Papillary thyroid microcarcinoma (PTMC) is a
papillary thyroid carcinoma which is ≤1.0 cm at the greatest
dimension, as defined by the World Health Organization (1). Due to the wide use of high-resolution
thyroid ultrasound (US) and fine needle aspiration cytology (FNAC)
under US guidance, a number of patients are diagnosed with PTMC
without palpable thyroid nodules, which may explain the high
incidence during previous years in Eastern China (2,3). The
prognosis for PTMC patients is favorable, however, there remains a
1% disease-associated mortality rate, a 5% lymph node recurrence
rate and a 2.5% distant metastasis rate (4–7). The
reported incidence of lymph node metastasis (LNM) has reached 40%:
The common sites of metastasis include the central compartment of
the neck (8). The revised American
Thyroid Association guidelines recommend that central neck lymph
node dissection (CND) should be considered in patients with
high-risk thyroid cancer (9).
However, it remains uncertain whether CND should be routinely
performed on cervical lymph node-negative (cN0) patients with PTMC.
Therefore, clinicopathological and US characteristic data of cN0
PTMC patients admitted into our department was retrospectively
analyzed to identify the risk factors for central LNM (CLNM).
Furthermore, a scoring system for differentiating between patients
with and without CLNM in Eastern China was established. Written
informed consent was obtained from the patients' families and
ethical approval was obtained from Wengzhou Medical University
(Wengzhou, China).
Patients and methods
Patients
Between May 2011 and October 2012, 1,090 patients
who were diagnosed with thyroid cancer by histological examination
underwent thyroidectomy at the First Affiliated Hospital of
Wengzhou Medical University (Wengzhou, China) for initial diagnosis
and treatment. Among these patients, 449 (41.2%) were diagnosed
with PTMC by pathology, and all the patients had preoperatively
undergone US. Inclusion criteria referred to the clinical
evaluation criteria of cervical lymph nodes defined by Kowalski
et al (10). The patients
matching the following conditions could be diagnosed as cN0 PTMC:
i) No palpable enlarged lymph node on clinical examination, or the
maximum diameter of the enlarged lymph node was <2 cm with a
soft texture; ii) no visible enlarged lymph node in the imaging
examination, the maximum diameter of the enlarged lymph node was
<1 cm or the maximum diameter was 1–2 cm with no central
liquefaction necrosis, peripheral enhancement or disappeared fat
gap adjacent to the lymph node. Using these criteria, a total of
392 patients who had undergone CND were enrolled in the present
study to evaluate risk factors that may predict CLNM. The study
group included 308 (78.6%) female and 84 (21.4%) male patients. The
mean age at diagnosis was 47.62 [range, 18–83; standard deviation
(SD), 9.77] and the average tumor size was 7.05 mm (range, 1–10 mm;
SD, 1.97). All the patients were from Eastern China (the majority
of patients were from Wenzhou). Table
I lists the demographic and clinicopathological data of the 392
patients. Age at diagnosis, gender, tumor size, location,
multifocality and bilaterality, extracapsular spread (ECS), chronic
lymphocytic thyroiditis and the status of the central lymph nodes
were recorded through the retrospective review of clinical data and
pathological reports. The status of the central lymph nodes was
diagnosed by a final pathological examination. Chronic lymphocytic
thyroiditis was confirmed by serological examination or frozen
biopsy with 100% sensitivity and 99.3% specificity. US was
performed with Acuson Sequoia and 128XP sonographic scanners
(Siemens Medical Solutions, Mountain View, CA, USA) equipped with
commercially available 8–13 MHz linear probes. Real-time US was
performed by experienced radiologists dedicated to thyroid imaging.
US findings of patients were further categorized according to
composition, echogenicity, calcifications, margin, shape and
width/length. When multiple PTMCs were found in the surgical
specimen, the characteristics of the largest or most dominant tumor
on the preoperative US were analyzed.
 | Table I.Clinicopathological characteristics of
392 patients. |
Table I.
Clinicopathological characteristics of
392 patients.
| Characteristics | Value |
|---|
| Total number | 392 |
| Age (years) |
|
| At
diagnosis | 47.62±9.77
(18–83) |
|
<45 | 132 (33.7) |
| ≥45 | 260 (66.3) |
| Gender |
|
|
Female | 308 (78.6) |
| Male | 84 (21.4) |
| Tumor size (mm) | 7.05±1.97 (1–10) |
| Location |
|
|
Upper | 129 (32.9) |
|
Middle | 146 (37.2) |
|
Lower | 101 (25.8) |
|
Isthmus | 16 (4.1) |
| Multifocality |
|
|
Unifocal | 332 (84.7) |
|
Multifocal | 60 (15.3) |
| Tumor
bilaterality |
|
|
Unilateral | 340 (86.7) |
|
Bilateral | 52 (13.3) |
| Extracapsular
spread |
|
|
Present | 38 (9.7) |
|
Absent | 354 (90.3) |
| Chronic lymphocytic
thyroiditis |
|
|
Present | 85 (21.7) |
|
Absent | 307 (78.3) |
| Central lymph node
metastasis |
|
| Yes | 159 (40.6) |
| No | 233 (59.4) |
Grouping
In the present study, total or almost total
thyroidectomy was performed on the patients. Ipsilateral CND for
unilateral cN0 PTMC patients or bilateral CND for bilateral cN0
PTMC patients was performed. According to the presence of CLNM,
patients were divided into two groups: CLNM-negative (Group I) and
CLNM-positive (Group II).
Statistical analysis
Statistical analysis was performed to assess the
differences between groups I and II with SPSS software, version
19.0 (SPSS, Inc., Chicago, IL, USA). All statistical tests were
two-sided and P<0.05 was considered to indicate a statistically
significant difference. Univariate analysis with the χ2
test was used for categorical data to analyze the statistical
correlation between the factors and CLNM. Multivariate logistic
regression analyses were performed to assess independent
associations of CLNM with all the factors that were observed to be
statistically significant by the univariate analysis. Odds ratios
(ORs) with the relative 95% confidence intervals (CIs) are
presented. Characteristics which were independent factors were
given different points to develop a score system according to the
multiple logistic regression analysis. Receiver operating
characteristic (ROC) curves were used to identify the optimal point
with a high sensitivity and low false-negative rate (100 -
specificity).
Results
Among the 392 patients enrolled in this study, 308
were female and 84 were male, aged 18–83 years with a median age of
47.62 years. The mean diameter of nodules was 7.05 mm (range, 1–10
mm; SD, 1.97). A total of 159 patients (40.6%) were demonstrated to
exhibit CLNM by the final pathological examination, while the other
233 patients (59.4%) did not. Table
II presents the comparison of clinicopathological and US
differences between CLNM-negative (Group I) and CLNM-positive
(Group II) groups. Male gender, age <45 years, maximum tumor
diameter >5 mm, lower lobe location and multifocal carcinoma
with total tumor diameter (TTD) >10 mm were significantly
correlated with the incidence of CLNM (P<0.001). ECS was
significantly associated with CLNM (P=0.022). Tumor bilaterality,
chronic lymphocytic thyroiditis and US characteristics did not
predict CLNM in PTMC. A multivariate logistic regression analysis
was performed on the factors that were revealed to be statistically
significant by the univariate analysis. Male gender (OR, 5.021;
P<0.001), tumor size >5 mm (OR, 4.842; P<0.001), age
<45 years (OR, 3.911; P<0.001), lower lobe location (OR,
4.652; P<0.001), ECS (OR, 3.885; P=0.002) and TTD >10 mm (OR,
8.553; P=0.016) were identified as statistically significant
independent predictive factors for CLNM (Table III). According to the multiple
logistic regression analysis, the patients were scored by giving an
index point to each characteristic that was significantly
associated with CLNM. The index score for each characteristic is
presented in Table IV. The mean
clinicopathological score was 1.23±0.722 in the CLNM-positive group
and 2.34±0.973 in the CLNM-negative group, which was a
statistically significant difference (P<0.001). ROC curves were
then used to identify that an index point ≥2 was the optimal point
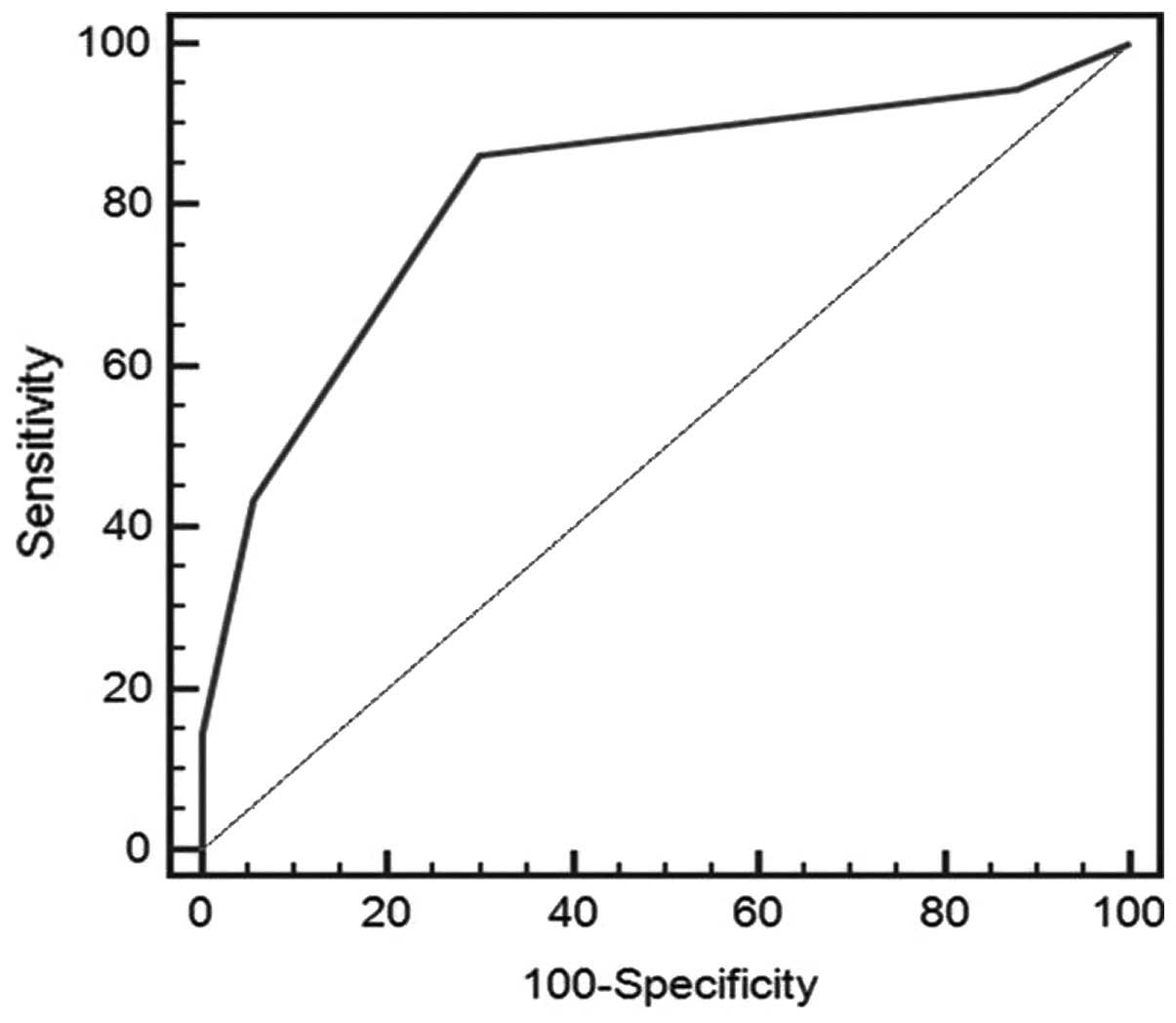
to distinguish between patients with and without CLNM (Fig. 1). The sensitivity and specificity
reached 86.2% and 70.4%, respectively, with an area under the ROC
curve of 0.816.
 | Table II.Clinicopathological and
ultrasonographic characteristics according to central lymph node
metastasis status. |
Table II.
Clinicopathological and
ultrasonographic characteristics according to central lymph node
metastasis status.
|
Characteristics | Group I, n=233
(%) | Group II, n=159
(%) | P-value |
|---|
| Age (years) |
|
|
<0.001a |
|
<45 | 55 (78.5) | 77 (53.5) |
|
|
≥45 | 178 (154.5) | 82 (105.5) |
|
| Gender |
|
|
<0.001a |
|
Female | 202 (183.1) | 106 (124.9) |
|
|
Male | 31 (49.9) | 53 (34.1) |
|
| Tumor size
(mm) |
|
|
<0.001a |
| ≤5 | 106 (78.5) | 26 (53.5) |
|
|
>5 | 127 (154.5) | 133 (158.5) |
|
| ≤7 | 140 (131.4) | 81 (89.6) | 0.079 |
|
>7 | 93 (101.6) | 78 (69.4) |
|
| Location |
|
|
<0.001a |
| Upper
lobe | 190 (163.5) | 85 (111.5) |
|
| Lower
lobe | 43 (69.5) | 74 (47.5) |
|
| Multifocality |
|
|
0.106 |
|
Unifocal | 203 (197.3) | 129 (134.7) |
|
|
Multifocal | 30 (35.7) | 30 (24.3) |
|
| TTD (mm) |
|
|
|
|
≤10 | 28 (27.6) | 17 (17.4) | 0.889 |
|
>10 | 2 (8.9) | 13 (6.1) |
<0.001a |
| Tumor
bilaterality |
|
|
0.137 |
|
Unilateral | 207 (202.1) | 133 (137.9) |
|
|
Bilateral | 26 (30.9) | 26 (21.1) |
|
| Extracapsular
spread |
|
|
0.022a |
|
Present | 16 (22.6) | 22 (15.4) |
|
|
Absent | 217 (210.4) | 137 (143.6) |
|
| Chronic lymphocytic
thyroiditis |
|
|
0.103 |
|
Present | 44 (50.5) | 41 (34.5) |
|
|
Absent | 189 (182.5) | 118 (124.5) |
|
| Composition |
|
|
0.222 |
|
Solid | 124 (226.5) | 157 (154.5) |
|
| Cystic
or mixed | 9 (6.5) | 2 (4.5) |
|
| Echogenicity |
|
|
0.248 |
|
Hyperchogonicy or
isoechoic | 227 (229.4) | 159 (156.6) |
|
|
Hypoeschogenicy | 6 (3.6) | 0 (2.4) |
|
| Margin |
|
|
1.000 |
|
Well-defined | 148 (148.0) | 101 (101.0) |
|
|
Ill-defined | 85 (85.0) | 58 (58.0) |
|
| Calcification |
|
|
0.105 |
|
Present | 138 (145.6) | 107 (99.4) |
|
|
Absent | 95 (87.4) | 52 (59.6) |
|
| Shape |
|
|
0.363 |
|
Non-parallel | 55 (58.8) | 44 (40.2) |
|
|
Parallel | 178 (174.2) | 115 (118.8) |
|
| Taller than
wide |
|
|
0.715 |
|
Yes | 126 (124.2) | 83 (84.8) |
|
| No | 107 (108.8) | 76 (74.2) |
|
 | Table III.Multivariate analysis of the
associations between central lymph node metastasis and
clinicopathological characteristics. |
Table III.
Multivariate analysis of the
associations between central lymph node metastasis and
clinicopathological characteristics.
|
|
|
| 95% confidence
interval |
|---|
|
|
|
|
|
|---|
|
Characteristics | P-value | Odds ratio | Lower | Upper |
|---|
| Male gender | <0.001 | 5.021 | 2.735 |
9.215 |
| Age (>45
years) | <0.001 | 3.911 | 2.316 |
6.604 |
| Tumor size (>5
mm) | <0.001 | 4.842 | 2.765 |
8.479 |
| Lower lobe | <0.001 | 4.652 | 2.707 |
7.992 |
| Total tumor
diameter (>10mm) |
0.016 | 8.553 | 1.486 | 49.226 |
| Extracapsular
spread present |
0.002 | 3.885 | 1.638 |
9.217 |
 | Table IV.Clinicopathological index points. |
Table IV.
Clinicopathological index points.
| Characteristic | Category | Points |
|---|
| Gender | Male | 1 |
|
| Female | 0 |
| Age (years) | <45 | 1 |
|
| ≥45 | 0 |
| Tumor size
(mm) | >5 mm | 1 |
|
| ≤5 mm | 0 |
| Tumor location | Upper lobe | 1 |
|
| Lower lobe | 0 |
| Total tumor
diameter (mm) | >10 | 1 |
|
| ≤10 | 0 |
| Extracapsular
spread | Present | 1 |
|
| Absent | 0 |
Discussion
Owing to the widespread use of thyroid US and
US-guided FNAC, the incidence of PTMC has increased in Eastern
China in previous years (2,11). Although PTMC is generally associated
with an excellent prognosis, CLNM is common in PTMC patients with
an incidence of 41–64% (12–17). There is no controversy in performing
therapeutic neck dissection on cervical lymph node-positive PTMC
patients to reduce the chance of persistence and recurrence
(18), however debate remains as to
whether CND should be performed on cN0 PTMC patients. A number of
authors have proposed that there should be routine CND in patients
with PTMC since there is evidence demonstrating that residual
metastases in cervical lymph nodes represent the most common site
of recurrent disease, and repeated operations may increase the
incidence of operative complications, including hypoparathyroidism
and damage to the laryngeal nerve (19). However, a previous study indicated
that CND is not required, as the presence of CLNM is not associated
with the disease-free survival rate of patients (20). The present study focused on
identifying the preoperative and intraoperative predictive factors
for CLNM in cN0 PTMC patients, which may aid in identifying
approaches to appropriate surgical treatment for patients in
Eastern China. Although the use of thyroid US for detecting CLNM is
limited (20), the present study also
investigated characteristics of US to identify any potential
predictors.
The incidence of CLNM in the present study was 40.6%
(n=159), which is consistent with previous studies (21,22).
Statistical analyses performed between the CLNM-positive and
-negative groups in the present study included eight
clinicopathological parameters. The results demonstrated that male
gender, age <45 years, maximum tumor diameter >5 mm, lower
lobe location and multifocal carcinoma with TTD >10 mm were
independent predictors for CLNM from the multivariate analysis.
Zeng et al (23) indicated
that certain US characteristics (upper pole location, no
well-defined margin and presence of calcifications) were predictive
factors for lateral LNM in patients with PTMC in Eastern China. Few
studies have reported the associations between US characteristics
and CLNM. In the present study, no significance was identified for
these associations, which is consistent with a previous study by
Kim et al (24).
A number of previous studies have reported that age
is not associated with CLNM in patients with PTMC (24,25).
However, Wang et al (26)
demonstrated that there is a significant difference between
patients <45 years old and ≥45 years old in cN0 PTMC. The
present study identified that age <45 years was significantly
associated with CLNM patients. Male gender has been considered to
be an important prognostic characteristic of CLNM by a number of
previous studies (21,27). The present study demonstrated a
significant association between male gender and CLNM, which is
consistent with these previous reports. Tumor size has been
previously confirmed as a prognostic feature of CLNM (20). Lee et al (28) demonstrated that PTMC with tumor size
>7 mm had a stronger association with poor prognosis compared
with those <7 mm. Lim et al (25) had reported that tumor size >5 mm
was frequently associated with CLNM. The results of the present
study demonstrated no difference between the ≤7 and >7 mm tumor
size groups. However, the univariate and multivariate analyses
confirmed tumor size >5 mm as an independent predictor of LNM. A
number of previous studies reported the upper pole location as an
independent predictive factor for lateral LNM (23,29). To
the best of our knowledge, few reports have defined the statistical
correlation between tumor location and CLNM in PTMC. Wang et
al (26) proposed that the CLNM
rate in the lower/middle pole group was significantly increased
compared with that of the upper pole group. A study by Choi et
al (30) demonstrated that tumors
located in the upper neck had an increased risk of lateral LNM,
while tumors in the lower neck had an increased risk of CLNM.
Similarly, the present study determined that lower lobe location
was significantly associated with CLNM in patients with cN0 PTMC.
The association between tumor multifocality and CLNM remains
controversial. In the present study, multifocality was not an
independent predictor of CLNM, which is not consistent with
previous reports (21,24). Therefore, the TTD, which was defined
as the sum of the maximal diameter of each lesion, was calculated.
Univariate and multivariate analyses revealed that TTD >10 mm
was statistically significantly associated with CLNM in the present
study, which was similar to the findings of a previous study by
Zhao et al (31). In the
present study, tumor bilaterality was not an independent predictive
factor for CLNM, although this has been demonstrated to be
significantly associated with CLNM in a previous study (24). ECS is an important prognostic
characteristic of CLNM. ECS in patients with CLNM was significantly
increased compared with that in those without CLNM in the present
study, which was confirmed by certain previous reports (27,32). In
the present study, coexisting chronic lymphocytic thyroiditis was
found in 21.7% of patients with PTMC. Patients with chronic
lymphocytic thyroiditis are considered to be more likely to exhibit
thyroid carcinoma compared with patients without this condition
(33,34). A previous report indicated that
coexisting chronic lymphocytic thyroiditis in patients with
papillary thyroid carcinoma had an inverse correlation with CLNM
(35). The present study demonstrated
that the frequency of CLNM was 48.2% (41/85 cases) in the chronic
lymphocytic thyroiditis group, which was reduced compared with the
control group. However, underlying chronic lymphocytic thyroiditis
was not statistically significantly associated with CLNM.
To improve the diagnostic accuracy, a scoring system
of independent predictors based on the multiple logistic regression
analysis was established. The ROC curves had high predictive
performance since the area under the ROC curves was 0.85 (95% CI,
0.81–0.90). Using the ROC curves, index point ≥2 was identified as
the optimal cut-off point to distinguish CLNM-positive from
CLNM-negative, with a sensitivity of 86.2% and a specificity of
70.4%. These results may aid surgeons to tailor the follow-up
treatment of each patient accurately.
There are certain potential limitations in the
current study. First, not all risk factors are included, such as
lateral LNM, since only 12 patients (3.06%) enrolled in the present
study underwent lateral neck dissection. Second, tumor recurrence
and disease-free survival rate were not investigated. Further
studies should be designed to confirm whether the scoring system is
useful for treatment outcome.
In conclusion, this scoring system for the
prediction of CLNM may be a practical and convenient diagnostic
approach for cN0 patients with PTMC in Eastern China. Patients with
an index point <2 may be considered as CLNM-negative. For
patients with index point ≥2, the present study indicates that a
CND may be routinely performed.
Acknowledgements
The present study was supported by a grant from the
National High Technology Research and Development Program 863 (no.
Y20120190).
References
|
1
|
Hedinger C, Williams ED and Sobin LH: The
WHO histological classification of thyroid tumors: A commentary on
the second edition. Cancer. 63:908–911. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiang J, Wu Y, Li DS, Shen Q, Wang ZY, Sun
TQ, An Y and Guan Q: New clinical features of thyroid cancer in
eastern China. J Visc Surg. 147:e53–e56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng P, Chen ED, Zheng HM, He QX and Li
Q: Ultrasound score to select subcentimeter-sized thyroid nodules
requiring ultrasound-guided fine needle aspiration biopsy in
eastern China. Asian Pac J Cancer Prev. 14:4689–4692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baudin E, Travagli JP, Ropers J, Mancusi
F, BrunoBossio G, Caillou B, Cailleux AF, Lumbroso JD, Parmentier C
and Schlumberger M: Microcarcinoma of the thyroid gland: The
gustave-roussy institute experience. Cancer. 83:553–559. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hay ID, Grant CS, van Heerden JA, Goellner
Jr, Ebersold JR and Bergstralh EJ: Papillary thyroid
microcarcinoma: A study of 535 cases observed in a 50-year period.
Surgery. 112:1139–1146; discussion 1146–1147. 1992.PubMed/NCBI
|
|
6
|
Yamashita H, Noguchi S, Murakami N, Toda
M, Uchino S, Watanabe S and Kawamoto H: Extracapsular invasion of
lymph node metastasis. A good indicator of disease recurrence and
poor prognosis in patients with thyroid microcarcinoma. Cancer.
86:842–849. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chow SM, Law SC, Chan JK, Au SK, Yau S and
Lau WH: Papillary microcarcinoma of the thyroid-Prognostic
significance of lymph node metastasis and multifocality. Cancer.
98:31–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moo TA, McGill J, Allendorf J, Lee J,
Fahey T III and Zarnegar R: Impact of prophylactic central neck
lymph node dissection on early recurrence in papillary thyroid
carcinoma. World J Surg. 34:1187–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F,
Schlumberger M, et al: American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer1; Revised American thyroid association management guidelines
for patients with thyroid nodules and differentiated thyroid
cancer. Thyroid. 19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kowalski LP, Bagietto R, Lara JR, Santos
RL, Silva JF Jr and Magrin J: Prognostic significance of the
distribution of neck node metastasis from oral carcinoma. Head
Neck. 22:207–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie WC, Chan MH, Mak KC, Chan WT and He M:
Trends in the incidence of 15 common cancers in Hong Kong,
1983–2008. Asian Pac J Cancer Prev. 13:3911–3916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kutler DI, Crummey AD and Kuhel WI:
Routine central compartment lymph node dissection for patients with
papillary thyroid carcinoma. Head Neck. 34:260–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moo TA, Umunna B, Kato M, Butriago D,
Kundel A, Lee JA, Zarnegar R and Fahey TJ III: Ipsilateral versus
bilateral central neck lymph node dissection in papillary thyroid
carcinoma. Ann Surg. 250:403–408. 2009.PubMed/NCBI
|
|
14
|
Sadowski BM, Snyder SK and Lairmore TC:
Routine bilateral central lymph node clearance for papillary
thyroid cancer. Surgery. 146:696–703; discussion 703–695. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YS, Lim YS, Lee JC, Wang SG, Kim IJ
and Lee BJ: Clinical implication of the number of central lymph
node metastasis in papillary thyroid carcinoma: Preliminary report.
World J Surg. 34:2558–2563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lim YS, Lee JC, Lee YS, Lee BJ, Wang SG,
Son SM and Kim IJ: Lateral cervical lymph node metastases from
papillary thyroid carcinoma: Predictive factors of nodal
metastasis. Surgery. 150:116–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao GZ and Gao L: Central lymph node
metastasis: Is it a reliable indicator of lateral node involvement
in papillary thyroid carcinoma? World J Surg. 34:237–241. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito Y, Higashiyama T, Takamura Y, Miya A,
Kobayashi K, Matsuzuka F, Kuma K and Miyauchi A: Risk factors for
recurrence to the lymph node in papillary thyroid carcinoma
patients without preoperatively detectable lateral node metastasis:
validity of prophylactic modified radical neck dissection. World J
Surg. 31:2085–2091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suliburk J and Delbridge L: Surgical
management of well-differentiated thyroid cancer: state of the art.
Surg Clin North Am. 89:1171–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ito Y, Tomoda C, Uruno T, Takamura Y, Miya
A, Kobayashi K, Matsuzuka F, Kuma K and Miyauchi A: Clinical
significance of metastasis to the central compartment from
papillary microcarcinoma of the thyroid. World J Surg. 30:91–99.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY,
Wang Y, Huang CP, Shen Q, Li DS and Wu Y: Risk factors for neck
nodal metastasis in papillary thyroid microcarcinoma: a study of
1066 patients. J Clin Endocrinol Metab. 97:1250–1257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim BY, Jung CH, Kim JW, Lee SW, Kim CH,
Kang SK and Mok JO: Impact of clinicopathologic factors on
subclinical central lymph node metastasis in papillary thyroid
microcarcinoma. Yonsei Med J. 53:924–930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng RC, Li Q, Lin KL, Zhang W, Gao EL,
Huang GL, Zhang XH and Zheng MH: Predicting the factors of lateral
lymph node metastasis in papillary microcarcinoma of the thyroid in
eastern China. Clin Transl Oncol. 14:842–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KE, Kim EK, Yoon JH, Han KH, Moon HJ
and Kwak JY: Preoperative prediction of central lymph node
metastasis in thyroid papillary microcarcinoma using
clinicopathologic and sonographic features. World J Surg.
37:385–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim YC, Choi EC, Yoon YH, Kim EH and Koo
BS: Central lymph node metastases in unilateral papillary thyroid
microcarcinoma. Br J Surg. 96:253–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Gu J, Shang J and Wang K:
Correlation analysis on central lymph node metastasis in 276
patients with cN0 papillary thyroid carcinoma. Int J Clin Exp
Pathol. 6:510–515. 2013.PubMed/NCBI
|
|
27
|
So YK, Son YI, Hong SD, Seo MY, Baek CH,
Jeong HS and Chung MK: Subclinical lymph node metastasis in
papillary thyroid microcarcinoma: A study of 551 resections.
Surgery. 148:526–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee KJ, Cho YJ, Kim SJ, Lee SC, Kim JG,
Ahn CJ and Lee DH: Analysis of the clinicopathologic features of
papillary thyroid microcarcinoma based on 7-mm tumor size. World J
Surg. 35:318–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY,
Park CS and Nam KH: Papillary microcarcinoma of the thyroid:
Predicting factors of lateral neck node metastasis. Ann Surg Oncol.
16:1348–1355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi YJ, Yun JS, Kook SH, Jung EC and Park
YL: Clinical and imaging assessment of cervical lymph node
metastasis in papillary thyroid carcinomas. World J Surg.
34:1494–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X
and Huang T: Multifocality and total tumor diameter predict central
neck lymph node metastases in papillary thyroid microcarcinoma. Ann
Surg Oncol. 20:746–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gulben K, Berberoğlu U, Celen O and Mersin
HH: Incidental papillary microcarcinoma of the thyroid-factors
affecting lymph node metastasis. Langenbecks Arch Surg. 393:25–29.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HS, Choi YJ and Yun JS: Features of
papillary thyroid microcarcinoma in the presence and absence of
lymphocytic thyroiditis. Endocr Pathol. 21:149–153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ
and Qu JM: The BRAF mutation is predictive of aggressive
clinicopathological characteristics in papillary thyroid
microcarcinoma. Ann Surg Oncol. 17:3294–3300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SS, Lee BJ, Lee JC, Kim SJ, Jeon YK,
Kim MR, Huh JE, Mok JY, Kim BH, Kim YK and Kim IJ: Coexistence of
Hashimoto's thyroiditis with papillary thyroid carcinoma: The
influence of lymph node metastasis. Head Neck. 33:1272–1277. 2011.
View Article : Google Scholar : PubMed/NCBI
|