Introduction
A tumor suppressor gene is defined as a gene whose
product normally inhibits tumor development (1,2). Tumor
suppressor gene mutation may predispose animals to cancer, which
plays a critical role in tumorigenesis (3). Previously, protein-coding genes were
considered to be the main compartment of tumor suppressors
(4). However, more recent studies
have revealed that non-coding RNAs, including microRNA and long
non-coding RNA (lncRNA) may also play a significant role in tumor
suppression (5,6).
LncRNA is defined as a non-protein-coding RNA
molecule longer than 200 nucleotides in length (7,8). In past
decades, these long non-coding transcripts were simply considered
as transcriptional ‘noise’ or cloning artifacts (9). However, accumulating evidence has
revealed that lncRNAs play key roles in a number of biological and
pathological processes (10). In
particular, the role of lncRNAs in human cancers has been
thoroughly researched and multiple lines of study have demonstrated
that lncRNAs act as oncogenes or tumor suppressors during
tumorigenisis (11).
LncRNA growth arrest-specific transcript 5 (GAS5)
was originally identified in 1988 (12–14). GAS5
was named due to its increased expression upon cell growth arrest
(12). Since its identification, a
number of studies have demonstrated that the expression of GAS5 is
decreased in various malignancies, including breast, gastric, lung
and prostate cancer (15–17). In this review, we discuss the current
knowledge on GAS5 lncRNA, with a focus on its status in human
tumors, mechanism in tumorigenesis, clinical implication, and
regulation of gene expression in carcinogenesis.
Structural characterization and biological
functions of GAS5
GAS5 was originally isolated in a study aimed at
screening potential tumor suppressor genes that were highly
expressed during growth arrest (14).
GAS5, located on chromosome 1q25, is approximately 630 nt in length
(18). GAS5 is classified as a
5′-terminal oligopyrimidine (5′TOP) gene since it possesses an
oligopyrimidine tract characteristic of the 5′TOP class of genes
(19). 5′TOP genes are defined as a
class of genes that have a uncommon pyrimidine-rich 5′-terminal
sequence (20). The mRNAs of 5′TOP
genes accumulate in messenger ribonucleoprotein particles (mRNPs)
during cell growth arrest. GAS5 comprises 12 exons, which are not
conserved between human and mouse homologs. GAS5 introns encode 10
small nucleolar RNAs (snoRNAs) and 2 mature lncRNA isoforms: GAS5a
and GAS5b. GAS5a is the main isoform at 77 nt in length whereas
GAS5b has only 45 nt, missing 32 nt. However, the putative open
reading frame is small and poorly conserved (13,21).
GAS5 is non-protein coding but its RNA is spliced,
polyadenylated, and associated with ribosomes (22). The essential biological activity of
GAS5 is mediated through the introns, which encode multiple snoRNAs
(12). The functions of GAS5 are not
well known as yet; however, it expresses multiple snoRNAs that
regulate the biosynthesis of ribosomal RNA. GAS5 transcript is
ubiquitously expressed in tissues, but it is unstable in
proliferating cells (23). Its
spliced RNA is low in growing cells, but is highly expressed during
growth arrest (serum starvation, density arrest) or under
inhibition of translation by cycloheximide, pactamycin or rapamycin
(12). The increased the transcript
level of GAS5 was considered to be caused by prolonged decay rates.
GAS5 has also proven to be necessary for normal growth arrest in
human T lymphocytes (24,25). A number of studies have been conducted
to elucidate the functions of GAS5 in humans. GAS5 is proven to be
associated with the cell cycle and cell progression, and critical
to normal growth arrest (26). GAS5
overexpression inhibits cell cycle progression while GAS5
inhibition decreases cell apoptosis and contributes to faster cell
cycle progression (27,28).
GAS5 status in cancers
Several lines of evidence have revealed aberrant
expression of GAS5 in numerous human cancers (15–17). GAS
expression is decreased in a number of cancer types, suggesting
that GAS5 may function as a tumor suppressor. In addition,
overexpression of GAS5 contributed to growth arrest in vitro
in numerous cancer lines. In this section, we discuss the current
findings of the expression of GAS5 in various human cancer types
(Table I).
 | Table I.Growth arrest-specific transcript 5
expression in various human cancers. |
Table I.
Growth arrest-specific transcript 5
expression in various human cancers.
| Cancer type | Expression | Method of
analysis | Reference |
|---|
| Breast cancer | Decreased | RT-qPCR | 15 |
| Lung cancer | Decreased | RT-qPCR | 23 |
| Gastric cancer | Decreased | RT-qPCR | 16 |
| Pancreatic
cancer | Decreased | RT-qPCR | 28 |
| Renal cell
carcinoma | Decreased | RT-qPCR | 32 |
| Bladder
carcinomas | Decreased | RT-qPCR | 27 |
| Prostate
cancer | Decreased | RT-qPCR | 17 |
| Malignant pleura
mesothelioma | Decreased | RT-qPCR | 22 |
| B-cell
lymphoma | Decreased | RT-qPCR | 19 |
| Leukemia | Decreased | RT-qPCR | 38 |
Breast cancer
GAS5 transcript levels were markedly decreased in
cancerous tissues compared with adjacent non-cancerous tissue,
using reverse transcription-quantitative polymerase chain reaction
analysis (15). The reduced
expression of GAS5 suggested its potential role in oncogenesis.
Certain GAS5 transcripts (GAS52B, GAS53A and GAS5O1) were proven to
induce growth arrest and apoptosis in human breast cancer cell
lines (29). GAS5 lncRNA promoted the
apoptosis of triple-negative and estrogen receptor-positive breast
cancer cells. Reduced GAS5 expression attenuated the cellular
responses to apoptotic stimuli in breast cancer. In addition, the
extent of breast cancer cell death was directly proportional to
cellular GAS5 levels, indicating a correlation between GAS5
expression and prognosis of breast cancer patients. There were a
negative correlation between miR-21 and GAS5 expression in breast
tumor specimens (30). It was
considered that miR-21 and GAS5 might regulate each other in a
similar way to the microRNA-mediated silencing of target mRNAs.
Nevertheless, the target genes of lncRNAs vary between specific
tissues and cell types. As a result, the target gene of GAS5 in
different cancer types differs. Further studies are needed to
identify the target genes of GAS5 in different cancers.
Lung cancer
GAS5 expression has also been associated with
carcinogenesis and metastasis in non-small cell lung cancer (NSCLC)
patients. In 72 specimens of NSCLC, GAS5 expression was reduced in
cancer samples compared with adjacent unaffected normal lung
tissues (P<0.05). The GAS5 expression level was correlated with
tumor size and tumor-node-metastasis stage (P<0.05).
Furthermore, a functional role of GAS5 in NSCLC was also
determined. GAS5 overexpression was demonstrated to promote cell
growth arrest and induce apoptosis in NSCLC in vitro and
in vivo (23).
Gastric cancer
A previous study compared HOX transcript antisense
RNA (HOTAIR) expression levels in 89 gastric cancer tissues with
expression levels in corresponding non-cancerous gastric tissue
(16). GAS5 expression was
significantly decreased in gastric cancer tissues. In addition,
GAS5 expression levels correlated with larger tumor size and
advanced pathological stage. Low GAS5 expression levels indicated
poorer prognosis, with shorter disease-free survival and overall
survival times, serving as an independent prognostic indicator for
gastric cancer. Functional analysis demonstrated that GAS5
inhibited gastric cancer cell proliferation and induced apoptosis
in vitro and in vivo. GAS5 overexpression decreased
E2F1 and cyclin D1 expression while it increased P21 expression in
gastric cells. In addition, GAS5 downexpression increased E2F1 and
cyclin D1 expression while it decreased P21 expression levels.
These results indicated that by regulating E2F1 and P21, GAS5
inhibited gastric cancer development through a mechanism of
post-transcriptional regulation (16).
Pancreatic cancer
Similar to the findings of GAS5 in gastric cancer,
studies of pancreatic cancer have demonstrated that GAS5 expression
was markedly decreased in pancreatic cancer tissues compared with
normal pancreatic ductal cells. GAS5 inhibited pancreatic cancer
cell proliferation. Furthermore, cyclin-dependent kinase 6 (CDK6)
expression was negatively regulated by GAS5 in vitro and
in vivo. Knockdown of CDK6 partially abrogates
GAS5-siRNA-induced cancer cell proliferation (28).
Renal cell carcinoma
The expression of GAS5 was significantly
downregulated in renal cell carcinoma cells compared with normal
control cells. Overexpression of GAS5 inhibited cell proliferation,
migration and invasion potential and induced cell apoptosis in
renal carcinoma cell lines (31,32).
Bladder cancer
GAS5 expression was lower in both bladder cancer
cell lines and human specimens. Overexpression of GAS5 inhibited
bladder cancer cell proliferation. GAS5 exerted its effect in
bladder cancer partly by regulating CDK6, since knockdown of GAS5
increased CDK6 mRNA and protein levels in bladder cancer cells.
GAS5 inhibition induces a significant decrease in the G0/G1 phase
and a notable increase in the S phase (27).
Prostate cancer
GAS5 gene expression was significantly downregulated
in prostate cell lines derived from metastases (LNCaP and PC-3)
compared with those derived from normal prostate tissue or primary
prostate cancer (17,33,34). In
addition, the cellular GAS5 levels decreased as prostate cancer
cells acquired castration resistance. GAS5 promoted the apoptosis
of prostate cells. GAS5 overexpression promoted cancer cell death
induced by UV-C irradiation and chemotherapeutic drugs, and
downregulation of GAS5 expression attenuated it. LncRNA GAS5 binds
to the corresponding domain on the androgen receptor and inhibits
transcriptional stimulation (35).
Considering that the androgen receptor plays a crucial role in the
survival of prostate cancer cells (36), this is of potential significance,
since downregulation of GAS5 levels may permit increased
pro-survival signaling through the androgen receptor pathway.
Malignant pleura mesothelioma
GAS5 expression was significantly decreased in
malignant pleura mesothelioma (MPM) cell lines compared with normal
controls. GAS5 was largely expressed in quiescent cancerous cells.
MPM cell growth arrest induced by drugs was accompanied with GAS5
accumulation in the nuclei. In addition, GAS5 expression was highly
expressed in podoplanin-positive MPM. Silencing of GAS5 shortened
the cell cycle length and increased the expression of
glucocorticoid responsive genes, glucocorticoid inducible
leucine-zipper and serum/glucocorticoid-regulated kinase-1
(22).
B-cell lymphoma
Nakamura et al reported a chromosomal
translocation of t (1;3) (q25;q27) in a case of diffuse large
B-cell lymphoma (19). The
chromosomal translocation of t (1;3) (q25;q27) led to the
expression of GAS5-B-cell lymphoma 6 (BCL6) chimeric transcripts.
In this case, the breakpoint in chromosome 1 was located within
GAS5 and the breakpoint in chromosome 3 at 4 kb upstream of BCL6
exon 1a. Promoter substitution leads to the inappropriate
expression of BCL6, which normally acts as a transcriptional
repressor (19,37). It was considered that the
inappropriate expression of BCL6, which was a result of the changes
in post-transcriptional regulation of the chimeric mRNA, was
responsible for lymphomagenesis (19).
Leukemia
Etomidate is an essential tool in a variety of
scenarios for intubation and procedural sedation. DNA microarray
assay indicated that etomidate increased the expression of GAS5 in
murine leukemia in vitro. Etomidate is cytotoxic due to its
induction of apoptosis. However, the mechanism for
etomidate-induced apoptosis remains unclear. GAS5 may play a role
in the process (38). Table I summarizes the expression of GAS5 in
various types of cancers.
GAS5 tumor-suppressive functions
The frequent downregulation of GAS5 in human cancers
has provided new insight into our understanding of its functions.
GAS5 suppresses tumors by regulating multiple cellular processes,
including growth arrest, apoptosis, proliferation, metastasis and
DNA damage repair. In this section, we discuss how GAS5
deregulations contribute to cancer development.
GAS5 regulates growth arrest and
apoptosis
Apoptosis, also called programmed cell death, is
critical in normal development, and in the initiation and
progression of cancers (39). Mutated
and deleted pro-apoptotic genes give rise to carcinogenesis and
tumor growth. A number of in vitro studies have demonstrated
that GAS5 is pro-apoptotic. In vitro and in vivo
experiments revealed that GAS5 induced growth arrest and apoptosis
in numerous cancers, including breast cancer, NSCLC, gastric
cancer, renal cancer and prostate cancer (15–17,23,31,40).
GAS5 regulates proliferation
GAS5 was demonstrated to inhibit tumor cell
proliferation in gastric cancer, pancreatic cancer, renal carcinoma
cell lines and bladder cancer cells (16,27,28,31).
GAS5 regulates metastasis
In addition to promoting tumor cell apoptosis and
inhibiting proliferation, GAS5 also inhibits tumorigenesis by
inhibiting metastasis. Overexpression of GAS5 inhibited migration
and invasion potential in renal carcinoma cell lines (31). GAS5 gene expression was markedly
decreased in prostate cancer cells derived from metastases when
compared with those derived from normal prostate tissue or primary
prostate cancer (34).
Molecular mechanism and action of GAS5
Although GAS5 has been reported as a tumor
suppressor, the underlying molecular mechanism remains largely
unknown. LncRNAs modulate the expression of various genes, playing
a number of significant biological roles, including dosage
compensation and genomic imprinting, histone-modification, gene
activation, gene repression, lineage determination and cell
proliferation (5,41).
GAS5 controls gene transcription
Aberrant gene expression plays a critical role in
the initiation and progression of cancer. Elucidation of the
lncRNA-mediated transcriptional or post-transcriptional gene
regulations has prompted investigations into the mechanisms of
cancer.
The negative correlation between miR-21 and GAS5
expression in breast tumor suggested that they might regulate each
other in a similar manner to the microRNA-mediated silencing of
target mRNAs (30). In gastric cells,
GAS5 overexpression decreased the expression of E2F1 and cyclin D1
and increased the expression of P21 (16). E2F1 was demonstrated to contribute to
tumorigenesis by influencing cell proliferation (23). Cyclin D1 is also associated with the
development of cancers through its involvement in cell cycle
regulation (42). Furthermore, GAS5
snoRNA levels are strongly correlated with p53 expression in
colorectal cancer (43). P53 plays a
prominent role in cancer by deregulating apoptosis (44). In addition, p21 is downregulated or
lost in several cancer types with its inhibitory control over the
cell cycle (45,46). GAS5 exerts its effect in bladder
cancer partly by regulating CDK6, since knockdown of GAS5 increased
CDK6 mRNA and protein levels (27,28). CDK6
regulated the cell cycle, and dysregulation of CDK6 is associated
with bladder cancer progression (47,48). In
addition, GAS5 expression was highly expressed in
podoplanin-positive MPM (22).
Podoplanin, a type I transmembrane sialomucin-like glycoprotein, is
highly expressed in MPM and induces platelet aggregation (49). The GAS5-BCL6 chimeric transcripts
resulting from the chromosomal translocation t (1;3) (q25;q27) led
to the inappropriate expression of BCL6 (19). It was considered to be responsible for
the initiation and development of lymphoma.
GAS5 acts as molecular decoy sequestering-specific
DNA-binding proteins. GAS5 could suppress glucocorticoid receptor
(GR)-induced transcriptional activity of endogenous
glucocorticoid-responsive genes by competing with DNA
glucocorticoid receptor response element (GRE) for binding to the
GR since it is structurally similar to GRE (34,43). The
GR regulates cell survival by binding the promoters of various
glucocorticoid-responsive genes, including apoptosis-related genes
(50).
Upstream regulation of GAS5
expression
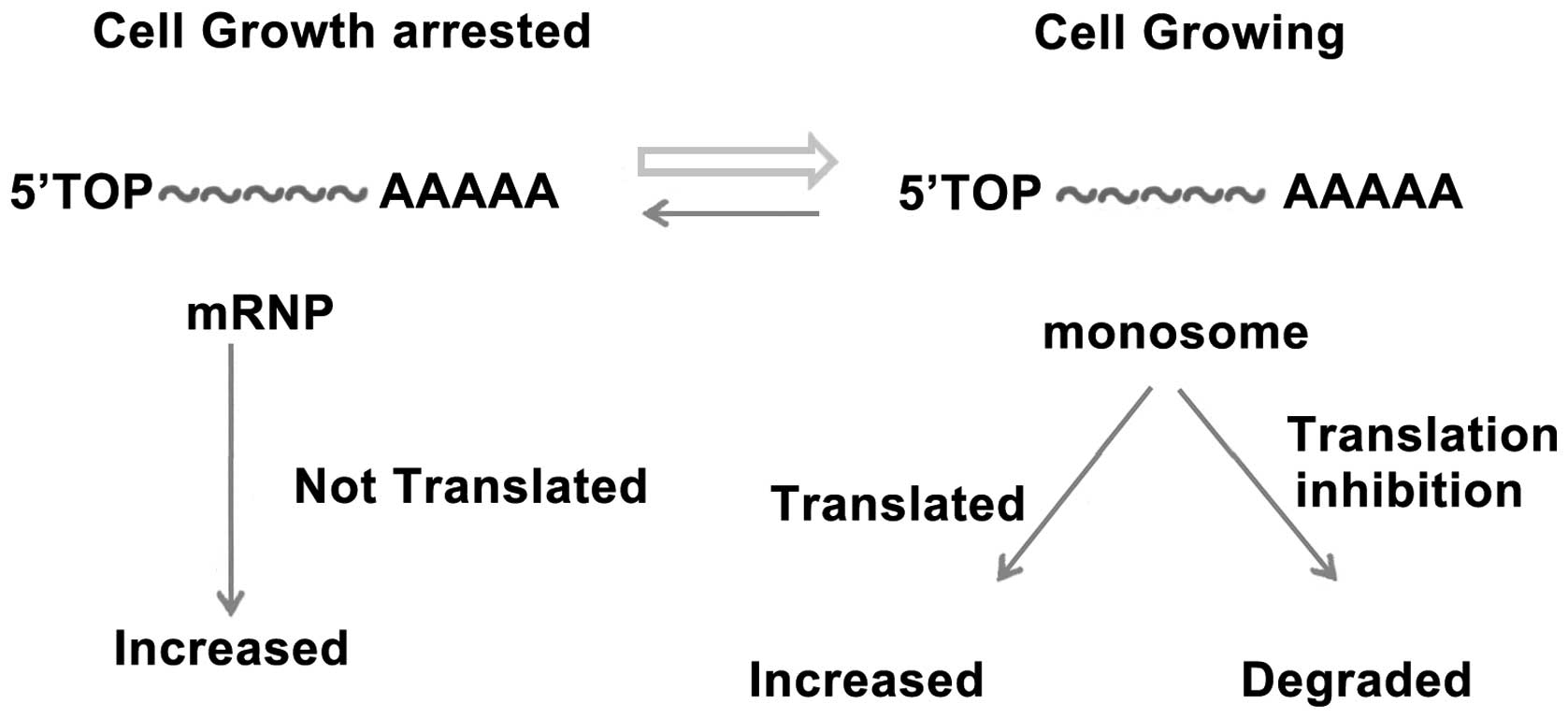
As mentioned above, GAS5 expression increased upon
cell growth arrest. GAS5 plays critical roles in normal growth
arrest, as well as in the growth arrest of a number of cancers.
However, how GAS5 expression increases upon cell growth arrest
remains unclear. The classification of GAS5 as a 5′TOP gene
provides a possible explanation as to why it functions as a growth
arrest (20). During arrested cell
growth, 5′TOP accumulates in mRNPs. In growing cells, spliced GAS5
RNA is associated with ribosomes, translated and rapidly degraded.
When cell growth is arrested and translation is inhibited, the
levels of GAS5 increase (Fig. 1).
The human genome integrity is constantly damaged by
genotoxic agents from the exogenous environment (chemicals and
ultraviolet, for example) and endogenous environment (replication
and oxidative stress) (51,52). The ability to repair damaged DNA is
critical for living organisms. GAS5-derived snoRNA expression was
induced by DNA damage in a p53-dependent manner in colorectal
cancer cell lines (43). It was
considered that GAS5-derived snoRNA might be involved in the DNA
damage repair.
Implications in cancer management
Given that aberrant GAS5 expression was reported in
various human cancers, it provides an attractive approach for
cancer management. Firstly, GAS5 may be used as a biomarker for
cancer screening. By monitoring the GAS5 level in an individual
with a suspected tumor, it is possible to predict the risk of
cancer development and progression, or the prognosis of the cancer.
Secondly, GAS5 is assumed to be a tumor suppressor, which inhibits
cell growth and metastasis and induces apoptosis in certain cancer
cells; therefore, re-introduction of the GAS5 gene provides us with
an attractive strategy to treat cancer.
Conclusions and future directions
GAS5, downregulated in a number of human cancers, is
a well-characterized tumor suppressor. GAS5 induces apoptosis and
inhibits proliferation and metastasis. The molecular mechanism of
GAS5 tumorigenesis remains to be investigated. Through regulating
the expression of various tumor-related genes and proteins,
including E2F1 and cyclin D1, GAS5 may exert its function in
tumorigenesis. Since the regulatory mechanisms are tissue-specific,
the effects of the polymorphisms on GAS5 expression and the
associated regulatory mechanisms have only been evaluated in a few
cancer tissues. Although previous studies have reported the
tumor-suppressing role of GAS5 in several cancer types, including
breast and gastric cancer, the expression and function of GAS5 in
other tumors, such as liver cancer, remains to be investigated.
References
|
1
|
Li Z, Lei H, Luo M, et al: DNA methylation
downregulated mir-10b acts as a tumor suppressor in gastric cancer.
Gastric Cancer. 18:43–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oliveira AM, Ross JS and Fletcher JA:
Tumor suppressor genes in breast cancer: the gatekeepers and the
caretakers. Am J Clin Pathol. 124 (Suppl):S16–S28. 2005.PubMed/NCBI
|
|
3
|
Banerjee R, Mani RS, Russo N, et al: The
tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2
overexpression in invasive squamous cell carcinoma. Oncogene.
30:4339–4349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen X, Cao X, Dong W, Luo S, Suo Z and
Jin Y: Expression of TIP30 tumor suppressor gene is down-regulated
in human colorectal carcinoma. Dig Dis Sci. 55:2219–2226. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tano K and Akimitsu N: Long non-coding
RNAs in cancer progression. Front Genet. 3:2192012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitagawa M, Kotake Y and Ohhata T: Long
non-coding RNAs involved in cancer development and cell fate
determination. Curr Drug Targets. 13:1616–1621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: a new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Y, Meng XM, Huang C, et al: Long
noncoding RNAs: Novel insights into hepatocelluar carcinoma. Cancer
Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gutschner T and Diederichs S: The
hallmarks of cancer: a long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith CM and Steitz JA: Classification of
gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member
of the 5′-terminal oligopyrimidine gene family reveals common
features of snoRNA host genes. Mol Cell Biol. 18:6897–6909.
1998.PubMed/NCBI
|
|
13
|
Muller AJ, Chatterjee S, Teresky A and
Levine AJ: The gas5 gene is disrupted by a frameshift mutation
within its longest open reading frame in several inbred mouse
strains and maps to murine chromosome. J. Mamm Genome. 9:773–774.
1998. View Article : Google Scholar
|
|
14
|
Coccia EM, Cicala C, Charlesworth A, et
al: Regulation and expression of a growth arrest-specific gene
(gas5) during growth, differentiation and development. Mol Cell
Biol. 12:3514–3521. 1992.PubMed/NCBI
|
|
15
|
MourtadaMaarabouni M, Pickard MR, Hedge
VL, Farzaneh F and Williams GT: GAS5, a non-protein-coding RNA,
controls apoptosis and is downregulated in breast cancer. Oncogene.
28:195–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun M, Jin FY, Xia R, et al: Decreased
expression of long noncoding RNA GAS5 indicates a poor prognosis
and promotes cell proliferation in gastric cancer. BMC Cancer.
14:3192014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pickard MR, MourtadaMaarabouni M and
Williams GT: Long non-coding RNA GAS5 regulates apoptosis in
prostate cancer cell lines. Biochim Biophys Acta. 1832:1613–1623.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fleming JV, Fontanier N, Harries DN and
Rees WD: The growth arrest genes gas5, gas6 and CHOP-10 (gadd153)
are expressed in the mouse preimplantation embryo. Mol Reprod Dev.
48:310–316. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura Y, Takahashi N, Kakegawa E, et
al: The GAS5 (growth arrest-specific transcript 5) gene fuses to
BCL6 as a result of t (1;3)(q25;q27) in a patient with B-cell
lymphoma. Cancer Genet Cytogenet. 182:144–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tcherkezian J, Cargnello M, Romeo Y, et
al: Proteomic analysis of cap-dependent translation identifies
LARP1 as a key regulator of 5′TOP mRNA translation. Genes Dev.
28:357–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raho G, Barone V, Rossi D, Philipson L and
Sorrentino V: The gas5 gene shows four alternative splicing
patterns without coding for a protein. Gene. 256:13–17. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Renganathan A, KresojaRakic J, Echeverry
N, et al: GAS5 long non-coding RNA in malignant pleural
mesothelioma. Mol Cancer. 13:1192014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi X, Sun M, Liu H, et al: A critical
role for the long non-coding RNA GAS5 in proliferation and
apoptosis in non-small-cell lung cancer. Mol Carcinog. Dec
19–2013.(Epub ahead of print).
|
|
24
|
MourtadaMaarabouni M, Hasan AM, Farzaneh F
and Williams GT: Inhibition of human T-cell proliferation by
mammalian target of rapamycin (mTOR) antagonists requires noncoding
RNA growth-arrest-specific transcript 5 (GAS5). Mol Pharmacol.
78:19–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
MourtadaMaarabouni M, Hedge VL, Kirkham L,
Farzaneh F and Williams GT: Growth arrest in human T-cells is
controlled by the non-coding RNA growth-arrest-specific transcript
5 (GAS5). J Cell Sci. 121:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010.PubMed/NCBI
|
|
27
|
Liu Z, Wang W, Jiang J, et al:
Downregulation of GAS5 promotes bladder cancer cell proliferation,
partly by regulating CDK6. PLoS One. 8:e739912013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu X, Fang Y, Wang Z, et al:
Downregulation of gas5 increases pancreatic cancer cell
proliferation by regulating CDK6. Cell Tissue Res. 354:891–896.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gee HE, Buffa FM, Camps C, et al: The
small-nucleolar RNAs commonly used for microRNA normalisation
correlate with tumour pathology and prognosis. Br J Cancer.
104:1168–1177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Zhu Z, Watabe K, et al: Negative
regulation of lncRNA GAS5 by miR-21. Cell Death Differ.
20:1558–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou S, Wang J and Zhang Z: An emerging
understanding of long noncoding RNAs in kidney cancer. J Cancer Res
Clin Oncol. 140:1989–1995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shain SA: Exogenous fibroblast growth
factors maintain viability, promote proliferation and suppress
GADD45alpha and GAS6 transcript content of prostate cancer cells
genetically modified to lack endogenous FGF-2. Mol Cancer Res.
2:653–661. 2004.PubMed/NCBI
|
|
34
|
Romanuik TL, Wang G, Morozova O, Delaney
A, Marra MA and Sadar MD: LNCaP Atlas: gene expression associated
with in vivo progression to castration-recurrent prostate cancer.
BMC Med Genomics. 3:432010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abbah SA, Lam CX, Ramruttun AK, Goh JC and
Wong HK: Fusion performance of low-dose recombinant human bone
morphogenetic protein 2 and bone marrow-derived multipotent stromal
cells in biodegradable scaffolds: a comparative study in a large
animal model of anterior lumbar interbody fusion. Spine (Phila Pa
1976). 36:1752–1759. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agirre X, VilasZornoza A, Jiménez-Velasco
A, et al: Epigenetic silencing of the tumor suppressor microRNA
Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis
in acute lymphoblastic leukemia. Cancer Res. 69:4443–4453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MourtadaMaarabouni M and Williams GT: Role
of GAS5 noncoding RNA in mediating the effects of rapamycin and its
analogues on mantle cell lymphoma cells. Clin Lymphoma Myeloma
Leuk. 14:468–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu RS, Wu KC, Yang JS, et al: Etomidate
induces cytotoxic effects and gene expression in a murine leukemia
macrophage cell line (RAW264.7). Anticancer Res. 31:2203–2208.
2011.PubMed/NCBI
|
|
39
|
He JF, Luo YM, Wan XH and Jiang D:
Biogenesis of MiRNA-195 and its role in biogenesis, the cell cycle
and apoptosis. J Biochem Mol Toxicol. 25:404–408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pickard MR and Williams GT: Regulation of
apoptosis by long non-coding RNA GAS5 in breast cancer cells:
implications for chemotherapy. Breast Cancer Res Treat.
145:359–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Zhang P, Wang L, Piao HL and Ma
L: Long non-coding RNA HOTAIR in carcinogenesis and metastasis.
Acta Biochim Biophys Sin (Shanghai). 46:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Z, Shen J, Wu WK, et al: Leptin induces
cyclin D1 expression and proliferation of human nucleus pulposus
cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PloS One.
7:e531762012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Krell J, Frampton AE, Mirnezami R, et al:
Growth arrest-specific transcript 5 associated snoRNA levels are
related to p53 expression and DNA damage in colorectal cancer. PloS
One. 9:e985612014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okada N, Lin CP, Ribeiro MC, et al: A
positive feedback between p53 and miR-34 miRNAs mediates tumor
suppression. Genes Dev. 28:438–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi OR and Lim IK: Loss of p21 (Sdi1)
expression in senescent cells after DNA damage accompanied with
increase of miR-93 expression and reduced p53 interaction with p21
(Sdi1) gene promoter. Biochem Biophys Res Commun. 407:406–411.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baldus SE, Schneider PM, Mönig SP, et al:
p21/waf1/cip1 in gastric cancer: associations with
histopathological subtypes, lymphonodal metastasis, prognosis and
p53 status. Scand J Gastroenterol. 36:975–980. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu J, Qian J, Li C, et al: miR-129
regulates cell proliferation by downregulating Cdk6 expression.
Cell Cycle. 9:1809–1818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
RodriguezOtero P, Román-Gómez J,
VilasZornoza A, et al: Deregulation of FGFR1 and CDK6 oncogenic
pathways in acute lymphoblastic leukaemia harbouring epigenetic
modifications of the MIR9 family. Br J Haematol. 155:73–83. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dadras SS, Skrzypek A, Nguyen L, et al:
Prox-1 promotes invasion of kaposiform hemangioendotheliomas. J
Invest Dermatol. 128:2798–2806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu I, Shin SC, Cao Y, et al: Selective
glucocorticoid receptor translational isoforms reveal
glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis.
4:e4532013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ghosal G and Chen J: DNA damage tolerance:
a double-edged sword guarding the genome. Transl Cancer Res.
2:107–129. 2013.PubMed/NCBI
|
|
52
|
Alexeyev M, Shokolenko I, Wilson G and
LeDoux S: The maintenance of mitochondrial DNA integrity-critical
analysis and update. Cold Spring Harb Perspect Biol. 5:a0126412013.
View Article : Google Scholar : PubMed/NCBI
|