Introduction
Hepatocellular carcinoma (HCC) is one of the leading
causes of mortality in Asian countries, including Japan, as well as
in Africa and Europe. Hepatitis B virus (HBV) and hepatitis C virus
(HCV) infections are known to be important risk factors for the
development of HCC (1,2). Carcinogenesis due to HBV and/or HCV
infection has been decreasing in Japan, due to the reduction in the
number of new viral infections. However, the number of HCC patients
with neither HBV nor HCV infection (NBNC HCC) has been increasing
annualy, currently accounting for 15% of all HCC cases in Japan
(3). In NBNC HCC cases,
carcinogenesis may be attributable to a history of alcohol abuse
(AL HCC) or non-alcoholic steatohepatitis (NASH HCC), in which a
progressively fatty liver leads to cirrhosis despite the absence of
a history of alcohol abuse. Furthermore, carcinogenesis may occur
in a minority of patients with normal background liver tissue and
no history of alcohol abuse. In addition, cases positive for the
hepatitis B core antibody (HBc-Ab), indicating a history of
infection, may be classified as NBNC HCC if they are negative for
the hepatitis B surface antigen (HBs-Ag). However, the criteria to
determine whether these cases should be classified as NBNC HCC have
not yet been clearly determined. The clinicopathological
characteristics of NBNC HCC have recently attracted attention. The
present study evaluated patients who underwent NBNC HCC resection
at a single center, with the purpose of comparing the
post-resection outcomes of NBNC HCC with those of virus-related HCC
according to disease stage. In addition, we aimed to elucidate the
factors contributing to poor prognosis in NBNC HCC, classify NBNC
HCC according to etiology (NASH, AL and non-NASH/non-AL) and
investigate its characteristics and outcomes.
Patients and methods
Patients
Of the patients diagnosed with HCC between March,
2001 and the end of March, 2013, 164 were negative for HBs-Ag and
HCV-Ab according to preoperative blood tests and underwent HCC
resection (NBNC group); 144 were positive for HBs-Ag and underwent
HCC resection (HBV group); and 550 were positive for HCV-Ag and
underwent HCC resection (HCV group). Patients who were positive for
HBc-Ab and those positive for both HBs-Ag and HCV-Ab were excluded
from the analysis. The diagnosis of HCC was based on abdominal
contrast-enhanced computed tomography, magnetic resonance imaging,
or ultrasonography findings. All the studies were approved by the
Committee of Medical Ethics of Meiwa Hospital (Nishinomiya,
Japan).
Study design
Study 1
The NBNC, HBV and HCV groups were compared with
regard to age, maximum tumor size, tumor number, disease stage
(Liver Cancer Study Group of Japan) (4), albumin level, total bilirubin level,
aspartate aminotransferase (AST) level, alanine aminotransferase
(ALT) level, white blood cell (WBC) count, platelet count,
prothrombin (PT) activity level, α-fetoprotein (AFP) level,
des-γ-carboxyprothrombin (DCP) level, indocyanine green retention
rate at 15 min (ICGR15), presence or absence of diabetes,
Child-Pugh classification and body mass index (BMI). Patients ‘with
diabetes’ were defined as those self-administering insulin and
those receiving oral antidiabetic drugs. The post-resection
outcomes were compared according to disease stage. The
Kruskal-Wallis and χ2 tests were used to compare
background factors among the groups. The Kaplan-Meier method and
the log-rank test were used to compare outcomes.
Study 2
Sixty-one patients with NBNC HCC succumbed after 2
years. The remaining patients were divided into two groups: 39
patients with a survival time of <2 years (short survival; SS
group) and 64 patients with a survival time of ≥2 years (long
survival; LS group). The tumor and patient characteristics were
compared between the two groups, and a multivariate analysis was
performed with prognostic factors achieving significance
(P<0.05) in the univariate analysis. The Mann-Whitney U test and
the χ2 test were used for comparisons between the two
groups, and multivariate logistic regression was used for the
multivariate analysis.
Study 3
Patients with NBNC HCC were divided into three
groups: 40 patients with pathologically diagnosed NASH (NASH
group), 80 patients with a history of alcohol abuse (>20 g per
day; AL group), and 44 patients without pathologically diagnosed
NASH or history of alcohol abuse (non-NASH/non-AL group). The tumor
and patient characteristics were compared among the three groups,
and the post-resection outcomes were analyzed. The Kruskal-Wallis
and χ2 tests were used for comparing background
characteristics, whereas the Kaplan-Meier method and the log-rank
test were used to evaluate outcomes. For each statistical test,
P<0.05 was considered to indicate statistically significant
differences. The statistical software used was JMP 9.0.2 (SAS
Institute Japan Ltd., Tokyo, Japan).
Results
Study 1
The patient characteristics according to HCC
etiology are summarized in Table I.
The average age in the NBNC group was the highest (70.3±7.8 years)
and that in the HBV group the lowest (60.3±10.8 years). Tumor size
was significantly larger in the NBNC (4.7±3.7 cm) and HBV (4.5±3.7
cm) groups compared with that in the HCV (3.2±2.2 cm) group. The
albumin, WBC and platelet count were significantly increased, and
AST and ALT levels were significantly decreased in the NBNC and HBV
groups compared with those in the HCV group. The bilirubin level
was significantly decreased and PT activity was significantly
increased in the NBNC group compared with those in the HBV and HCV
groups. ICGR15 was significantly decreased in the NBNC group
compared with that in the HCV group, and the Child Pugh class A was
significantly increased in the NBNC group compared with that in the
HBV and HCV groups. The prevalence of diabetes was significantly
higher in the NBNC group compared with that in the HBV and HCV
groups. BMI was significantly higher in the NBNC group compared
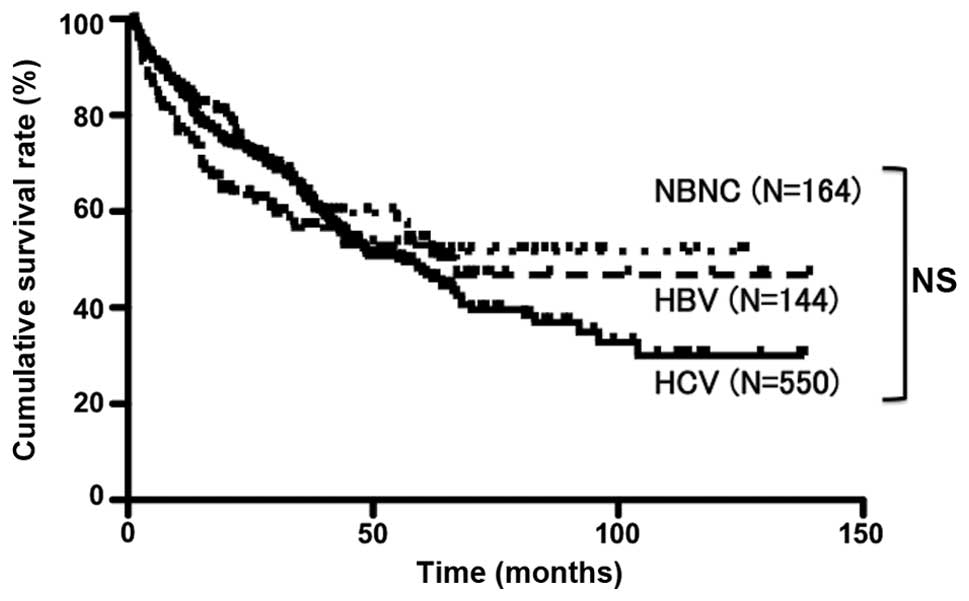
with that in the HBV and HCV groups (Table I). For the entire series, the
cumulative survival rates at 1, 3 and 5 years were 85.0, 63.6 and
54.0%, respectively, in the NBNC group; 75.6, 56.5 and 53.0%,
respectively, in the HBV group; and 83.5, 63.1 and 47.4%,
respectively, in the HCV group. These differences were not
significantly different (Fig. 1). On
comparison according to disease stage, the cumulative survival
rates for stage II disease at 1, 3 and 5 years were 97.2, 81.4 and
70.1%, respectively, in the NBNC group and 93.9, 75.8 and 57.3%,
respectively, in the HCV group. Thus, the NBNC group exhibited a
significantly more favorable prognosis (P=0.04). No significant
differences were observed for stages I, III and IV (Fig. 2).
 | Table I.Patient characteristics according to
hepatocellular carcinoma etiology. |
Table I.
Patient characteristics according to
hepatocellular carcinoma etiology.
| Characteristics | NBNC (n=164) | HBV (n=144) | HCV (n=550) |
|---|
| Age
(years)a | 70.3±7.8b,c |
60.3±10.8c | 69.4±7.6 |
| Gender
(male/female) | 149/32c | 111/33 | 401/149 |
| Maximum tumor
diameter (cm)a | 4.7±3.7c | 4.5±3.7c | 3.2±2.2 |
| Tumors
(n)a | 1.9±2.8 | 2.0±1.6 | 2.0±2.1 |
| Disease
stagea | 2.2±0.6 | 2.3±0.6c | 2.1±0.7 |
| Albumin
(g/dl)a | 4.0±0.5c | 3.9±0.5c | 3.7±0.4 |
| Total bilirubin
(mg/dl)a | 0.7±0.3b,c | 0.8±0.4 | 0.8±0.4 |
| AST
(IU/l)a | 41±36c | 46±32c | 58±34 |
| ALT
(IU/l)a | 36±36c | 39±32c | 52±37 |
| White blood cell
count (/µl)a |
5,586±1,817c |
5,325±1,956c | 4,593±1,685 |
| Platelet count
(x104/µl)a | 18.4±9.9c | 16.0±8.6c | 13.1±6.9 |
| Prothrombin activity
(%)a | 87±13b,c | 79±13 | 82±16 |
| α-fetoprotein
(ng/ml)a |
14,072±110,966c |
17,559±122,993c | 2,406±18,702 |
| DCP
(mAU/ml)a |
11,827±74,826b,c | 6,726±45,768 | 1,707±8,522 |
| ICGR15
(%)a | 15±11c | 16±11 | 21±14 |
| Diabetes (+/-) | 87/94
(48%)b,c | 33/111 (23%) | 151/399 (27%) |
| Child-Pugh
Classification (A/B) | 166/15b,c | 116/28 | 425/125 |
| BMI
(kg/m2) | 23.9±3.8b,c | 22.7±3.8 | 22.5±3.1 |
Study 2
The results of the univariate analysis for the SS
and LS groups are presented in Table
II. The groups differed significantly with regard to tumor
size, tumor number, albumin, total bilirubin, DCP, AFP and AST
levels, PT activity, ICGR15, Child-Pugh classification, tumor
differentiation, degree of liver fibrosis and presence or absence
of portal vein tumor thrombus (Table
II). In the multivariate analysis of these factors, high AFP
level, poorly differentiated HCC and advanced liver fibrosis were
found to be independent risk factors (Table III).
 | Table II.Univariate analysis of factors
affecting prognosis in patients with NBNC hepatocellular
carcinoma. |
Table II.
Univariate analysis of factors
affecting prognosis in patients with NBNC hepatocellular
carcinoma.
| Characteristics | SS group (n=39) | LS group (n=64) | P-value |
|---|
| Age
(years)a | 69±8 | 69±7 | 0.47 |
| Gender
(male/female) | 34/5 | 52/12 | 0.43 |
| Maximum tumor
diameter (cm)a | 7.2±5.8 | 4.2±3.0 | 0.005 |
| Tumors
(n)a | 2.5±2.5 | 1.5±1.1 | 0.01 |
| Albumin
(g/dl)a | 3.8±0.5 | 4.2±0.4 | 0.01 |
| Total bilirubin
(mg/dl)a | 0.8±0.4 | 0.7±0.3 | 0.01 |
| AST
(IU/l)a | 58±52 | 34±22 | 0.001 |
| ALT
(IU/l)a | 42±44 | 36±40 | 0.50 |
| White blood cell
count (/µl)a | 5,397±2,054 | 5,870±1,684 | 0.06 |
| Platelet count
(x104/µl)a | 19.3±7.4 | 18.5±7.2 | 0.69 |
| Prothrombin
activity (%)a | 85.5±12.8 | 90.7±11.9 | 0.04 |
| α-fetoprotein
(ng/ml)a | 64,222±236,188 | 201±847 | 0.0001 |
| DCP
(mAU/ml)a | 54,224±162,995 | 1,677±5,669 | 0.02 |
| ICGR15
(%)a | 18.1±10.1 | 12.5±7.9 | 0.003 |
| Diabetes (+/-) | 16/23 | 33/31 | 0.29 |
| Child-Pugh
Classification (A/B,C) | 32/7 | 61/3 | 0.02 |
| Pathological
differentiation |
| (poor or
moderate/high) | 15/24 | 1/63 | 0.0001 |
| Fibrosis
stagea | 2.9±0.9 | 2.1±1.2 | 0.003 |
| BMI
(kg/m2) | 23.4±3.5 | 24.1±3.6 | 0.61 |
| Tumor thrombus in
portal vein (+/-) | 18/11 | 7/57 | 0.0001 |
 | Table III.Multivariate analysis of factors
affecting prognosis in patients with NBNC hepatocellular
carcinoma. |
Table III.
Multivariate analysis of factors
affecting prognosis in patients with NBNC hepatocellular
carcinoma.
|
Characteristics | P-value | Hazard ratio | 95% confidence
interval |
|---|
| Maximum tumor
diameter (cm) | 0.86 | 1.02 | −0.24–0.28 |
| Tumors (n) | 0.97 | 1.01 | −0.38–0.41 |
| Albumin (g/dl) | 0.22 | 0.34 | −0.29–0.66 |
| Total bilirubin
(mg/dl) | 0.26 | 2.81 | −0.74–3.14 |
| AST (IU/l) | 0.34 | 1.01 | −0.01–0.04 |
| α-fetoprotein
(ng/ml) | 0.04 | 1.89 | 0.001–1.33 |
| DCP (mAU/ml) | 0.10 | 2.13 | −0.15–1.75 |
| ICGR15 (%) | 0.55 | 1.02 | −0.05–0.09 |
| Child-Pugh
Classification (A/B,C) | 0.88 | 0.81 | −3.14–2.53 |
| Pathological
differentiation |
| (poor or
moderate/high) | 0.003 | 18.70 | 0.89–6.01 |
| Fibrosis stage | 0.004 | 2.08 | 0.03–1.54 |
| Tumor thrombus in
portal vein (+/-) | 0.26 | 2.50 | −0.72–2.57 |
Study 3
The patient characteristics in the NASH, AL and
non-NASH/non-AL groups are presented in Table IV. The proportion of female patients
was significantly higher in the NASH group and the proportion of
male patients was significantly higher in the AL group. There were
no significant differences in the other background data (Table IV). The cumulative survival rates
following surgery at 1, 3 and 5 years were 83.0, 62.2 and 54.5%,
respectively, for the NASH group; 87.0, 69.7 and 56.3%,
respectively, for the AL group; and 80.8, 42.0 and 33.6%,
respectively, for the non-NASH/non-AL group. These differences were
not statistically significant (Fig.
3).
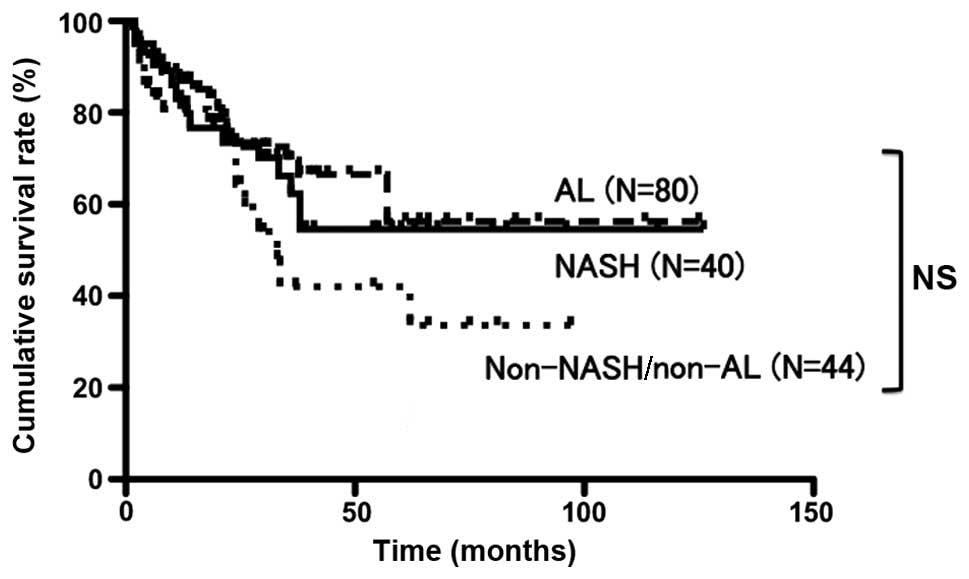 | Figure 3.Post-surgical cumulative survival
rates over 5 years for the NASH, AL and non-NASH/non-AL groups were
54.5, 56.3 and 33.6%, respectively. The prognosis was not
significantly different. Continuous line, NASH; short dotted line,
non-NASH/non-AL; and long dotted line, AL. NASH, HCC patients with
non-alcoholic steatohepatitis; AL, HCC patients who consumed >20
g alcohol daily; non-NASH/non-AL, HCC patients without NASH or AL;
NS, non-significant. |
 | Table IV.Patient characteristics of NBNC
hepatocellular carcinoma according to underlying liver
background. |
Table IV.
Patient characteristics of NBNC
hepatocellular carcinoma according to underlying liver
background.
|
Characteristics | NASH (n=40) | AL (n=80) | Non-NASH/non-AL
(n=44) |
|---|
| Age
(years)a | 71.6±8.1 | 68.9±7.3 | 71.3±8.1 |
| Gender
(male/female) | 29/11b | 76/4c | 31/13 |
| Maximum tumor
diameter (cm)a | 5.0±3.1 | 4.3±3.1 | 5.2±4.7 |
| Tumors
(n)a | 2.3±2.1 | 1.7±1.4 | 1.7±1.7 |
| Disease
stagea | 2.6±0.8 | 2.3±0.8 | 2.6±0.8 |
| Albumin
(g/dl)a | 3.9±0.7 | 4.0±0.4 | 4.0±0.4 |
| Total bilirubin
(mg/dl)a | 0.6±0.2 | 0.7±0.4 | 0.8±0.3 |
| AST
(IU/l)a | 42±44 | 34±34 | 51±37 |
| ALT
(IU/l)a | 33±30 | 35±36 | 45±48 |
| White blood cell
count (/µl)a | 5,455±1,568 | 5,910±1,887 | 5,439±1,967 |
| Platelet count
(x104/µl)a | 16.9±7.0 | 21.5±11.0 | 17.6±7.2 |
| Prothrombin
activity (%)a | 84±16 | 90±14 | 87±12 |
| α-fetoprotein
(ng/ml)a | 8,884±44,271 | 65±40,398 | 43,646±222,264 |
| DCP
(mAU/ml)a | 2,373±5,235 | 1,103±87,819 | 17,558±88,206 |
| ICGR15
(%)a | 17.2±13.3 | 13.1±11.5 | 14.7±11.4 |
| Diabetes (+/-) | 15/25 | 41/39 | 21/23 |
| Child-Pugh
Classification (A/B,C) | 35/5 | 74/6 | 42/2 |
| Fibrosis
stagea | 2.3±1.3 | 2.2±1.1 | 2.4±1.3 |
| BMI
(kg/m2) | 23.8±3.6 | 24.6±3.9 | 23.9±4.2 |
Discussion
The incidence of virus-related HCC has recently
exhibited a decreasing trend due to the reduction in hepatitis
virus transmission during blood transfusions and significant
advances in antiviral therapy. However, the incidence of NBNC HCC
has been increasing in Japan (2);
consequently, the rate of resected NBNC HCCs in Meiwa Hospital has
also been increasing. Between 2001 and 2003, the percentage of
cases was 14% (NBNC HCC/virus-related HCC, 23/139); however this
percentage increased to 22% (61/216) between 2010 and 2012,
prompting the investigation of the clinicopathological
characteristics of the NBNC HCC cases surgically treated at Meiwa
Hospital.
The analysis of the characteristics of NBNC HCC
indicated that the mean age, rate of complications associated with
diabetes and BMI were significantly higher in these cases compared
with cases of virus-related HCC, in agreement with previous reports
(5–12). Patients with NBNC HCC exhibited a good
liver function, although a number of patients presented with a
large tumor size (6), possibly as
they were not examined periodically due to the absence of hepatitis
virus infection.
Comparisons according to disease stage revealed no
significant difference in stage I cases between the NBNC, HBV and
HCV groups, possibly due to the small sample size, although
early-stage carcinoma (stages I and II) tended to exhibit a
favorable prognosis. This may be explained by the fact that
repetitive therapy was possible, as these patients exhibited a good
liver function reserve compared with those with virus-related HCC
(13–15). However, the prognosis was not
significantly different between patients with NBNC HCC and those
with virus-related HCC for stage III and IV cases.
The NBNC HCC prognostic factors in the present study
included a high AFP value, poorly differentiated HCC and high
degree of liver fibrosis. Distance from the resection margin, tumor
multiplicity, the presence or absence of diabetes (12,16) and
HBV DNA expression (17–20) are also reported to be factors
associated with NBNC HCC prognosis. Thus, the prognostic factors
for NBNC HCC identified in the present study are similar to those
for virus-related HCC (21,22).
Furthermore, when patient characteristics were
compared, it was revealed that the NASH group included a lower
proportion of male patients compared with the AL and
non-NASH/non-AL groups, and had a similar liver function reserve
compared with the AL and non-NASH/non-AL groups. Clinically,
patients with a history of alcohol abuse may be easily identified;
however, it is more difficult to screen patients with NASH at high
risk of carcinogenesis. In the present study, the mean BMI was 23.8
kg/m2, the platelet count was 16.9×104/µl,
the AST and ALT levels were 42 and 33 IU/l, respectively, and the
rate of diabetes complications was 38% (15/40) in the NASH group.
Collectively, these findings suggest that diagnostic imaging should
be considered in cases of abnormal AST or ALT levels.
The prognosis was not significantly different
between the NASH, AL and non-NASH/non-AL groups. A history of
alcohol abuse has been reported to increase the rate of HCC
recurrence, as it promotes cirrhosis (23). However, in the present study, patients
with a history of alcohol abuse did not have a worse prognosis;
guidance was provided following surgery to help them quit, which
may have improved their prognosis. The present study has helped
elucidate the characteristics of NBNC HCC; however, it is necessary
to establish a screening system for patients at high risk of
hepatic carcinogenesis. A study including a large number of
patients is required to identify high-risk groups and evaluate
their treatment outcomes.
References
|
1
|
Ikeda K, Saitoh S, Koida I, et al: A
multivariate analysis of risk factors for hepatocellular
carcinogenesis: a prospective observation of 795 patients with
viral and alcoholic cirrhosis. Hepatology. 18:47–53. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Umemura T and Kiyosawa K: Epidemiology of
hepatocellular carcinoma in Japan. Hepatol Res. 37 Suppl
2:S95–S100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagaoki Y, Hyogo H, Aikata H, et al:
Recent trend of clinical features in patients with hepatocellular
carcinoma. Hepatol Res. 42:368–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liver Cancer Study Group of Japan, .
General Rules for the Clinical and Pathological Study of Primary
Liver Cancer. 2nd English Edition. Kanehara & Co., Ltd.; Tokyo:
pp. 1102010
|
|
5
|
Abe H, Yoshizawa K, Kitahara T, Aizawa R,
Matsuoka M and Aizawa Y: Etiology of non-B non-C hepatocellular
carcinoma in the eastern district of Tokyo. J Gastroenterol.
43:967–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takamatsu S, Noguchi N, Kudoh A, et al:
Influence of risk factors for metabolic syndrome and non-alcoholic
fatty liver disease on the progression and prognosis of
hepatocellular carcinoma. Hepatogastroenterology. 55:609–614.
2008.PubMed/NCBI
|
|
7
|
Kusakabe A, Tanaka Y, Orito E, et al: A
weak association between occult HBV infection and non-B non-C
hepatocellular carcinoma in Japan. J Gastroenterol. 42:298–305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Honda T, Miyaaki H, Ichikawa T, et al:
Clinical characteristics of hepatocellular carcinoma in elderly
patients. Oncol Lett. 2:851–854. 2011.PubMed/NCBI
|
|
9
|
Li T, Qin LX, Gong X, et al: Hepatitis B
virus surface antigen-negative and hepatitis C virus
antibody-negative hepatocellular carcinoma: clinical
characteristics, outcome and risk factors for early and late
intrahepatic recurrence after resection. Cancer. 119:126–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaneda K, Kubo S, Tanaka H, et al:
Features and outcome after liver resection for non-B non-C
hepatocellular carcinoma. Hepatogastroenterology. 59:1889–1892.
2012.PubMed/NCBI
|
|
11
|
Kim SK, Marusawa H, Eso Y, et al: Clinical
characteristics of non-B non-C hepatocellular carcinoma: a
single-center retrospective study. Digestion. 84 Suppl 1:43–49.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawamura Y, Ikeda K, Arase Y, et al:
Diabetes mellitus worsens the recurrence rate after potentially
curative therapy in patients with hepatocellular carcinoma
associated with nonviral hepatitis. J Gastroenterol Hepatol.
23:1739–1746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamanaka N, Tanaka T, Tanaka W, et al:
Correlation of hepatitis virus serologic status with
clinicopathologic features in patients undergoing hepatectomy for
hepatocellular carcinoma. Cancer. 79:1509–1515. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoi Y, Suzuki S, Baba S, Inaba K, Konno
H and Nakamura S: Clinicopathological features of hepatocellular
carcinomas (HCCs) arising in patients without chronic viral
infection or alcohol abuse: a retrospective study of patients
undergoing hepatic resection. J Gastroenterol. 40:274–282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaibori M, Ishizaki M, Matsui K and Kwon
AH: Clinicopathologic characteristics of patients with non-B non-C
hepatitis virus hepatocellular carcinoma after hepatectomy. Am J
Surg. 204:300–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinkawa H, Uenishi T, Takemura S, et al:
Risk factors for postoperative recurrence of non-B non-C
hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 17:291–295.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishikawa H, Osaki Y, Arimoto A, Kita R
and Kimura T: Relation between antibody to hepatitis B core antigen
and survival after curative therapy for non-B non-C hepatocellular
carcinoma. Anticancer Res. 33:2211–2219. 2013.PubMed/NCBI
|
|
18
|
Shi Y, Wu YH, Wu W, Zhang WJ, Yang J and
Chen Z: Association between occult hepatitis B infection and the
risk of hepatocellular carcinoma: a meta-analysis. Liver Int.
32:231–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ikeda K, Kobayashi M, Someya T, et al:
Occult hepatitis B virus infection increases hepatocellular
carcinogenesis by eight times in patients with non-B, non-C liver
cirrhosis: a cohort study. J Viral Hepat. 16:437–443. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakai T, Shiraishi O, Kawabe T, Ota H,
Nagano H and Shiozaki H: Significance of HBV DNA in the hepatic
parenchyma from patients with non-B, non-C hepatocellular
carcinoma. World J Surg. 30:1338–1343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cescon M, Cucchetti A, Grazi GL, et al:
Role of hepatitis B virus infection in the prognosis after
hepatectomy for hepatocellular carcinoma in patients with
cirrhosis: a Western dual-center experience. Arch Surg.
144:906–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanazaki K, Kajikawa S, Koide N, Adachi W
and Amano J: Prognostic factors after hepatic resection for
hepatocellular carcinoma with hepatitis C viral infection:
univariate and multivariate analysis. Am J Gastroenterol.
96:1243–1250. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamagishi Y, Horie Y, Kajihara M, et al:
Hepatocellular carcinoma in heavy drinkers with negative markers
for viral hepatitis. Hepatol Res. 28:177–183. 2004. View Article : Google Scholar : PubMed/NCBI
|