Introduction
Osteosarcoma (OS) is considered to be one of the
most common and aggressive primary bone tumors of the
musculoskeletal system, and predominantly occurs in childhood and
adolescence (1,2). There are various potential treatments
for OS, including radiotherapy, surgery and chemotherapy; however,
the results of these available treatments remain unsatisfactory
(3). Significant nephrotoxic and
cardiotoxic side-effects are induced by chemopreventative
medicines, thereby limiting the efficacy of their use in the
treatment of OS (4). Manipulation of
apoptosis is one of the major targets in the treatment of cancer.
Apoptosis describes genetically-dependent programmed cell death
type I, and is characterized by cell shrinkage, signal transduction
(5), nucleic condensation (6,7) and DNA
and cellular protein degradation (8).
Previous studies have revealed that few therapeutic treatments
exist that result in an enhanced ability of human tumor cell lines
to undergo apoptosis (9,10). Therefore, the development of novel
agents to induce or increase the phenomenon of apoptosis present a
promising approach for the development of cancer treatments. Novel
inducers of apoptosis may provide alternative and efficacious
therapeutic anticancer strategies.
Flavonoids are associated with multiple biological
effects, including antitumor, anti-oxidation, anti-inflammation,
antiviral and hepatoprotective activities, as well as in the
prevention of cardiovascular diseases (11–16).
Eupatilin (5,7-dihydroxy-3′,4′,6-trimethoxyflavone) is extracted
from Artemisia asiatica (A. asiatica) Nakai, and this
isolated flavonoid contains pharmacologically active ingredients.
Eupatilin has been demonstrated to exert anticancer, anti-oxidative
and anti-inflammatory effects (17).
A previous report indicated that Stillen™ (DA-9601), produced from
the ethanol extract of A. asiatica, contained the
pharmacologically active flavonoid compound eupatilin (17). DA-9601 demonstrated cytoprotective
effects against gastric mucosal damage and ulcerative proctitis.
Eupatilin has exhibited positive effects in the treatment of
oxidant-dependent gastric disorders (18). Eupatilin, apigenin, wogonin and
baicalein are all members of the same family of flavonoids.
Although the flavones, apigenin, wogonin and baicalein, have
previously been used in the treatment of OS (19–21), the
molecular mechanisms underlying eupatilin-mediated apoptosis of the
U-2 OS cell line have remained to be elucidated. Therefore, the
present study aimed to aid the elucidation of the underlying
mechanism involved in eupatilin-induced apoptosis of U-2 OS cells.
This was achieved via cytotoxicity experiments, apoptosis studies
and the analysis of changes in protein expression associated with
apoptotic cell death.
Materials and methods
Reagents
Eupatilin was provided by Dong-A Pharmaceutical Co.
Ltd (Cheoin-gu, South Korea). Antibodies against PARP, Bax, Bcl-2
and cytochrome c were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Caspase inhibitors
Z-DEVE-FMK, Z-IETD-FMK and Z-LEHD-FMK for caspase-3, caspase-8 and
caspase-9 were obtained from R&D Systems (Minneapolis, MP,
USA). These inhibitors were dissolved in dimethyl sulfoxide (DMSO;
Xi'an Chemical Co., Ltd., Boaji, China) and diluted prior to use in
cell culture. The present study was approved by the ethics
committee board of Mudanjiang Medical University (Mudanjiang,
China). The remaining reagents and solvents used were of analytical
grade and purchased from Xi'an Chemical Co., Ltd.
Cells and culture
The U-2 OS human OS cells were purchased from the
Cell Bank of Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The cells were
maintained in RPMI-1640 medium supplemented with fetal calf serum
(FCS; 10%), glutamine (2 mM), penicillin (100 U/ml) and
streptomycin (10 µg/ml) in a humidified atmosphere at 37°C with 5%
CO2.
MTT assay
The viability of U-2 OS cells was determined using
an MTT assay. U-2 OS cells were seeded at a density of
5×104 cells/well in 96-well culture plates. Each well
contained 100 µl medium supplemented with 10% FCS, and the cells
were incubated at 37°C for 24 h prior to treatment with various
concentrations of eupatilin. Following 24 h of incubation, the
medium was removed and replaced with 10% FCS containing various
concentrations of eupatilin (0, 50, 100, 200 and 400 µg/ml) and
incubated at 37°C for 96 h. Subsequently, 50 µl MTT solution was
added to each well and incubated for a further 4 h as described
previously (22). The formazan
crystals obtained were dissolved in 100 µl DMSO and the absorbance
was recorded using an ELISA microplate reader (model 550; Bio-Rad,
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 570
nm.
Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) double staining
Cell apoptosis was detected using Annexin V-FITC/PI
double staining. In brief, 1×105 untreated (control) or
treated cells were harvested following trypsinization and
centrifugation (500 × g for 5 min). The cells were then washed
twice with ice-cold phosphate-buffered saline (PBS) and stained
with an Annexin V-FITC/PI apoptosis kit (BD Pharmingen, San Diego,
CA, USA), according to the manufacturer's instructions.
Subsequently, the samples were analyzed using the FACS Calibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Double-staining was used for the quantification of the apoptosis of
U-2 OS cells following treatment with eupatilin, by measurement of
phosphatidylserine expression on the outer surface of the plasma
membrane identified by Annexin V-FITC binding. The results of the
assay were analyzed with the exclusion of PI, the plasma membrane
integrity probe (23).
Flow cytometry and cell cycle
analysis
Eupatilin-treated cells were harvested using 0.25%
trypsin and then washed with PBS twice prior to fixation with 70%
ethanol for ~30 min at 4°C. The cells were pelleted by centrifuging
at 500 × g for 5 min and resuspended in 1 ml PBS containing 100 µl
RNase (10 mg/ml) and 100 µl PI (0.5 mg/ml) for ~20 min at 37°C for
cytoplasmic or nuclear DNA staining. The stained cells were then
analyzed for DNA content using the FACS Calibur as previously
described (24). Annexin V- and
PI-positive cells were considered to be necrotic type cells,
whereas Annexin-V positive and PI-negative cells were considered to
be apoptotic cells.
Mitochondrial membrane potential (ΔΨm)
determination
The ΔΨm of the U-2 OS cells was analyzed by flow
cytometry, using rhodamine 123 (Rh123), a fluorescent dye which has
been shown to be selectively accumulated within the mitochondria of
live cells (25). In brief, the
eupatilin-treated and untreated cells were incubated with Rh123 dye
(1 mg/ml in DMSO) for 30 min at 37°C in 5% CO2.
Subsequently, the cells were washed twice with PBS, resuspended in
PBS, stained with 2 µg/ml PI and immediately subjected to flow
cytometric analysis. The loss of ΔΨm was calculated as a percentage
using CellQuest™ software (version 5.1; BD Biosciences).
Caspase-3, -8 and -9 activities
The activities of caspase-3, -8 and -9 were
evaluated using a caspase colorimetric assay kit (BioTek
Instruments, Inc., Winooski, VT, USA) according to the
manufacturer's instructions. U-2 OS cells were seeded in 12-well
culture plates at a density of 2×105 cells/well prior to
incubation with eupatilin for 48 h. The cells were then harvested,
lysed with lysis buffer for ~5 min in an ice bath and then
centrifuged at 10,000 × g for 10 min. Subsequently, the reaction
buffer was added to the supernatant solutions containing the
proteins (100 µg). Caspase-3, -8 and -9 colorimetric substrates (5
µl each) were then added for 2 h at 37°C in a CO2
incubator, prior to quantification of the optical density (OD) of
the mixture at 405 nm using a spectrophotometer. Their respective
activities were expressed relative to the theoretical OD value,
calculated using the Sigma-Aldrich Caspase 9 Assay Kit,
Colorimetric Technical Bulletin (www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Bulletin/casp8cbul.pdf).
Western blot analysis
Cells were subjected to eupatilin treatment, and
cell proteins were subsequently obtained by incubation for 1 h in
200 µl lysis buffer containing NaCl (300 mM), Tris HCl (50 mM; pH
7.6), Triton X-100 (0.5%), phenylmethanesulfonyl fluoride (2 mM),
aprotinin (2 µl/ml) and leupeptin (2 µl/ml) at 4°C. A bicinchoninic
acid protein assay kit (Pierce Biotechnology, Inc.; Thermo Fisher
Scientific, Rockford, IL, USA) was used to quantify the protein
expression levels, according to the manufacturer's instructions.
Equal quantities of each protein (20 µg) were separated by 12%
SDS-PAGE and then electrotransferred onto polyvinylidene difluoride
membranes. The membranes were then incubated for 1 h with PBS
containing 5% non-fat milk as a blocking solution at room
temperature, prior to incubation with polyclonal rabbit anti-mouse
poly(ADP-ribose)polymerase (PARP; 1:1,000 dilution; cat no. 9542),
B cell lymphoma-2 (1:1,000 dilution; cat no. 2876), Bcl-2-like
protein 4 (Bax; 1:1,000 dilution; cat no. 2772) and cytochrome
c (1:1,000 dilution; cat no. 4272) antibodies (all from Cell
Signaling Technology, Inc., Danvers, MA, USA) diluted with blocking
solution at 4°C overnight. The membranes were subsequently
incubated with horseradish peroxidase-conjugated rabbit anti-mouse
secondary antibody (1:2,000–5,000) for ~2 h at room temperature.
The developed immunoblots were then visualized with an enhanced
chemiluminescence detecting system (GE Healthcare Life Sciences,
Chalfont, UK). Expression was analyzed relatively, based on the
ratio between the target protein and that of polyclonal rabbit
β-actin (1:1,000 dilution; cat no. 4967; Cell Signaling Technology,
Inc.).
Statistical analysis
All the quantitative results obtained are presented
as the mean ± standard deviation. Student's t-test was used to
calculate statistical differences between the control and
eupatilin-treated cells, using GraphPad Prism 3.03 software
(GraphPad Software, Inc., La Jolla, CA, USA) and P<0.05 was
considered to indicate a statistically significant difference. All
the studies were performed at least in triplicate.
Results
Eupatilin inhibits growth of U-2 OS
cells
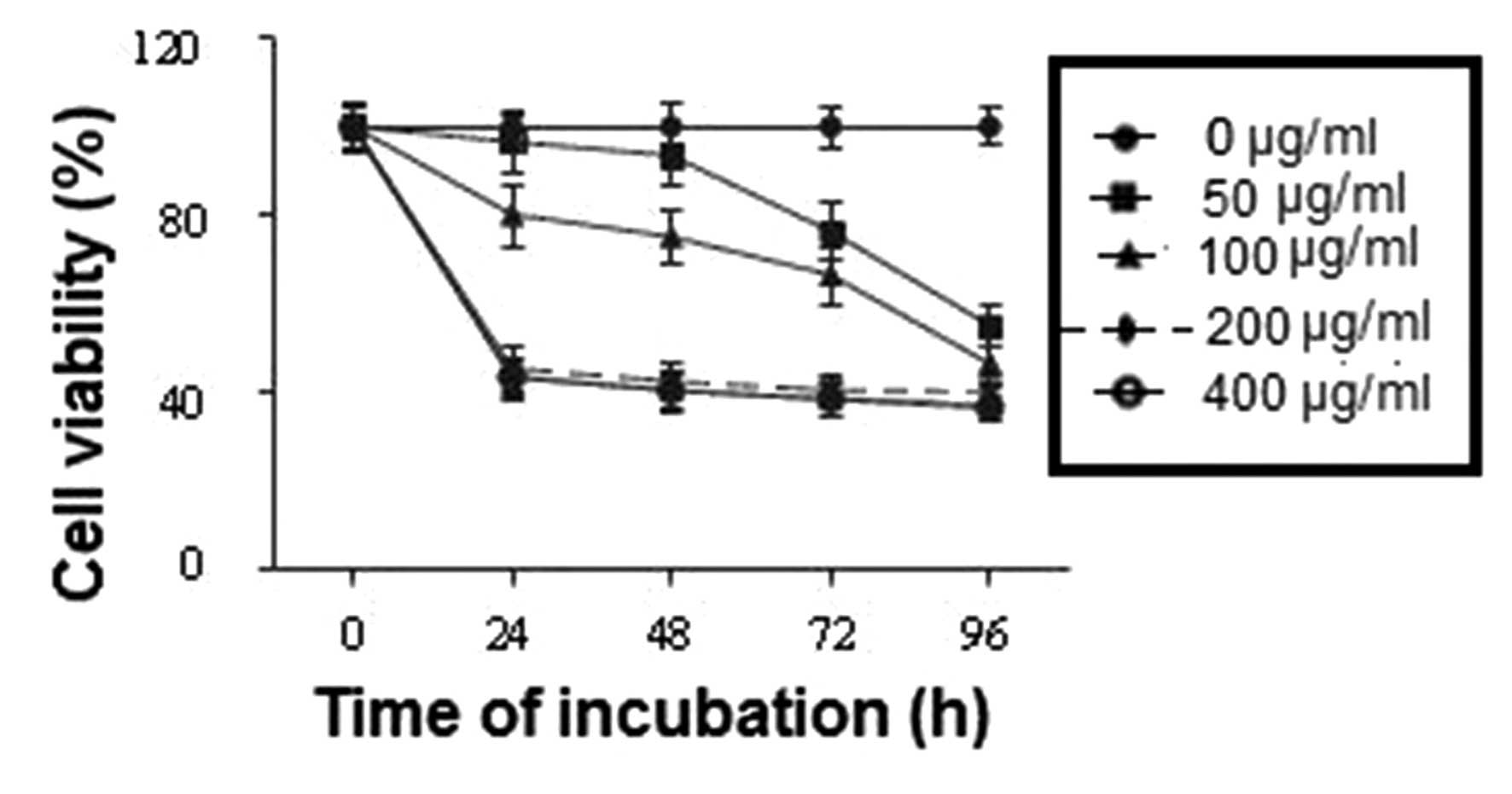
The effects of eupatilin on U-2 OS cell
proliferation were evaluated by MTT assay. The cells were treated
with various concentrations of eupatilin (0, 50, 100, 200 and 400
µg/ml) for various time-periods (0, 24, 48, 72 and 96 h) and cell
proliferation under these conditions was evaluated. The results
revealed a dose-dependent inhibitory effect of eupatilin on U-2 OS
cell proliferation 24 h post-treatment (Fig. 1). The inhibitory effect of eupatilin
was demonstrated to be significant at a concentration of 100 µg/ml,
whereas the most marked inhibition was observed in cells which were
treated with eupatilin at concentrations of 200 and 400 µg/ml.
These results confirmed the antiproliferative effect
of eupatilin on U-2 OS cells, and in order to elucidate the
mechanism underlying this effect, eupatilin at a concentration of
100 µg/ml was selected for use in the subsequent experiments.
Eupatilin alters the cell cycle
distribution and induces apoptosis of U-2 OS cells
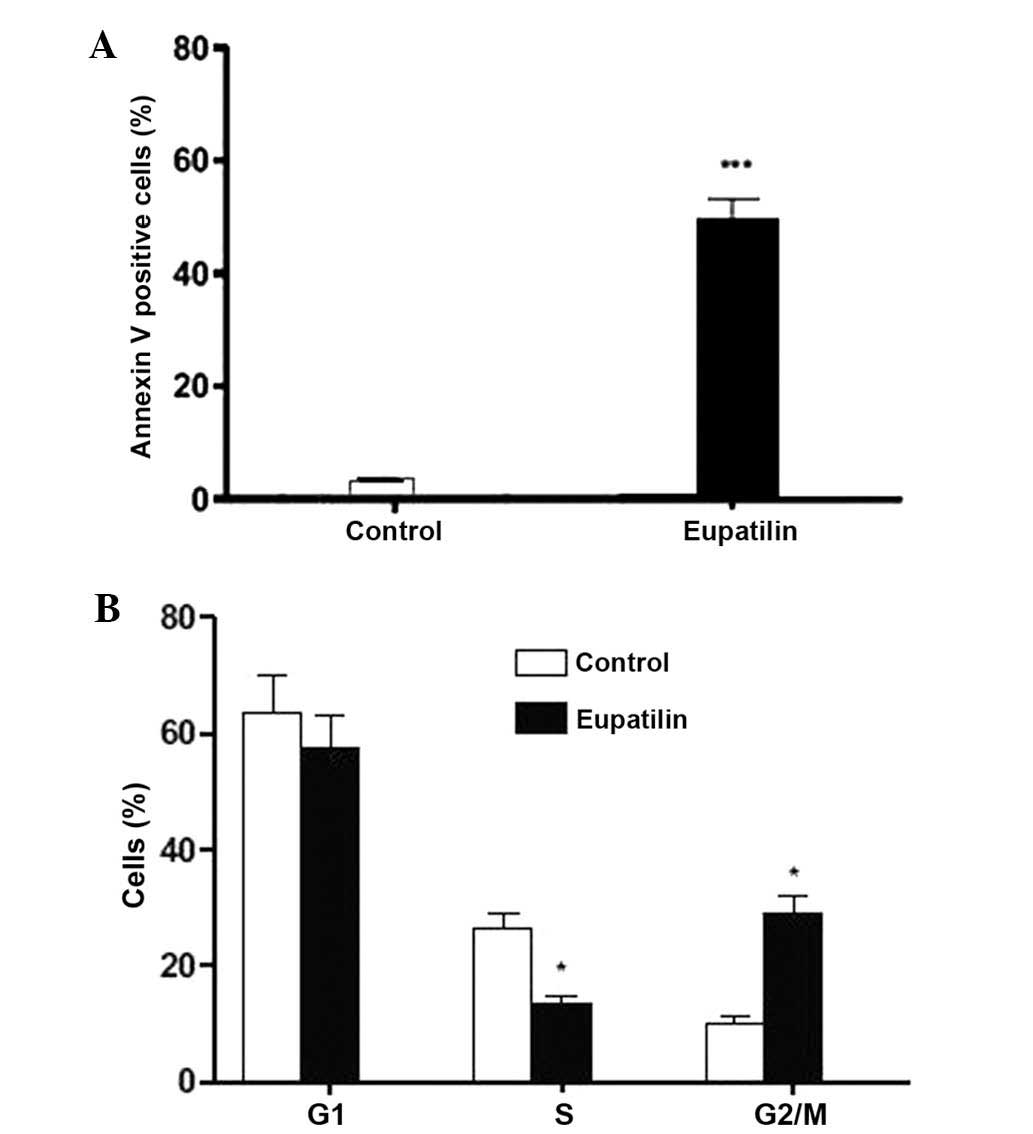
To ascertain whether eupatilin inhibited U-2 OS cell
proliferation via induction of apoptosis, the cells were treated
with eupatilin and then subjected to flow cytometric analysis. The
results revealed a significant increase in Annexin V-FITC cell
binding following 100 µg/ml eupatilin treatment, compared with that
of the control cells, indicating the initiation of apoptosis.
Fig. 2A indicates that the apoptotic
cell proportion was significantly increased from 3.23% in untreated
U-2 OS cells to 49.75% in eupatilin-treated U-2 OS cells. These
results confirmed that the cell death observed in U-2 OS cells
following eupatilin treatment occurred via the induction of
apoptosis. In addition, flow cytometric analysis revealed that
eupatilin treatment for 24 h significantly reduced the proportion
of cells in S phase, compared with that of the control cells
(Fig. 2B). Furthermore, elevated
accumulation of cells in the G2/M apoptotic phase was observed,
with respect to control cells, while the cell population in G1
phase demonstrated a slight decrease. These results revealed that
the eupatilin responses of U-2 OS cells are correlated with
apoptotic cell death.
Eupatilin induces mitochondrial
dysfunction and cytochrome c release
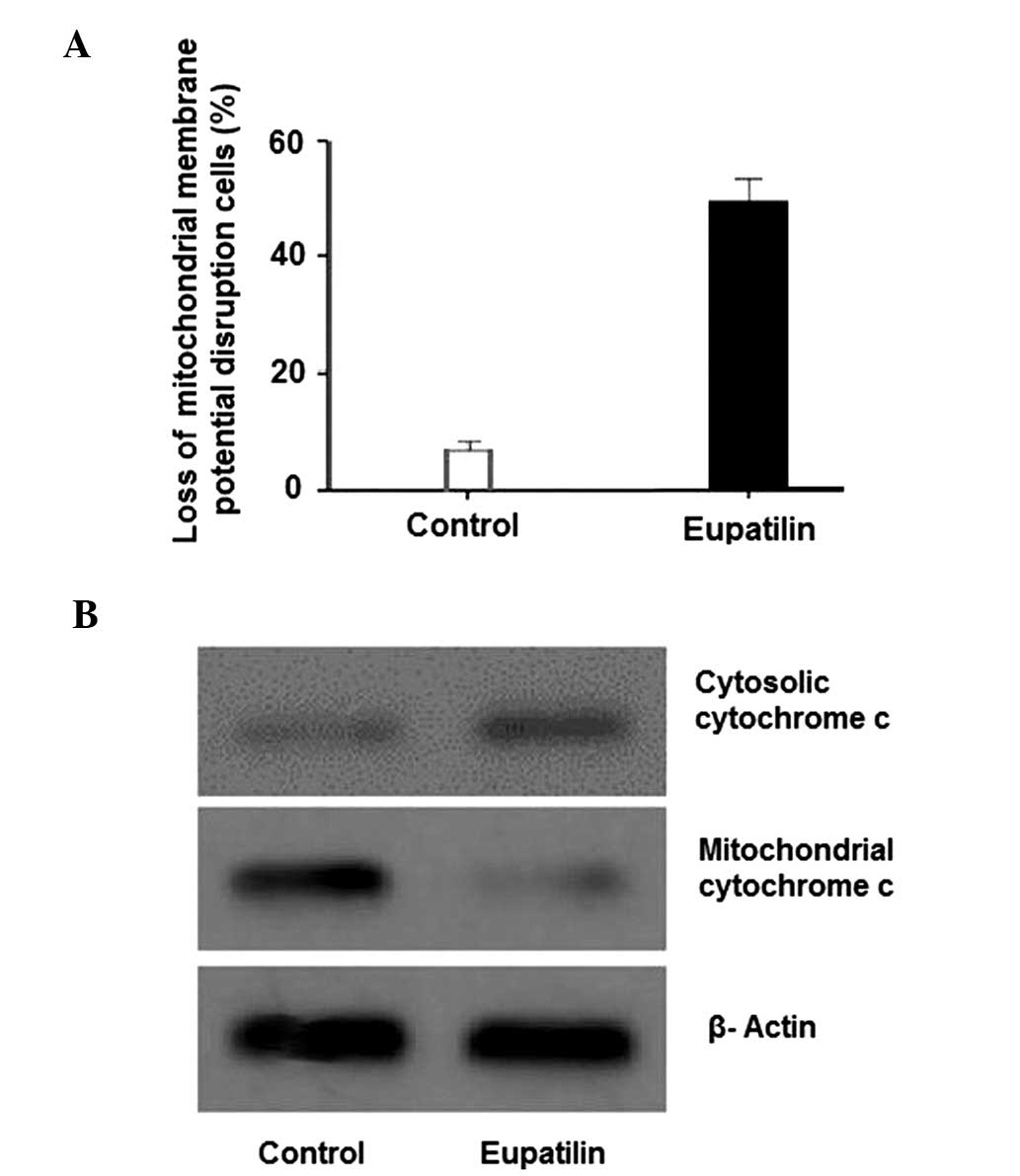
In order to confirm whether mitochondrial
dysfunction was involved in eupatilin-induced apoptosis, changes in
ΔΨm were evaluated using Rh123 fluorescent dye. Rh123 was used as
it is able to cross the mitochondrial membrane and thus accumulates
in the mitochondrial matrix. However, this accumulation only occurs
when the transmembrane potential is maintained (26). As indicated in Fig. 3A, following exposure of U-2 OS cells
to 100 µg/ml eupatilin for 24 h, a significant reduction in ΔΨm was
observed. The release of cytochrome c from the mitochondria
to the cytosol is typically associated with ΔΨm depolarization
(27). In addition, cytochrome
c has been demonstrated to have a vital role in apoptosis
(28,29). Therefore, the expression of cytochrome
c was analyzed by western blotting. Fig. 3B indicates an increase in cytosolic
cytochrome c and a decrease in mitochondrial cytochrome
c in eupatilin-treated cells, when compared with control
cells. These results indicated that eupatilin-induced apoptosis
involves mitochondrial dysfunction, associated with a loss of ΔΨm
and cytosolic cytochrome c release.
Eupatilin induces caspase activation
and PARP cleavage in U-2 OS cells
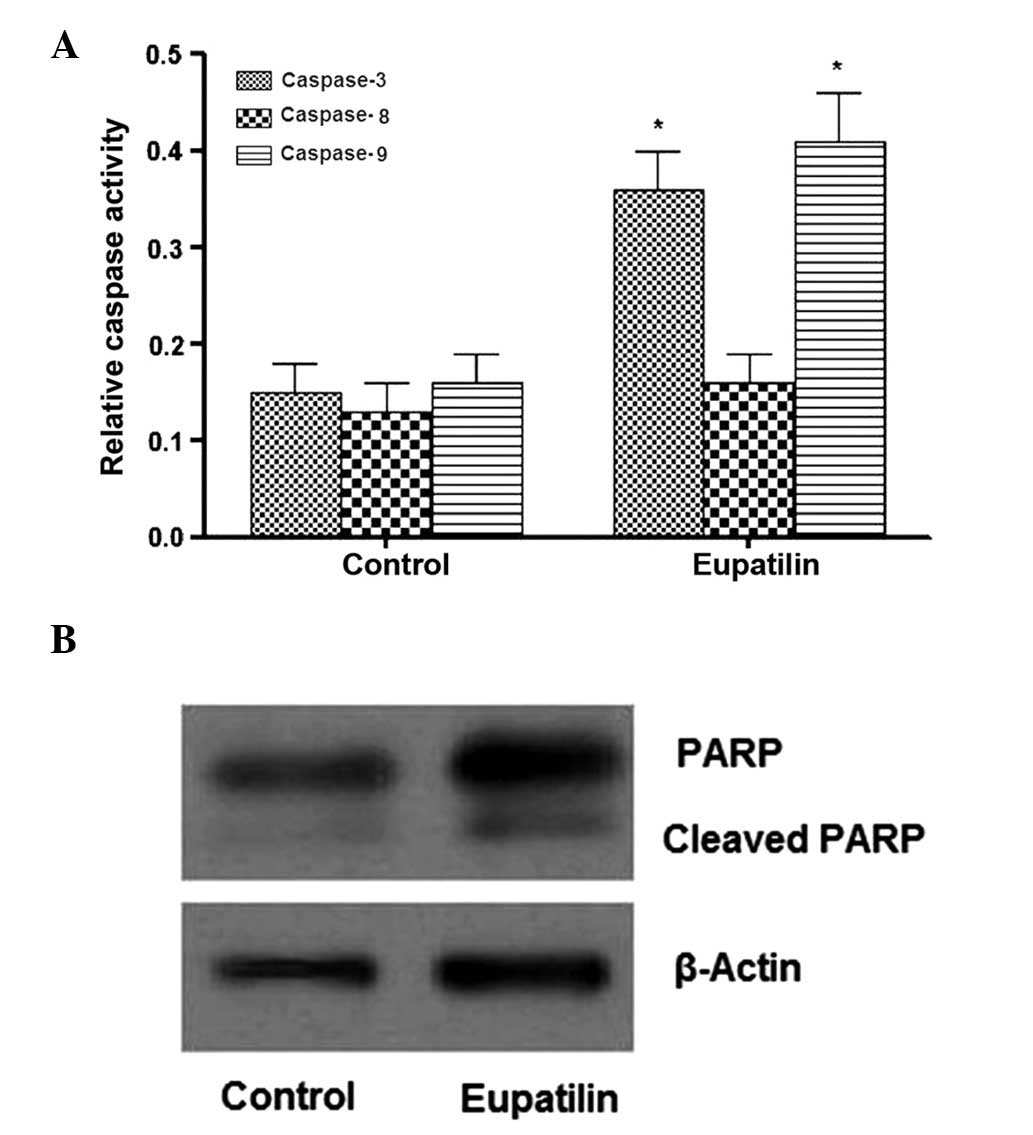
Subsequently, the effects of eupatilin on caspase-3,
-8 and -9 activation and PARP cleavage were evaluated, in order to
determine the death receptor involvement and mitochondrial pathways
in eupatilin-induced apoptosis. The results revealed that eupatilin
treatment of U-2 OS cells for 24 h induced activation of caspase-3
and -9, but not caspase-8 (Fig. 4A).
Furthermore, as indicated in Fig. 4B,
an increase in PARP cleavage was detected following eupatilin
treatment. To further investigate the role of caspase activation in
eupatilin-induced apoptosis, the effects of caspase inhibitors
z-DEVE-FMK, Z-IETD-FMK, and Z-LEHD-FMK for caspase-3, -8 and -9 on
apoptosis in U-2 OS cells were evaluated. Significant inhibition of
eupatilin-induced apoptosis was observed following pretreatment
with caspase-3 and -9 inhibitors, whereas the levels of early
apoptosis remained unchanged with respect to caspase-8 inhibition
(data not shown). These observations suggested that eupatilin
induced caspase-dependent apoptosis in U-2 OS cells via a
mitochondrial-dependent pathway.
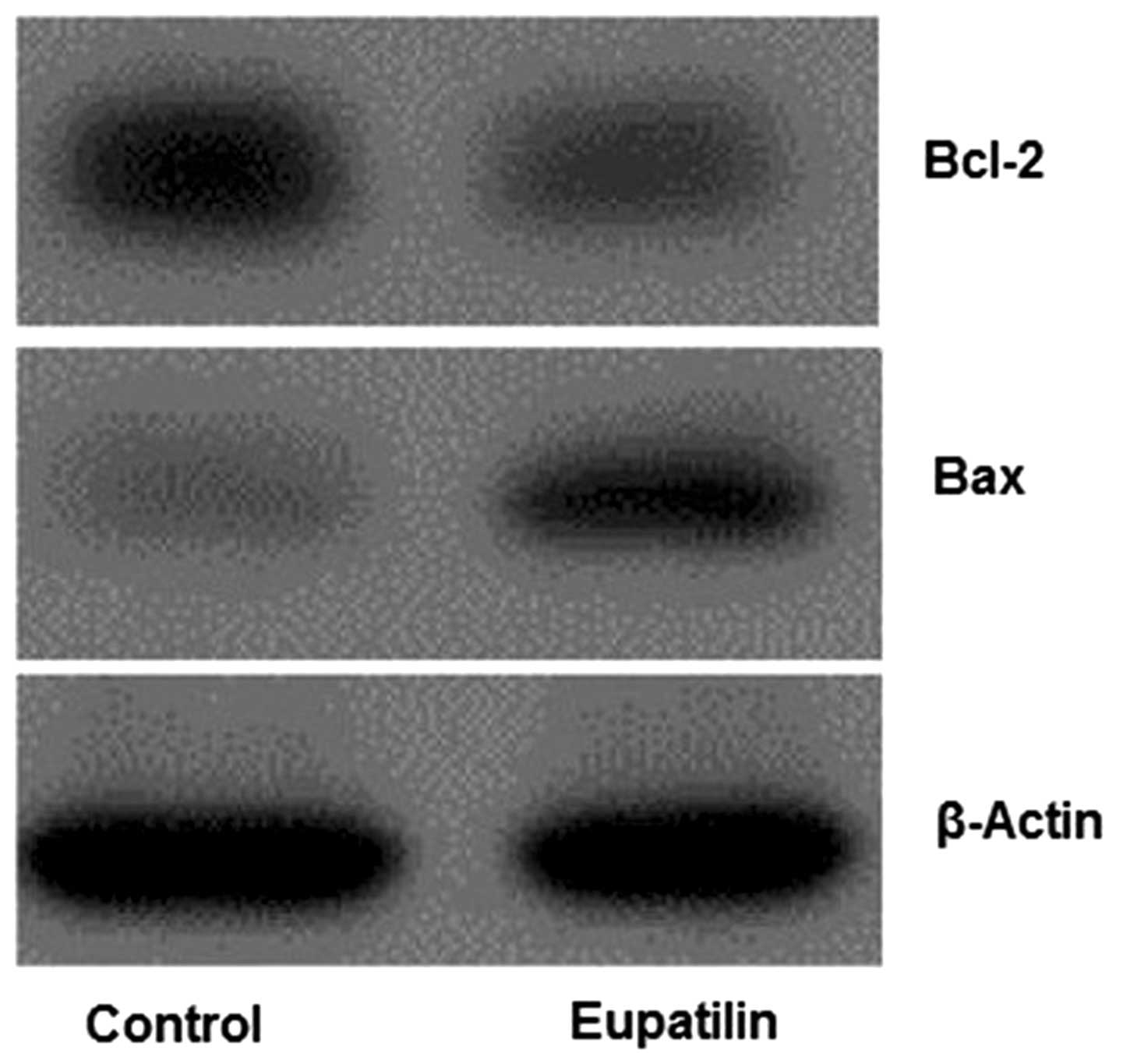
Eupatilin upregulates Bax and
downregulates Bcl-2 in U-2 OS cells
The effects of eupatilin on Bax and Bcl-2 expression
in U-2 OS cells were subsequently examined via analysis of Bax and
Bcl-2 protein expression levels following 24 h of treatment.
Western blot analysis indicated a marked increase in Bax expression
levels in eupatilin-treated cells, whereas a significant decrease
was observed in Bcl-2 expression (Fig.
5). This high ratio of Bax/Bcl-2 may contribute to the
induction of apoptosis by eupatilin via the mitochondrial-dependent
pathway.
Discussion
Apoptosis is a genetically mediated mechanism of
type 1 programmed cell death. Shrinkage of cells, plasma membrane
blebbing and chromatin condensation associated with DNA cleavage
into ladders comprise the major characteristics of apoptosis
(30,31).
Accumulating evidence has indicated that the
antitumor effects of a wide variety of compounds and herbal
medicines obtained from natural products, which exhibit anticancer
effects, are able to induce apoptosis in numerous human tumor cell
lines (32,33). Previous studies have indicated a
cytoprotective effect of A. asiatica ethanol extract against
gastric mucosa damage and ulcerative colitis. A. asiatica is
also known to be effective in the treatment of oxidant-dependent
gastric disease (18,34). In the present study, the anticancer
efficacy of eupatilin and its underlying mechanism in human
osteosarcoma U-2 OS cells, in vitro, was evaluated. The MTT
assay results demonstrated that eupatilin effectively suppressed
the proliferation of U-2 OS cells in a concentration- and
time-dependent response. The results of FACS analysis indicated
that eupatilin induced apoptosis in U-2 OS cells and also increased
the proportion of cells at G2/M phase. These results demonstrated
that the eupatilin was able to potently trigger apoptosis in U-2 OS
cells. Subsequently, the present study aimed to identify the
apoptotic mechanism the effects of eupatilin in U-2 OS cells.
Mitochondrial integrity disruption is one of the most common and
earliest intracellular events to occur following apoptosis
initiation (35). Increasing evidence
suggests that this mitochondrial dysfunction may activate
particular cell signaling pathways, resulting in the induction of
apoptosis, as well as the reduction in ΔΨm associated with
mitochondrial dysfunction. For this reason, the loss of ΔΨm is
significant during mitochondrial-dependent apoptosis (36–38), as,
in turn, it induces the efflux of cytochrome c into the
cytosol from mitochondria. Following release into the cytosol,
cytochrome c is able to initiate caspase activation, which
aids termination of the cells by apoptosis. The results of the
present study indicated that eupatilin exposure induced ΔΨm loss
and an increase in cytochrome c release to the cytosol from
the mitochondria in U-2OS cells, which indicated that
eupatilin-induced cell death potentially occurred via the
mitochondrial apoptotic pathway.
Previous studies have indicated that the
mitochondria-mediated pathway for apoptosis is regulated by
proteins of the Bcl-2 family (39,40). It
was suggested that the balance between Bax and Bcl-2 is significant
in conferring cell susceptibility to apoptosis (41). In order to further elucidate the
mechanisms underlying the anticancer effects of eupatilin, the
expression levels of two major apoptotic signaling proteins, Bax
and Bcl-2, were evaluated. An increase in Bax:Bcl-2 ratio was
observed in the eupatilin-treated cells, suggesting that
eupatilin-induced apoptosis was associated with alterations in Bax
and Bcl-2 expression. Apoptosis is controlled by cell suicide
mechanisms induced by specific external and internal signals.
Currently, two major pathways associated with the induction of
apoptosis are known: The mitochondrial signaling pathway and the
cell-suicide receptor pathway, controlled by caspase-9 and -8,
respectively (42). Accumulating
evidence has revealed the essential roles of caspase action in the
apoptotic cascade. In the mitochondrial pathway (the intrinsic
pathway), cytochrome c is released from the mitochondria to
the cytosol, and is then able to bind with Apaf-1 and activate
caspase-9. Activated caspase-9 subsequently activates the
downstream caspases, caspase-3 and/or -7, which in turn aids the
cleavage or degradation of various cellular substances, including
PARP, inducing apoptosis (43–48). In
the cell suicide pathway (the extrinsic pathway), the death
receptors that are present on the cell surface (Fas/FasL) are
activated, triggering caspase-8 activation (49,50). To
elucidate which of these signaling pathways was involved in
eupatilin-induced apoptosis, the apoptosis-associated protein
expression of casapases-3, -8, -9 and PARP were investigated in U-2
OS cells. The results indicated that apoptosis was induced by
caspase-3 and -9 activation, but not caspase-8 activation.
Furthermore, the identification of PARP cleavage confirmed the
participation of caspase-3 in the induction of apoptosis in the
eupatilin-treated cells. In addition, apoptosis was significantly
attenuated in the presence of Z-DEVE-FMK and Z-LEHD-FMK inhibitors
of caspase-3 and -9, respectively. By contrast, the number of
eupatilin-induced early apoptotic U-2 OS cells remained unchanged
with respect to caspase-8 inhibitor response. These results
revealed that eupatilin-induced apoptosis in U-2 OS cells occurred
via the intrinsic pathway, associated with caspase-3 and -9
activation and PARP cleavage.
In conclusion, the results of the present study
indicated that eupatilin perturbed U-2 OS cell growth in a
dose-dependent fashion. The decrease in cell viability occurred as
a result of G2/M phase cell cycle arrest and the induction of
apoptosis, hallmarks of the intrinsic apoptotic pathway in U-2 OS
cells. Furthermore, eupatilin triggered apoptosis via the
mitochondria-mediated pathway, which involved the inhibition of
Bcl-2 expression and the induction of Bax expression for the
degradation of the outer mitochondrial membrane and release of
cytochrome c. Eupatilin also induced caspase-3 and -9
activation, but not caspase-8 activation. Finally, eupatilin
induced PARP cleavage, which is the substrate for caspase-3
activation following eupatilin treatment. In vivo studies of
eupatilin effects on U-2 OS xenografts in nude tumor mice are
currently underway. The results of the present study aid the
elucidation of the molecular mechanisms involved in the antitumor
effects of eupatilin in OS, and confirmed that eupatilin may be
effective as a drug for use in the treatment of OS.
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou AJ and Gorlick R: Chemotherapy
resistance in osteosarcoma: current challenges and future
directions. Expert Rev Anticancer Ther. 6:1075–1085. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson PM, Tomaras M and McConnell K:
Mifamurtide in osteosarcoma - a practical review. Drugs Today
(Barc). 46:327–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D'Adamo DR: Appraising the current role of
chemotherapy for the treatment of sarcoma. Semin Oncol. 38 (Suppl
3):S19–S29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orlov SN, Dam TV, Tremblay J and Hamet P:
Apoptosis in vascular smooth muscle cells: Role of cell shrinkage.
Biochem Biophys Res Commun. 221:708–715. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shimizu T, Maeno E and Okada Y:
Prerequisite role of persistent cell shrinkage in apoptosis of
human epithelial cells. Sheng Li Xue Bao. 59:512–516.
2007.PubMed/NCBI
|
|
8
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barry MA, Behnke CA and Eastman A:
Activation of programmed cell death (apoptosis) by cisplatin, other
anticancer drugs, toxins and hyperthermia. Biochem Pharmacol.
40:2353–2362. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoffman B and Liebermann DA: Molecular
controls of apoptosis: Differentiation/growth arrest primary
response genes, proto-oncogenes and tumor suppressor genes as
positive and negative modulators. Oncogene. 9:1807–1812.
1994.PubMed/NCBI
|
|
11
|
Guo Q, Zhao L, You Q, Yang Y, et al:
Anti-hepatitis B virus activity of wogonin in vitro and
in vivo. Antiviral Res. 74:16–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cárdenas M, Marder M, Blank VC and Roguin
LP: Antitumor activity of some natural flavonoids and synthetic
derivatives on various human and murine cancer cell lines. Bioorg
Med Chem. 14:2966–2971. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burda S and Oleszek W: Antioxidant and
antiradical activities of flavonoids. J Agric Food Chem.
49:2774–2779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
González-Gallego J, Sánchez-Campos S and
Tuñón MJ: Anti-inflammatory properties of dietary flavonoids. Nutr
Hosp. 22:287–293. 2007.PubMed/NCBI
|
|
15
|
Yao P, Nussler A, Liu L, Hao L, et al:
Quercetin protects human hepatocytes from ethanol-derived oxidative
stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J
Hepatol. 47:253–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tijburg LB, Mattern T, Folts JD,
Weisgerber UM and Katan MB: Tea flavonoids and cardiovascular
disease: A review. Crit Rev Food Sci Nutr. 37:771–785. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo HJ and Surh YJ: Eupatilin: A
pharmacologically active flavone derived from Artemisia
plants, induces apoptosis in human promyelocytic leukemia cells.
Mutat Res. 496:191–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huh K, Kwon TH, Shin US, Kim WB, et al:
Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic
lesions and gastric xanthine oxidase activity in rats. J
Ethnopharmacol. 88:269–273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CC, Chuang YJ, Yu CC, Yang JS, Lu CC,
Chiang JH, Lin JP, Tang NY, Huang AC and Chung JG: Apigenin induces
apoptosis through mitochondrial dysfunction in U-2 OS human
osteosarcoma cells and inhibits osteosarcoma xenograft tumor growth
in vivo. J Agric Food Chem. 60:11395–11402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CC, Kuo CL, Lee MH, et al: Wogonin
triggers apoptosis in human osteosarcoma U-2 OS cells through the
endoplasmic reticulum stress, mitochondrial dysfunction and
caspase-3-dependent signaling pathways. Int J Oncol. 39:217–224.
2011.PubMed/NCBI
|
|
21
|
Ding L, He S and Sun X: HSP70 desensitizes
osteosarcoma cells to baicalein and protects cells from undergoing
apoptosis. Apoptosis. 19:1269–1280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moalic S, Liagre B, Labrousse F and
Beneytout JL: Enhanced apoptosis in retrovirally transfected
osteosarcoma cells after exposure to sodium butyrate. Int J Oncol.
16:695–700. 2000.PubMed/NCBI
|
|
23
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: A
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moalic S, Liagre B, Le Bail JC and
Beneytout JL: Dose-dependent modulation of apoptosis and
cyclooxygenase-2 expression in human 1547 osteosarcoma cells by
NS-398, a selective cyclooxygenase-2 inhibitor. Int J Oncol.
18:533–540. 2001.PubMed/NCBI
|
|
25
|
Cao J, Liu Y, Jia L, Zhou HM, Kong Y, Yang
G, et al: Curcumin induces apoptosis through mitochondrial
hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free
Radic Biol Med. 43:968–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnson LV, Walsh ML, Bockus BJ and Chen
LB: Monitoring of relative mitochondrial membrane potential in
living cells by fluorescence microscopy. J Cell Biol. 88:526–535.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
VonAhsen O, Waterhouse NJ, Kuwana T,
Newmeyer DD and Green DR: The harmless release of cytochrome
c. Cell Death Differ. 7:1192–1199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Skulachev VP: Cytochrome c in the
apoptotic and antioxidant cascades. FEBS Lett. 423:275–280. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Ko LJ, Jayaraman L and Prives C:
p53 levels, functional domains and DNA damage determine the extent
of the apoptotic response of tumor cells. Genes Dev. 10:2438–2451.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Z, Chen J, Ford BN, Brackley ME and
Glickman BW: Human DNA repair systems: An overview. Environ Mol
Mutagen. 33:3–20. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huh JE, Lee EO, Kim MS, Kang KS, Kim CH,
Cha BC, et al: Penta-O-galloyl-beta-D-glucose suppresses tumor
growth via inhibition of angiogenesis and stimulation of apoptosis:
Roles of cyclooxygenase-2 and mitogen-activated protein kinase
pathways. Carcinogenesis. 26:1436–1445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taraphdar AK, Roy M and Bhattacharya RK:
Natural products as inducers of apoptosis: Implication for cancer
therapy and prevention. Curr Sci. 80:1387–1396. 2001.
|
|
34
|
Ahn BO, Ko KH, Oh TY, Cho H, Kim WB, et
al: Efficacy of use of colonoscopy in dextran sulfate sodium
induced ulcerative colitis in rats: The evaluation of the effects
of antioxidant by colonoscopy. Int J Colorectal Dis. 16:174–181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dikmen M, Ozturk N and Ozturk Y: The
antioxidant potency of Punica granatum L. fruit peel reduces
cell proliferation and induces apoptosis on breast cancer. J Med
Food. 14:1638–1646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi F, Li A, Zhao L, Xu H, Inagaki Y, Wang
D, et al: Cinobufacini, an aqueous extract from Bufo bufo
gargarizans Cantor, induces apoptosis through a
mitochondria-mediated pathway in human hepatocellular carcinoma
cells. J Ethnopharmacol. 128:654–661. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han J, Goldstein LA, Gastman BR and
Rabinowich H: Interrelated roles for Mcl-1 and BIM in regulation of
TRAIL-mediated mitochondrial apoptosis. J Biol Chem.
281:10153–10163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu YR, Yi ZJ, Yan YR and Qiu ZY:
Hydroxycamptothecin-induced apoptosis in hepatoma SMMC-7721 cells
and the role of mitochondrial pathway. Mitochondrion. 6:211–217.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Green DR and Evan Gl: A matter of life and
death. Cancer Cell. 1:19–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Walesnky LD: BCL-2 in the crosshairs:
Tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hui KK, Kanungo AK, Elia AJ and Henderson
JT: Caspase-3 deficiency reveals a physiologic role for Smac/DIABLO
in regulating programmed cell death. Cell Death Differ.
18:1780–1790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang HY and Yang X: Proteases for cell
suicide: Functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stennicke HR and Salvesen GS: Properties
of the caspases. Biochim Biophys Acta. 1387:17–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cain K, Brown DG, Langlais C and Cohen GM:
Caspase activation involves the formation of the aposome, a large
(approximately 700 kDa) caspase-activating complex. J Biol Chem.
274:22686–22692. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun XM, MacFarlane M, Zhuang J, Wolf BB,
Green DR and Cohen GM: Distinct caspase cascades are initiated in
receptor-mediated and chemical-induced apoptosis. J Biol Chem.
274:5053–5060. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang XL, Yang XY, Jung HJ, Kim SY, Jung
SY, Choi DY, et al: Asiatic acid induces colon cancer cell growth
inhibition and apoptosis through mitochondrial death cascade. Biol
Pharm Bull. 32:1399–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zou H, Li Y, Liu X and Wang X: An APAF-1
cytochrome c multimeric complex is a functional apoptosome
that activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Debatin KM and Krammer PH: Death receptors
in chemotherapy and cancer. Oncogene. 23:2950–2966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|