Introduction
Breast cancer accounts for ~20% of all malignancies
in women. Its various subtypes and behaviors have been well
documented in recent decades (1).
Treatments and outcomes have markedly improved with the
introduction of efficient screening programs and the use of novel
adjuvant hormonal therapy, chemotherapy and biological agents
(2,3).
Nevertheless, not all cases are curable. The need to improve
understanding of the mechanisms underlying the disease and to
provide the most appropriate therapy has prompted researchers to
attempt to identify novel prognostic and predictive tumor markers
that may serve as targets for future treatments. One such
predictive marker is the 78-kDa glucose-regulated protein (GRP78)
protein. GRP78 is a key regulator of the unfolded protein response
mechanism that underlies endoplasmic reticulum stress and protects
cells against apoptosis (4). A number
of studies have demonstrated that during endoplasmic reticulum
stress, cells overexpress GRP78, inducing its translocation to the
cell surface (5–7). Cell surface GRP78 has been identified in
various types of cancer, including prostate (8), gastric (9)
and ovarian cancer (10), as well as
melanoma (11) and astrocytoma
(12). Numerous previous studies have
identified a correlation between high GRP78 levels and high
pathological grade, recurrence and poor survival in various
malignancies (9–13); however, other studies have reported
the opposite (14,15).
Preclinical studies have indicated that high levels
of GRP78 protein may predict resistance to chemotherapy
(doxorubucin) or, by contrast, response to a specific type of
chemotherapy (taxane) (7,16,17).
The aim of the present study was to determine the
impact of GRP78 expression in early and locally advanced breast
cancer and to analyze cell surface GRP78 expression compared with
traditional prognostic and predictive parameters, as well as
Oncotype DX, a validated predictive gene profile test (18).
Materials and methods
Patients and design
The present study included patients with American
Joint Committee on Cancer stage I–III (19) breast cancer who were referred to Rabin
Medical Center (RMC), Beilinson Campus (Petah Tikva, Israel) with
sufficient residual tumor for GRP78 staining. All patients were
diagnosed and treated between 2005 and 2012. From this cohort, two
patient groups were assigned: Group 1, patients with operable T1,2,
node-negative cancer who were assessed with the Oncotype DX gene
profile in addition to standard assessment by the local
pathologist; group 2, patients who mainly possessed locally
advanced tumors, who were assigned to receive neoadjuvant systemic
treatment in addition to surgery. Only patients who underwent their
initial biopsy study and final surgery at the RMC were
included.
The study was approved by the Institutional Review
Board of the RMC, in accordance with the Helsinki Declaration of
1975 (20).
Data collection
Data on patient and tumor characteristics, including
age, stage, grade, estrogen receptor (ER) and progesterone receptor
(PR), human epidermal growth factor 2 receptor (HER2), Ki-67 status
and p53 protein expression were collected. For group 1 (early
breast cancer), the Oncotype DX score was also recorded. For group
2 (locally advanced cancer), the characteristics of the tissues
were recorded prior to and following systemic treatment, in
addition to the type of chemotherapy received (± trastuzumab) and
the rate of response to treatment. A complete pathological response
was defined as no invasive residual tumor in the breast or axillary
lymph nodes. An almost complete pathological response was defined
as residual invasive disease in the breast, measuring <0.1 cm
and no residual lymph-node involvement. Disease-free survival (DFS)
was reported for all patients.
Breast cancer subtypes were classified according to
a gene-expression-profile-validated immunohistochemistry surrogate
panel (21,22) as follows: Luminal A, ER-positive
and/or PR-positive, HER2-negative and Ki-67 <14%; luminal B,
ER-positive and/or PR-positive, HER2-negative and Ki-67 ≥14%;
luminal/HER2, ER-positive and/or PR-positive and HER2-positive
regardless of Ki-67 status; HER2-enriched, ER- and PR-negative,
HER2-positive; triple-negative, ER-, PR- and HER2-negative.
GRP78 staining
Samples were immediately fixed with 4%
paraformaldehyde and then embedded in paraffin. The paraffin
sections were stored at room temperature. Antigen retrieval was
performed using an anti-GRP78 BiP antibody (Rabbit immunoglobulin
G; Thermo Fisher Scientific, Waltham, MA, USA), according to the
manufacturer's instructions. Briefly, histological sections were
deparaffinized with xylene (100%; Sigma-Aldrich, Rehovot, Israel)
for 20 min and dehydrated in an ethyl alcohol series (100 and 70%;
Finkelman Ltd. Chemicals, Petach Tikva, Israel). Antigen unmasking
was performed by heating in citrate buffer (pH 6.0; Sigma-Aldrich)
using a Biocare Medical Decloaking Chamber™ (Biocare Medical, LLC,
Concord, CA, USA). Following antigen unmasking, the sections were
cooled to room temperature, washed with wash buffer (Zytomed
Systems, Berlin, Germany), submerged in H2O2
(3%) for 10 min, washed with tap water and rinsed with wash buffer.
The slides were then incubated overnight with 1 µg/ml anti-GRP78
antibody in phosphate-buffered saline (pH 7.5) in a moist chamber
at 4°C. The next day, the slides were washed with wash buffer and
incubated with horseradish peroxidase-conjugated anti-rabbit
secondary antibody (ZytoChem-Plus, Berlin, Germany) for 30 min.
This procedure was followed by washing in wash buffer and
incubation for 1 min with stable diaminobenzidene solution (Innovex
Biosciences, Richmond, CA, USA), prepared according to the
manufacturer's instructions. The slides were then washed with
distilled water for 5 min, counterstained with Harris hematoxylin
(Sigma-Aldrich) and permanently mounted with mount medium. Control
staining was performed without the primary antibody for nonspecific
staining, and was negative.
GRP78 expression score
GRP78 expression was analyzed in 15–20 areas of
infiltrative carcinoma cells in whole biopsy sections. Analyses
were performed separately for cytoplasmic and cell surface staining
at x400 magnification (BX-43 microscope; Olympus America, Inc.,
Center Valley, PA, USA). Cytoplasmic GRP78 staining was graded on a
4-point scale as follows: 0, none; 1, weak; 2, moderately intense;
3, very intense. Cell surface GRP78 staining was recorded as the
percentage of cell surface GRP78-positive tumor cells in the whole
slide. Membranous staining of <10% of cells was considered
negative, and >10% of cells, positive. All scoring was performed
by a single investigator blinded to the findings for other
pathological stains and patient outcome. Representative images of
the slides were captured with an Olympus DP72 camera (lens, x40;
Olympus, Tokyo, Japan) using the Cell A software program, version
3.2 (Olympus Soft Imaging Solutions, Münster, Germany).
Determination of known tumor
markers
Staining for ER, PR, p53, Ki-67 and HER2 was
performed using the Ventana Benchmark XT automated immonostainer
(Ventana, Tuscon AZ, USA) with the standard cell conditioner (CC1)
protocol for 30 min. Following deparaffinization and the CC1
protocol, ready-to-use ER rabbit monoclonal antibody [anti-ER
(6F11) primary antibody; Ventana] was applied for 40-min incubation
at 37°C; PR rabbit monoclonal antibody (clone 16; Novocastra,
Newcastle, UK) was employed at a 1:100 dilution with 40-min
incubation at 37°C; Ki-67 rabbit monoclonal antibody (clone SP6;
Thermo Fisher Scientific) was used at a 1:100 dilution for 40 min
at 37°C; and ready-to-use PATHWAY HER2 anti-HER2/neu rabbit
monoclonal antibody (4B5) (Ventana) was utilized with 32-min
incubation at 37°C. For HER2 fluorescence in situ
hybridization (FISH) assay, the slides were hybridized with probes
to locus-specific identifier (LSI) HER2/neu and to centromere 17
using the PathVysion HER-2 DNA Probe kit (Abbott Molecular, Abbott
Park, IL, USA) according to the manufacturer's instructions. Slides
were counterstained with 4,6-diamidino-2-phenylindole
(Sigma-Aldrich), and the stained material was visualized under a
BX51 fluorescence microscope (Olympus). The signals were analyzed
manually.
The ER and PR staining was scored using a modified
version of the H-SCORE method: (1 × percentage of weakly staining
nuclei + 2 × percentage of moderately staining nuclei + 3 ×
percentage of intensely staining nuclei)/100, yielding a range of
0–3 (23).
Ki-67 and p53 were evaluated by the percentage of
positively stained nuclei (0–100%). HER2 positivity was defined as
an IHC of 3. If IHC equaled 2, an amplification ratio ≥2.0 with
FISH, was considered positive.
Statistical analysis
The expression of cytoplasmic and cell surface GRP78
was compared between patients with early breast cancer and patients
who required neoadjuvant systemic treatment, prior to and following
the administration of treatment. For categorical variables,
Fisher's exact test or χ2 test was used to analyze
differences in mean values between groups. For ordinal variables,
Spearman's nonparametric correlation coefficient was used.
Differences in mean parameters prior to and following treatment
were analyzed with the Wilcoxon signed rank test. A Kaplan-Meier
plot was created for DFS and Cox regression analysis was performed
to examine the impact of the variables on DFS. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological data
Forty-eight patients with breast cancer were
included in the study, 27 with operable early cancer (group 1) and
21 who received neoadjuvant chemotherapy (group 2). In addition,
20/21 patients in group 2 presented with locally advanced
tumors.
The operable early cancers consisted of luminal A/B
subtypes only: Luminal A, 56% and luminal B, 44%. Twenty patients
in this group (74%) had stage I disease and 26% had stage II
disease. As per the inclusion criteria, none exhibited lymph node
involvement. The tumors in group 2, the neoadjuvant group,
consisted of various subtypes: Luminal A, 14%; luminal B, 47%;
luminal HER2, 24%; HER2-enriched, 5%; and triple-negative, 10%. Of
the 21 patients in this group, 15 (71%) presented with stage III
disease, 5 (24%) with stage II and one with stage I (5%). All
patients in group 2 received anthracycline- and taxane-based
regimens. Trastuzumab was administered to 3/6 patients (50%) with
HER2-positive disease. Two patients (10%) reached a pathological
complete response and 5 (24%), an almost pathological complete
response.
No significant differences in
cytoplasmic GRP78 were detected between groups
The cytoplasmic GRP78 was evaluated in histological
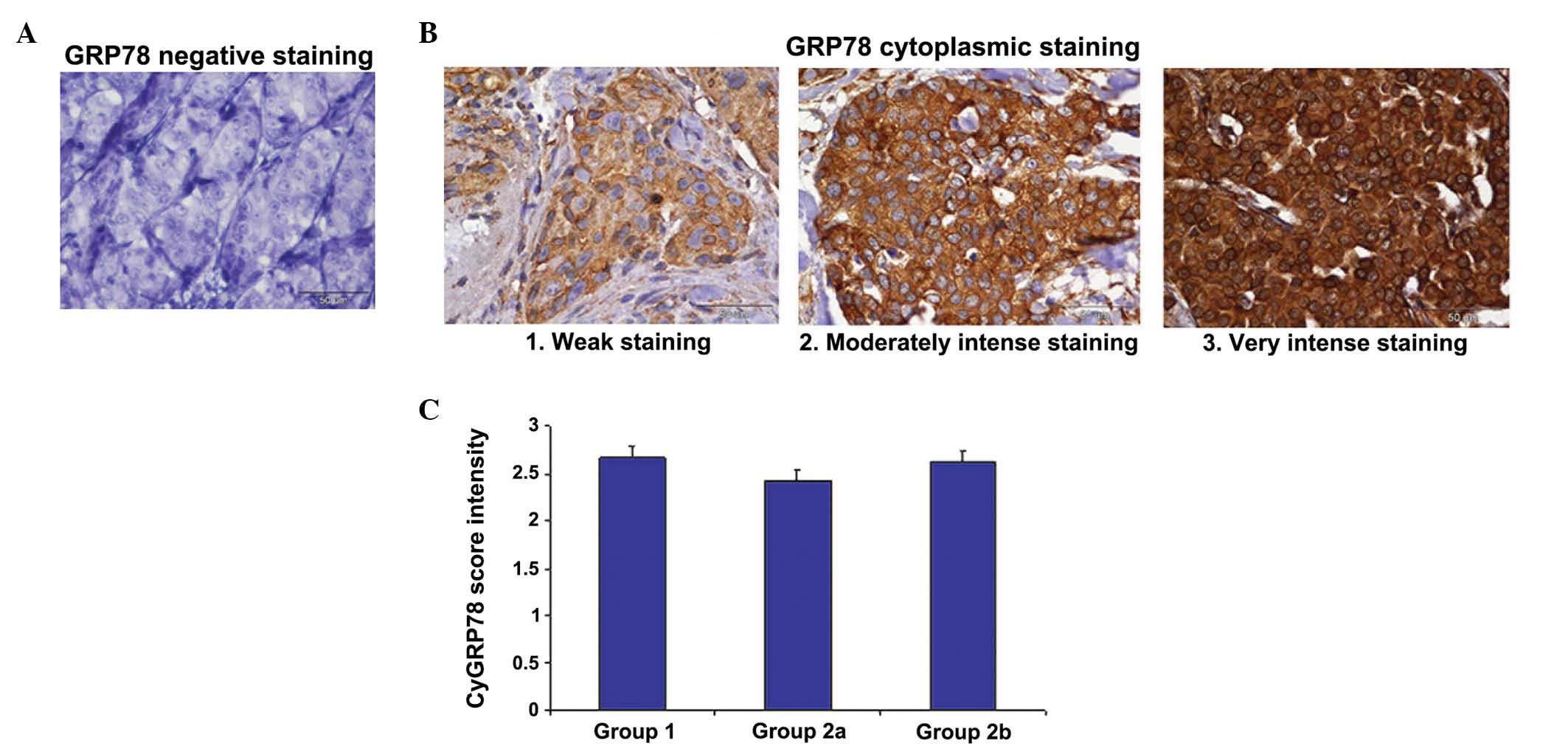
sections of the breast cancer patients (Fig. 1). The mean scores for cytoplasmic
GRP78 expression were 2.7±0.12 in group 1 (patients with
early-stage disease), 2.43±0.11 in group 2 (patients prior to
systemic therapy) and 2.65±0.13 in group 2 following systemic
therapy. No significant differences were observed between the
groups (P>0.5) (Fig. 1C). Fig. 1A depicts the negative control
cytoplasmic and cell surface GRP78 staining, while Fig. 1B illustrates the various intensities
of GRP78 staining, according to the scores described in the
materials and methods section.
Cell surface GRP78 expression varies
between groups
Since no significant differences were observed in
GRP78 cytoplasmic determination, all further analyses were based on
cell surface GRP78 staining only. A representative sample of
positive cell surface GRP78 expression is presented in Fig. 2A and the distinction between positive
and negative GRP78 staining is demonstrated in Fig. 2B. In group 1, 74.1% of the cells were
positive for cell surface GRP78 and 25.9% were negative; while in
group 2, the percentage of positive cell surface GRP78 expression
was 36% prior to neoadjuvant systemic treatment, which
significantly increased to 62.5% following treatment (P=0.039)
(Fig. 2C). Group 1, which included
patients with ER-positive disease but no lymph node involvement,
demonstrated the highest percentage of patients with cell surface
GRP78 expression. Patients in group 2 were significantly less
likely to present positive cell surface GRP78 expression prior to
systemic treatment compared with afterwards.
The results obtained for cell surface GRP78
expression in group 1 (the luminal, node negative group members who
were referred to up-front surgery) were compared by χ2
tests to the post neoadjuvant-treated patients (group 2), and no
significant differences were observed (P=0.32). By contrast, a
significant difference was observed between group 1 and the
pre-chemotherapy group 2 (P=0.039).
Cell surface GRP78 expression is
correlated with PR staining
Table I summarizes the
results obtained for the whole cohort. No significant differences
were observed between cell surface GRP78 expression and age, tumor
size, grade, ER, Ki167 and Oncotype DX score.
 | Table I.Cell surface GRP78 expression
correlation with breast cancer prognostic parameters. |
Table I.
Cell surface GRP78 expression
correlation with breast cancer prognostic parameters.
| Parameter | Cell surface
GRP78 | n | Mean ± SD | P-value |
|---|
| Age | N | 19 |
57.94±14.1 | 0.97 |
|
| P | 29 |
57.79±10.7 |
|
| Tumor size | N | 7 |
1.77±0.53 | 0.79 |
|
| P | 20 |
1.70±0.63 |
|
| Grade | N | 16 |
2.25±0.44 | 0.84 |
|
| P | 28 |
2.28±0.59 |
|
| ER | N | 19 |
2.02±0.99 | 0.99 |
|
| P | 29 |
2.02±0.83 |
|
| PR | N | 19 |
0.35±0.57 | 0.021 |
|
| P | 29 |
0.97±1.03 |
|
| p53 | N | 18 |
1.61±2.59 | 0.022 |
|
| P | 29 |
16.17±25.8 |
|
| Ki-67 | N | 18 |
31.11±20.54 | 0.31 |
|
| P | 29 |
24.31±22.65 |
|
| Oncotype DX
score | N | 7 |
28.57±5.5 | 0.75 |
|
| P | 20 |
26.30±18.18 |
|
A direct correlation between GRP78 expression and
the level of PR staining was observed. GRP78 expression was
observed in 44.8% of samples with a higher PR score (PR ≥1) and in
10.52% of samples with a lower PR score (PR <1). Positive
staining for cell surface GRP78 was therefore more likely to be
significantly associated with a higher PR score than with negative
staining (P=0.021).
Positive cell surface GRP78 was detected in 61% of
the samples with higher p53 protein expression as compared with 39%
of the samples with lower p53 protein expression. In this
experiment, a higher level of positive cell surface GRP78
correlated significantly with a higher level of p53 expression
(P=0.022).
Cell surface GRP78 expression is
associated with improved DFS
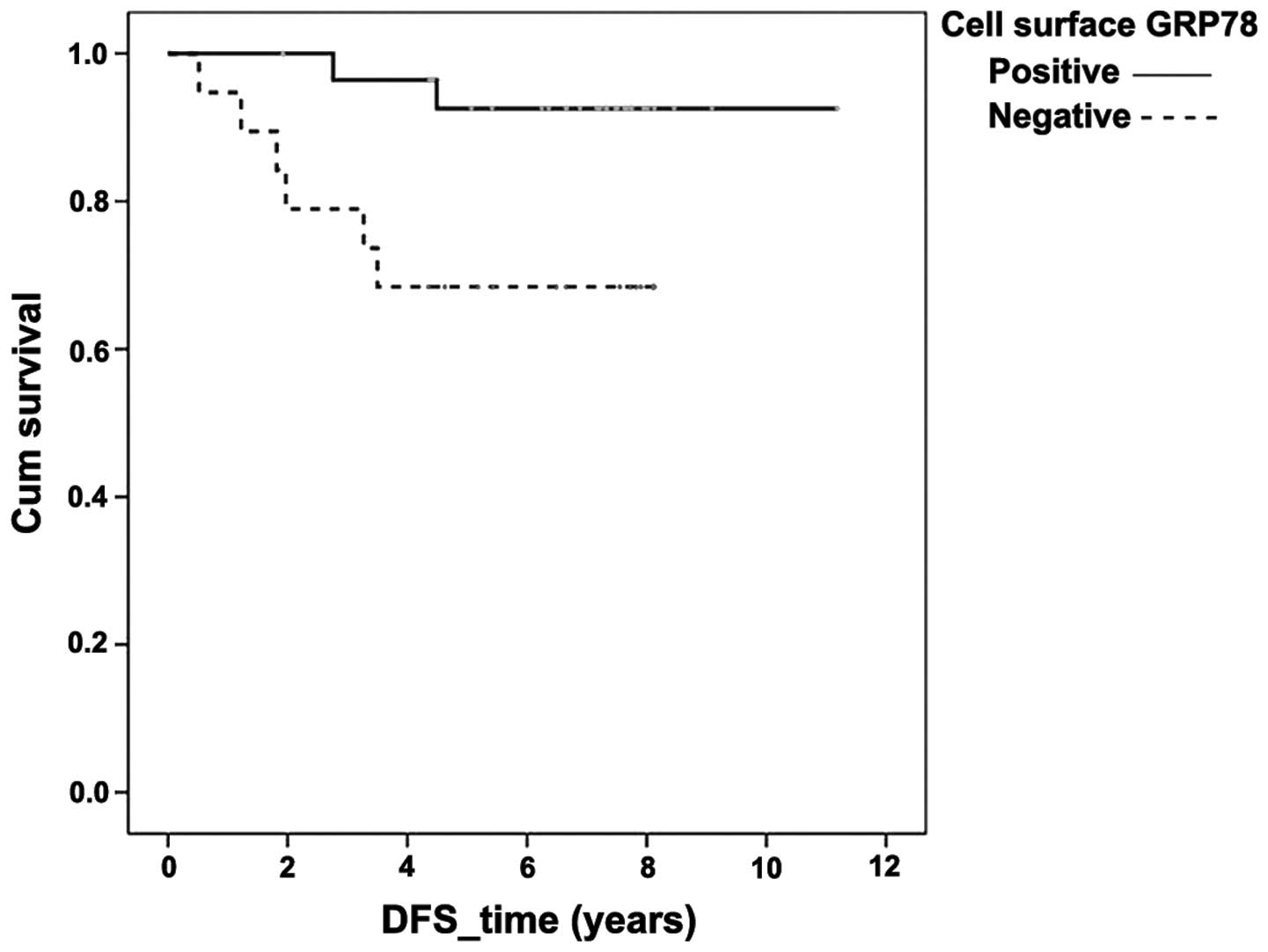
ER positivity was associated with an improved DFS
based on both univariate and multivariate analyses at P=0.004 and
P=0.047, respectively (data not shown). Positive cell surface GRP78
expression was also associated with an improved DFS (Fig. 3), P=0.047 in univariate analysis, but
demonstrated only a trend in the same direction in multivariate
analysis (P=0.070).
Association between cell surface GRP78
expression status and predictive parameters
A trend towards an inverse association between cell
surface GRP78 expression and an Oncotype DX score was observed,
which is predictive of beneficial adjuvant chemotherapy. Sixty-five
percent of patients with high expression of cell surface GRP78
(15/27) had a low Oncotype DX score, while 71.4% (19/27) of
patients with a high Oncotype DX score demonstrated low expression
of cell surface GRP78. Cell surface GRP78 positivity was therefore
associated with a lower gene profile score (P=0.185).
In the patients of the neoadjuvant group (group 2),
where all tumors except one were locally advanced, a complete or
almost complete pathological response was less likely to be
associated with GRP78 positivity prior to systemic treatment
(P=0.195). Only 25% of the patients with positive cell surface
GRP78 expression achieved a complete or almost complete
pathological response, compared with 50% of the patients negative
for GRP78 expression.
Discussion
The present study demonstrated that cell surface
GRP78 expression may serve as a novel prognostic and predictive
marker in breast cancer, to improve the estimation of the
recurrence risk and to predict the benefits of systemic treatment.
Good prognostic and predictive markers are critical in early and
locally advanced breast cancer since the aim of treatment is to
cure with minimal toxicity.
One of the challenges of investigating a novel tumor
marker is to establish a valid, reproducible scoring method. The
scoring method in the present study was based on that of previous
publications (13,17,24).
In the present study, negative cell surface GRP78
expression was significantly associated with locally advanced
disease, in contrast to previous studies in which positive GRP78
expression was associated with an aggressive phenotype and poor
prognosis (24–26). However, in those earlier studies there
was no clear distinction between cytoplasmic and cell surface GRP78
expression. To the best of our knowledge, the present study was the
first to differentiate between cytoplasmic and cell surface GRP78
expression in breast cancer patients. The present results are
supported by those of an earlier study (27) in which cell surface GRP78-positive
tumor cells separated by magnetic beads were characterized and
their reduced growth and metastatic potential was demonstrated.
The extensive analyses of the present study are
consistent with the finding that cell surface GRP78 expression is a
good prognostic factor (14,15). The most notable result was the
observation that DFS was significantly improved in cases which were
positive, as opposed to negative, for cell surface GRP78, as
depicted in the Kaplan-Meier graph. These findings were supported
by correlational analysis, which demonstrated an association
between positive cell surface GRP78 expression and high PR
expression, a known marker for good prognosis (28).
An additional correlation was observed between
positive cell surface GRP78 expression and high expression of p53
protein, which has been demonstrated to be associated with poor
prognosis in breast cancer patients (29). However, studies have revealed that the
p53 levels observed by immunohistochemical staining may be
misleading as a prognostic factor, since its significance depends
on the breast cancer subtype and may be influenced by the type of
p53 mutation (30). Therefore, this
specific finding requires further investigation.
A preclinical study reported that high GRP78
expression has a predictive value for resistance to doxorubicin
(17), although this finding was not
consistent in all studies (24).
These studies indicated benefits of the use of taxanes in breast
cancer, while others have demonstrated that GRP78-positive tumors
may be specifically resistant to topoisomerase inhibitors (16,31–33).
At present, guidelines for systemic adjuvant
chemotherapy incorporate the use of expensive gene profiling for
prognostic and predictive purposes (34–36). In
the clinic, patients with node-negative breast cancer who are
candidates for adjuvant chemotherapy are routinely offered gene
profiling; those with a high score are considered at high risk of
recurrence but may benefit from systemic chemotherapy. Since this
has become the standard of care, the correlation between the novel
tumor marker GPR78 and a popular gene set, the Oncotype DX, was
studied. The results demonstrated that 65% of the patients with
positive cell surface GRP78 expression had a low Oncotype DX score.
Translated into clinical practice, this result indicates that
measuring GRP78 expression may aid the identification of a subgroup
of patients with a favorable prognosis, who will not benefit from
adjuvant (prophylactic) chemotherapy.
Gene profiles, including as Oncotype DX, however, do
not yet serve a role in the decision-making process for systemic
neoadjuvant chemotherapy. In the present study, all patients in the
neoadjuvant subgroup received anthracycline- and taxane-based
regimens. A trend towards an improved pathological response to
treatment was noted in tumors with low levels of cell surface GRP78
expression. These results are in line with the well-established
finding that although aggressive breast cancer tumors indicate poor
patient prognosis, they respond better to chemotherapy (37,38).
At the completion of the neoadjuvant systemic
therapy, the residual tumor was significantly more likely to
exhibit positive cell surface GRP78 expression, compared with the
pre-treatment tissue. This finding may be attributed to the fact
that chemotherapy treatment activated the endoplasmic reticulum
stress response, inducing the unfolding protein response key
protein GRP78 and specifically its cell surface expression. This
effect has previously been demonstrated in breast cancer cell lines
(39). These cells may also be less
proliferative and metastatic, as was previously demonstrated
(27,39). In addition, the residual tumor with
high GRP78 expression may represent residual resistant clones that
do not respond to chemotherapy. To the best of our knowledge, this
is the first study to investigate cell surface GRP78 expression in
a neoadjuvant setting.
Despite the limitation of the present study, which
is its relatively small sample size, the value of the GRP78
biomarker was highlighted by the various analyses.
In conclusion, literature regarding the prognostic
value of high/low levels of cell surface GRP78 expression in
malignancies remains controversial. However, the present study
indicated that cell surface GRP78 positivity was an indicator of a
good prognosis and may serve as a marker for potential benefit from
chemotherapy in breast cancer.
Acknowledgements
The present study was supported by the Israel Cancer
Association (ICA) with the assistance of the ICA-USA (grant no.
2012016).
References
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA and
Wolmark N: Four-year follow-up of trastuzumab plus adjuvant
chemotherapy for operable human epidermal growth factor receptor
2-positive breast cancer: Joint analysis of data from NCCTG N9831
and NSABP B-31. J Clin Oncol. 29:3366–3373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group, . Polychemotherapy for early breast cancer: An
overview of the randomised trials. Lancet. 352:930–942. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sato M, Yao VJ, Arap W and Pasqualini R:
GRP78 signaling hub a receptor for targeted tumor therapy. Adv
Genet. 69:97–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arap MA, Lahdenranta J, Mintz PJ, Hajitou
A, Sarkis AS, Arap W and Pasqualini R: Cell surface expression of
the stress response chaperone GRP78 enables tumor targeting by
circulating ligands. Cancer Cell. 6:275–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee AS: GRP78 induction in cancer:
Therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J and Lee AS: Stress induction of
GRP78/BiP and its role in cancer. Curr Mol Med. 6:45–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
GonzalezGronow M, Cuchacovich M, Llanos C,
Urzua C, Gawdi G and Pizzo SV: Prostate cancer cell proliferation
in vitro is modulated by antibodies against
glucose-regulated protein 78 isolated from patient serum. Cancer
Res. 66:11424–11431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rauschert N, Brändlein S, Holzinger E,
Hensel F, Müller-Hermelink HK and Vollmers HP: A new tumor-specific
variant of GRP78 as target for antibody-based therapy. Lab Invest.
88:375–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chinni SR, Falchetto R, GercelTaylor C,
Shabanowitz J, Hunt DF and Taylor DD: Humoral immune responses to
cathepsin D and glucose-regulated protein 78 in ovarian cancer
patients. Clin Cancer Res. 3:1557–1564. 1997.PubMed/NCBI
|
|
11
|
Papalas JA, Vollmer RT, GonzalezGronow M,
Pizzo SV, Burchette J, Youens KE, Johnson KB and Selim MA: Patterns
of GRP78 and MTJ1 expression in primary cutaneous malignant
melanoma. Mod Pathol. 23:134–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang LH, Yang XL, Zhang X, Cheng JX and
Zhang W: Association of elevated GRP78 expression with increased
astrocytoma malignancy via Akt and ERK pathways. Brain Res.
1371:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni M, Zhang Y and Lee AS: Beyond the
endoplasmic reticulum: Atypical GRP78 in cell viability, signalling
and therapeutic targeting. Biochem J. 434:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uramoto H, Sugio K, Oyama T, Nakata S, Ono
K, Yoshimastu T, Morita M and Yasumoto K: Expression of endoplasmic
reticulum molecular chaperone Grp78 in human lung cancer and its
clinical significance. Lung Cancer. 49:55–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Tsao
PN, Lee H, Lin MT, Lai HS, Chen CN, Lai DM, et al: GRP78 expression
correlates with histologic differentiation and favorable prognosis
in neuroblastic tumors. Int J Cancer. 113:920–927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong D, Ko B, Baumeister P, Swenson S,
Costa F, Markland F, Stiles C, Patterson JB, Bates SE and Lee AS:
Vascular targeting and antiangiogenesis agents induce drug
resistance effector GRP78 within the tumor microenvironment. Cancer
Res. 65:5785–5791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee E, Nichols P, Spicer D, Groshen S, Yu
MC and Lee AS: GRP78 as a novel predictor of responsiveness to
chemotherapy in breast cancer. Cancer Res. 66:7849–7853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yarbro JW, Page DL, Fielding LP, Partridge
EE and Murphy GP: American Joint Committee on Cancer prognostic
factors consensus conference. Cancer. 86:2436–2446. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iyalomhe GB and Imomoh PA: Ethics of
clinical trials. Niger J Med. 16:301–306. 2007.PubMed/NCBI
|
|
21
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheang MC, Voduc D, Bajdik C, Leung S,
McKinney S, Chia SK, Perou CM and Nielsen TO: Basal-like breast
cancer defined by five biomarkers has superior prognostic value
than triple-negative phenotype. Clin Cancer Res. 14:1368–1376.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weidner N, Cote RJ, Suster S and Weiss LM:
Modern Surgical Pathology. 2nd. Elsevier Saunders; Philadelphia,
PA: 2009, View Article : Google Scholar
|
|
24
|
Baptista MZ, Sarian LO, Vassallo J, Pinto
GA, Soares FA and de Souza GA: Prognostic significance of GRP78
expression patterns in breast cancer patients receiving adjuvant
chemotherapy. Int J Biol Markers. 26:188–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Yang S, Liu J, Wang X, Ji J, Cao
Y, Lu K, Wang J and Gao Y: Expression of GRP78 predicts
taxane-based therapeutic resistance and recurrence of human gastric
cancer. Exp Mol Pathol. 96:235–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng YZ, Cao ZG, Hu X and Shao ZM: The
endoplasmic reticulum stress markers GRP78 and CHOP predict
disease-free survival and responsiveness to chemotherapy in breast
cancer. Breast Cancer Res Treat. 145:349–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hardy B, Raiter A, Yakimov M, Vilkin A and
Niv Y: Colon cancer cells expressing cell surface GRP78 as a marker
for reduced tumorigenicity. Cell Oncol (Dordr). 35:345–354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Díaz Flaqué MC, Galigniana NM, Béguelin W,
Vicario R, Proietti CJ, Russo R, Rivas MA, Tkach M, Guzmán P, Roa
JC, et al: Progesterone receptor assembly of a transcriptional
complex along with activator protein 1, signal transducer and
activator of transcription 3 and ErbB-2 governs breast cancer
growth and predicts response to endocrine therapy. Breast Cancer
Res. 15:R1182013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyle DP, McArt DG, Irwin G,
WilhelmBenartzi CS, Lioe TF, Sebastian E, McQuaid S, Hamilton PW,
James JA, Mullan PB, et al: The prognostic significance of the
aberrant extremes of p53 immunophenotypes in breast cancer.
Histopathology. 65:340–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coates AS, Millar EKA, O'Toole SA, Molloy
TJ, Viale G, Goldhirsch A, Regan MM, Gelber RD, Sun Z,
Castiglione-Gertsch M, et al: Prognostic interaction between
expression of p53 and estrogen receptor in patients with
node-negative breast cancer: Results from IBCSG trials VIII and IX.
Breast Cancer Res. 14:R1432012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee E, Nichols P, Groshen S, Spicer D and
Lee AS: GRP78 as potential predictor for breast cancer response to
adjuvant taxane therapy. Int J Cancer. 128:726–731. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu KD, Huang AJ, Fan L, Li WF and Shao ZM:
Genetic variants in oxidative stress-related genes predict
chemoresistance in primary breast cancer: A prospective
observational study and validation. Cancer Res. 72:408–419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roller C and Maddalo D: The molecular
chaperone grp78/bip in the development of chemoresistance:
Mechanism and possible treatment. Front Pharmacol. 4:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Health Quality Ontario, . Gene expression
profiling for guiding adjuvant chemotherapy decisions in women with
early breast cancer: An evidence-based and economic analysis. Ont
Health Technol Assess Ser. 10:1–57. 2010.
|
|
35
|
van de Vijver MJ, He YD, van't Veer LJ,
Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C,
Marton MJ, et al: A gene-expression signature as a predictor of
survival in breast cancer. N Engl J Med. 347:1999–2009. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes - dealing with the diversity of breast cancer: Highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rouzier R, Perou CM, Symmans WF, Ibrahim
N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P,
et al: Breast cancer molecular subtypes respond differently to
preoperative chemotherapy. Clin Cancer Res. 11:5678–5685. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Raiter A, Yerushalmi R and Hardy B:
Induction of cell surface GRP78 contributes to apoptosis in triple
negative breast cancer cells. Oncotarget. 5:11452–1163.
2014.PubMed/NCBI
|