Introduction
Uterine cervical cancer (UCC) is one of the main
causes of cancer-associated mortality in women. The main identified
factor involved with disease development is chronic infection by
high-risk human papilloma virus (HPV) (1). Prevention by vaccination and
premalignant lesion treatment are the best tools for decreasing the
incidence of this condition. However, the diagnosis of UCC at the
advanced stages is frequent in developing countries. In these
conditions, the existing treatments are not effective and affect
the quality of life; as a consequence, the requirement for novel
treatments continues to be necessary. Inflammation has been
identified as a critical component during tumor proliferation,
progression and dissemination (2,3).
Inflammation is induced and maintained mainly by the activity of
the cyclooxygenase 2 (COX-2) enzyme, and as a result, its
inhibition may be adequately employed for cancer treatments. Among
the pharmaceuticals with high inhibition activity for COX-2,
non-steroidal anti-inflammatory drugs (NSAIDs) are considered to
play a significant role (1,4–6).
The chronic administration of different NSAIDs may
contribute to reducing the incidence of diverse neoplasias
(7) and these actions represent
potential treatments. In this context, the fenamates, such as the
meclofenamic acid, represent a group of the most potent NSAID COX-2
inhibitors. Meclofenamic acid is a phenamate derivative that is not
only a potent inhibitor of aldoketoreductases (AKRs) (8), enzymes that regulate the androgen,
estrogen and progestin concentration, but also catalyzes the
reduction of ketosteroids (9). The
exerted inhibition of the NSAIDs over these enzymes has been
considered as a proposed mechanism of the antineoplastic effect
(10). Several anti-inflammatories
have also been probed experimentally as a UCC treatment, however,
the obtained results were varied and it is difficult to establish
the substance that has the greatest antineoplastic effect (11). In spite of the fact that meclofenamic
acid has previously been proposed as a promising antineoplastic
drug, the fenamates have not yet been investigated in UCC. The aim
of the present study was to evaluate the antineoplastic effect of
several NSAIDs, including the fenamates, in in vitro and
in vivo assays of UCC.
Materials and methods
Cell lines and drugs
The cell lines used in the present study, which were
supplied by the Health Sciences Research and Development Center of
Nuevo Leon Autonomous University (Monterrey, Mexico), were the
human UCC HeLa, VIPA, INBL (HPV-18+) and SiHa
(HPV-16+) cell lines, and the mouse TC-1
(HPV-16+) cell line (12,13), which
were manipulated in a class II A2 laminar flow cabinet and
maintained in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% (v/v)
fetal bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA,
USA) at 37°C, with 5% CO2 and 97% relative humidity
(14,15). Celecoxib, sulindac, nimesulide,
meclofenamic acid, flufenamic acid and mefenamic acid were obtained
as powder salts with a pureness of 98% (Sigma-Aldrich). The
dexamethasone was acquired in solution for intravenous application
(Chinoin Pharmaceutical Products, Aguascalientes, Mexico).
Initially, the pharmaceutical salts were prepared in highly
concentrated solutions. Celecoxib, sulindac and nimesulide were
dissolved in dimethyl sulfoxide (Sigma-Aldrich), while meclofenamic
acid was dissolved in ethanol at 70%, mefenamic acid was dissolved
in 0.1 M NaOH and flufenamic acid was dissolved in absolute ethanol
(Sigma-Aldrich).
Cytotoxicity assay (in vitro)
The cells were plated in 96-well plates (Corning
Inc., Corning, NY, USA) at a density of 1×103 cells per
well, containing DMEM with 2% FBS. Subsequent to an initial 12-h
period, the cells were exposed to the different drugs (16) and incubated for 3 days. The
cytotoxicity was determined with the Alamar Blue® reagent,
according to the manufacturer's instructions (DIAsource
ImmunoAssays, Nivelles, Belgium) (17). The cell viability was compared with
untreated cells, which were considered to represent 100% viability.
The assays were performed with two repetitions. In a first
selection assay, celecoxib, sulindac, nimesulide, dexamethasone and
meclofenamic acid were used in concentrations of 100 µM. In a
subsequent assay, the fenamates, meclofenamic acid, flufenamic acid
and mefenamic acid were used in concentrations of 0, 7.5, 15, 30,
60, 120 and 240 µM.
Murine model assays in UCC (in
vivo)
A total of 1×105 human HeLa [human
papillomavirus (HPV)-18+] cells were injected in the
dorsal region of immunodeficient Foxn1(nu) female mice (nude), aged
4–6 weeks (Harlan Mexico, Mexico City, Mexico). After 10–15 days,
when the tumors reached a length of 4 mm (small tumors), the mouse
population was divided into two groups: Control group (treatment
with saline solution) and experimental group (treatment with
meclofenamic acid). The intraperitoneal drug application was
performed over 30 days, at a dose of 10 mg/kg/day (tolerated dose
in humans and mice) in a volume of 100 ml (16). The tumor was measured every 5 days up
to completion of the 30-day treatment. In addition, a second assay
was performed with two variations with respect to the previous
experiments: i) A immunocompetent murine model was used; and ii)
treatment was initiated when the tumors reached a larger size (a
diameter of 10 mm). A total of 4×105 TC-1 cells
(HPV-16+) were injected into the dorsal region of
C57Bl/6 female mice (nude), aged 4–6 weeks (Harlan Mexico, Mexico
City, Mexico). After 20–25 days, once the tumors had reached a
diameter of 10 mm (large tumors), the mouse population was divided
into a control group and an experimental group, to start the
application of meclofenamic acid at the previously described dose
for a period of 25 days, with measurements of the tumor every 3
days. The antitumor efficiency of meclofenamic acid was evaluated
with the increment of the tumor volume and the survival curves. The
tumor size was registered by measuring three dimensions (length,
width and height) with a vernier gauge to compute the tumor volume,
according to previously reported procedure (18). In consideration of the study protocols
and ethics, the mice were sacrificed when the tumor reached 25 mm
on any of its dimensions, following the Mexican norms
(NOM-062-ZOO-1999) that regulate the use of laboratory animals
(19,20). The study was approved by the ethics
committee of the State Cancer Institute of Colima, Colima, Mexico
(Certificate of Ethical Approval: REV1/ANTICEL/2013).
Statistical analysis
The cytotoxicity data in the cell lines were
analyzed by descriptive statistics. The tumor growth was analyzed
by statistical techniques for curves of tumor growth with no
parametrical methods (free distribution) (19). Mouse survival was analyzed by Kaplan
Meier curves, with the MedCalc statistical program, version 10 for
Windows Vista (MedCalc Software, Ostend, Belgium). P<0.05 was
used to indicate a statistically significant difference.
Results
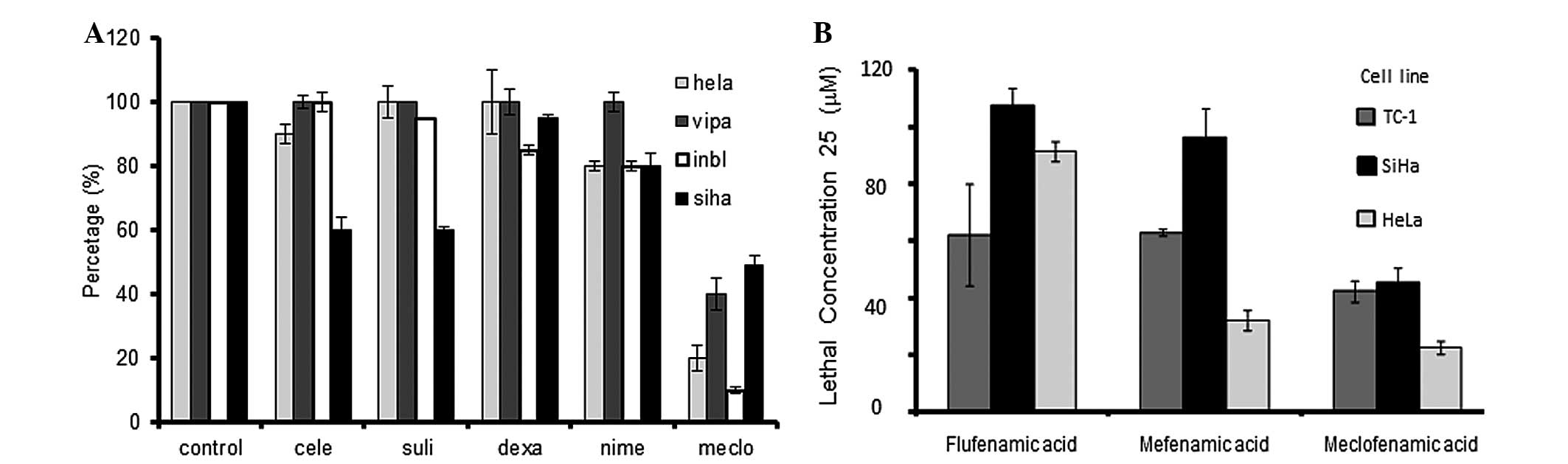
Fig. 1A shows the
viability of the different UCC cell lines exposed to different
anti-inflammatory drugs. Meclofenamic acid exhibited the highest
cytotoxicity in the presence of the four tested cell lines.
Celecoxib, sulindac and nimesulide also presented with partial
cytotoxic activity, but only in certain cell lines. To evaluate if
the meclofenamic acid effect was similar to the other drugs, a
second assay was carried out employing multiple concentrations in
the SiHa, HeLa and TC-1 cell lines. Upon calculation of the
necessary drug concentration lethal to 25% of cells
(LC25) (Fig. 1B), it was
observed that meclofenamic acid required a lower concentration to
generate the same level of cell death. The LC50 could
not be calculated, as only meclofenamic acid induced >50% cell
death in all studied cell lines. This fact demonstrated that
meclofenamic acid was the most cytotoxic drug among those studied,
and the in vivo essays were consequently performed with this
drug.
Two animal models with tumors associated with HPV
were generated. The first model was immunodeficient with neoplasia
of human HeLa HPV-18+ cells, in which treatment was
started when the tumors were small (4 mm diameter). A second model
was immunocompetent with neoplasia of murine TC-1
HPV-16+ cells, in which treatment was started when the
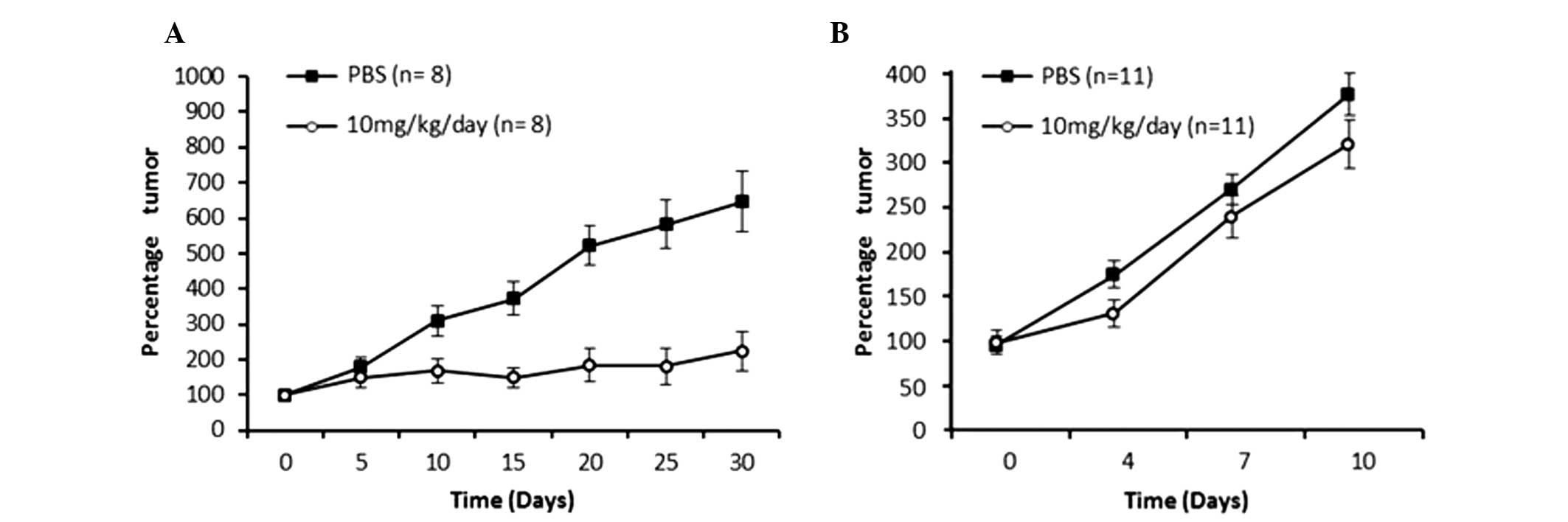
tumors were large (10 mm diameter). As shown in Fig. 2A, the small tumor model using HeLa
cells displayed a clear reduction in tumor growth starting from day
10 of the treatment period, and at the day 30, it was observed that
the mice treated with meclofenamic acid presented with tumors that
were 4-fold smaller in size than the control mice (administration
with saline solution). The difference in the percentage tumor
growth curve was statistically significant (P=0.0001). As shown in
Fig. 2B, the tumor volume growth
curve of the mice treated with meclofenamic acid was significantly
lower than that of the control group (P=0.009). By contrast, in the
large tumor model using TC-1 cells, the neoplastic growth curve was
performed until day 10. Although treatment was administered for 25
days, only 10 days of data are indicated in Fig. 2B, as 100% of the mice were alive
during this period. Subsequently, mice began to die. It is
noteworthy that although the differences are statistically
significant, they are not as marked as those observed in the
intitial animal model. It is noteworthy that although the
differences are statistically significant, they are not as marked
as those observed in the first animal model. In accordance with the
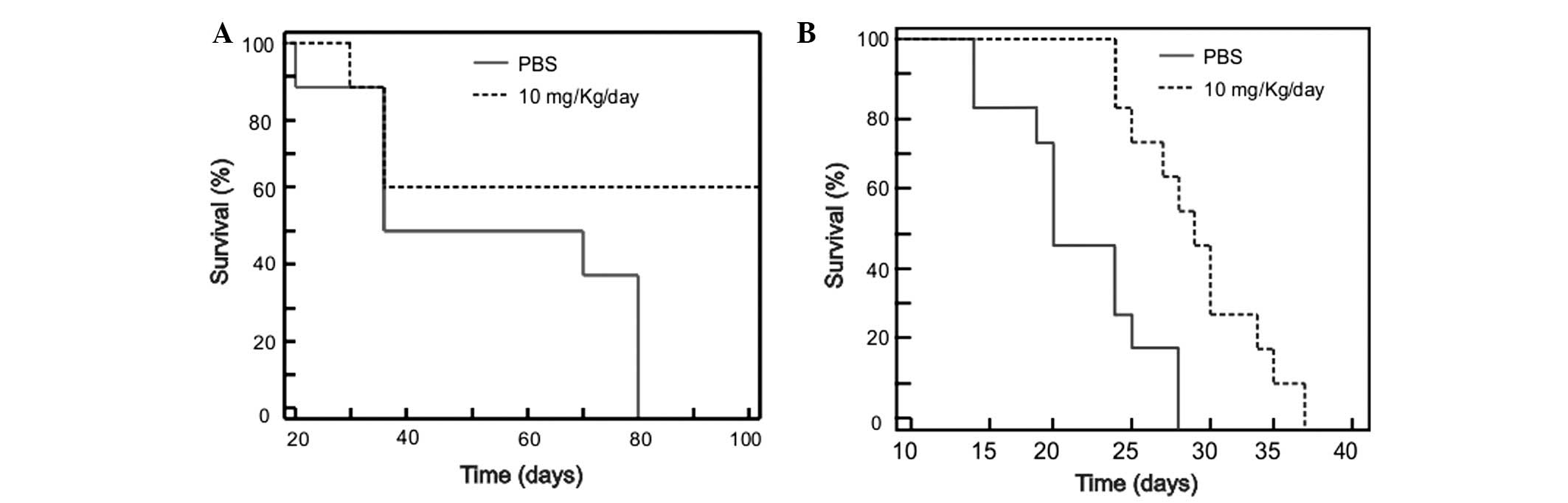
previous results of the present study, the survival times of the
mice treated with meclofenamic acid were significantly superior to
the mice of the control group. The median survival times were 100
vs. 52 days (treated vs. control) for the small tumor model using
HeLa cells (Fig. 3A; P=0.001), and 29
vs. 19 days for the large tumor model using TC-1 cells (Fig. 3B; P=0.038).
Discussion
Meclofenamic acid was revealed to be an efficient
antineoplastic and antitumor agent in in vitro and in
vivo models of UCC in the present study. Epidemiological
studies have demonstrated that the chronic administration of
several individually prescribed NSAIDs reduced the incidence of
certain neoplasias (21,22). It has been postulated that the COX-2
inhibition caused by NSAIDs generates anti-proliferative and
pro-apoptotic effects in diverse cell lines, which could explain
the therapeutic effects found in the present study. Nevertheless,
there are few studies on the NSAID effects in UCC. Sulindac,
aspirin, ibuprofen and celecoxib have exhibited apoptotic effects
in UCC cell lines (in vitro) (23). In the present study, it was confirmed
that the meclofenamic acid has a higher cytotoxic effect than
sulindac or celecoxib. It is noteworthy that celecoxib, one of the
most studied antineoplastic NSAIDs (16,24), did
not exhibit an significant effect when compared with meclofenamic
acid. This fact is in agreement with the poor benefits found in
clinic assays where celecoxib was employed in UCC patients
(25).
Notably, the drugs used in the present study (NSAIDs
and dexamethasone) exhibited varied antineoplastic effects, which
confirms that the inhibition of inflammation is not enough to
generate an effect against cancer. The antitumor activity of
meclofenamic acid was recently reported in prostate cancer
(11). It was proposed that the
effect is not only caused by COX-2 inhibition, but also by the
strong inhibition of AKRs. Meclofenamic acid is one of the
strongest NSAIDs in the inactivation of COX-2, and it is also one
of the most efficient to inactivate the AKRs. Therefore, all these
observations have lead to the proposition that NSAIDs are a
causative mechanism of antineoplastic effects (10).
In the in vitro and in vivo essays in
the present study, the highest drug effect was observed in the HeLa
(HPV-18+) cells, which have a glandular origin
(originally from cervix adenocarcinoma). This fact is in agreement
with the suggested proposal that this meclofenamic acid may have
stronger activity in neoplasias with a glandular origin (11). Nonetheless, the results of the present
study showed the antineoplastic action in human cells of a squamous
nature (SiHa, HPV-16+). In addition, the same
effectiveness was observed when the drug was applied to
HPV-16+ and HPV-18+ cells. The enhanced
action of meclofenamic acid showed significant benefits as a
cytotoxic-effect drug in a model in which the treatment was started
when the tumors were of a small size, as well as when mice with
large tumors were treated. Although the best effect was evidently
generated in the small tumor model of HeLa cells, the used models
were not comparable; the immunodeficient and the immunocompetent
models are characterized by tumor cells with different origins,
since they precede from different mouse strains. However, these
experiments confirm an antitumor effect even in the different
studied models.
The dose employed in the animals, in accordance with
previous results (11), did not have
systemic toxic effects; in addition, it is notable that the
immunodeficient model confirmed that the antitumor effect was
caused directly by the drug action, without implicating the immune
system.
In conclusion, the present study determined that
meclofenamic acid is a potential antineoplastic agent against UCC
or HPV-dependent neoplasias. The clinical utility of the drug,
possibly as a coadjuvant, requires further investigation in future
clinical trials.
Acknowledgements
The present study was completed with grants provided
by SEP-CONACYT via projects 61137 and 226165. The manuscript
publication costs were covered in part by support from PIFI-2013
(CA-UAZ-207).
References
|
1
|
Howley P and Lowy D: Papillomaviruses and
their replicationFields Virology. Knipe DM and Howley PM:
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 2197–2229.
2001
|
|
2
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hold GL and El-omar EM: Genetic aspects of
inflammation and cancer. Biochem J. 410:225–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vane J: Towards a better aspirin. Nature.
367:215–216. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
FitzGerald GA and Patrono C: The coxibs,
selective inhibitors of cyclooxygenase-2. N Engl J Med.
345:433–442. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gierse JK, Hauser SD, Creely DP, Koboldt
C, Rangwala SH, Isakson PC and Seibert K: Expression and selective
inhibition of the constitutive and inducible forms of human
cyclo-oxygenase. Biochem. J. 305:479–484. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gruber BM, Bubko I, KrzysztonRussjan J and
Anuszewska EL: Synergistic action of doxorubicin and sulindac in
human cervix carcinoma cells - studies on possible mechanisms. Med
Sci Monit. 16:BR45–BR51. 2010.PubMed/NCBI
|
|
8
|
Crum C, Muovo G and Lee K: The
cervixSternberg S: Diagnostic Surgical Pathology. 3rd. Lippincott
Williams & Wilkins; Philadelphia, PA: pp. 2155–2202. 1999
|
|
9
|
Bauman DR, Steckelbroeck S and Penning TM:
The roles of Aldo-Keto reductases in steroid hormone action. Drug
News Perspect. 17:563–578. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauman DR, Rudnick SI, Szewczuk LM, et al:
Development of nonsteroidal anti-inflammatory drug analogs and
steroid carboxylates selective for human aldo-keto reductase
isoforms: Potential antineoplastic agents that work independently
of cyclooxygenase isozymes. Mol Pharmacol. 67:60–68. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soriano-Hernández AD, Galvan-Salazar HR,
Montes-Galindo DA, et al: Antitumor effect of meclofenamic acid on
human androgen-independent prostate cancer: A preclinical
evaluation. Int Urol Nephrol. 44:471–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rahbari R, Sheahan T, Modes V, Collier P,
Macfarlane C and Badge RM: A novel L1 retrotransposon marker for
HeLa cell line identification. Biotechniques. 46:277–284.
2009.PubMed/NCBI
|
|
13
|
Watson DM: The Virginian-Pilot: HeLa
Cancer cells killed Henrietta Lacks - then made her immortal.
http://hamptonroads.com/2010/05/cancer-cells-killed-henrietta-lacks-then-made-her-immortalAccessed.
September 17–2014.
|
|
14
|
Kim KM, Song JJ, An JY, Kwon YT and Lee
YJ: Pretreatment of acetylsalicylic acid promotes tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis by
down-regulating BCL-2 gene expression. J Biol Chem.
280:41047–41056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blaheta RA, Weich E, Marian D,
BereiterHahn J, Jones J, Jonas D, Michaelis M, Doerr HW and Cinatl
J Jr: Human cytomegalovirus infection alters PC3 prostate carcinoma
cell adhesion to endothelial cells, and extracellular matrix.
Neoplasia. 8:807–816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SH, Song SH, Kim SG, Chun KS, Lim SY,
Na HK, Kim JW, Surh YJ, Bang YJ and Song YS: Celecoxib induces
apoptosis in cervical cancer cells independent of cyclooxygenase
using NF-kappaB as a possible target. J Cancer Res Clin Oncol.
130:551–560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Nasiry S, Geusens N, Hanssens M, Luyten
C and Pijnenborg R: The use of Alamar Blue assay for quantitative
analysis of viability, migration and invasion of choriocarcinoma
cells. Hum Reprod. 22:1304–1309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stephenson RA, Dinney CP, Gohji K, Ordonez
NG, Killion JJ and Fidler IJ: Metastatic model for human prostate
cancer using orthotopic implantation in nude mice. J Natl Cancer
Inst. 84:951–957. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koziol JA, Maxwell DA, Fukushima M,
Colmerauer ME and Pilch YH: A distribution-free test for
tumor-growth curve analyses with application to an animal tumor
immunotherapy experiment. Biometrics. 37:383–390. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
SAGARPA: Technical specifications for the
care and use of laboratory animals of the Mexican Official Standard
(NOM-062-ZOO-1999). https://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF(In
Spanish). Accessed. June 17–2015.
|
|
21
|
Quann EJ, Khwaja F, Zavitz KH and Djakiew
D: The aryl propionic acid R-flurbiprofen selectively induces
p75NTR-dependent decreased survival of prostate tumor cells. Cancer
Res. 67:3254–3262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adachi M, Sakamoto H, Kawamura R, Wang W,
Imai K and Shinomura Y: Nonsteroidal anti-inflammatory drugs and
oxidative stress in cancer cells. Histol Histopathol. 22:437–442.
2007.PubMed/NCBI
|
|
23
|
Sakonlaya D, Tapanadechopone P, Poomkokruk
A and Charoenvilaisiri S: Do NSAID's Inhibit growth of precancerous
cervical cells in vitro? J Med Assoc Thai. 95:(Suppl 1). S65–S73.
2012.PubMed/NCBI
|
|
24
|
Wang AH, Tian XY, Yu JJ, Mi JQ, Liu H and
Wang RF: Celecoxib radiosensitizes the human cervical cancer HeLa
cell line via a mechanism dependent on reduced cyclo-oxygenase-2
and vascular endothelial growth factor C expression. J Int Med Res.
40:56–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gaffney DK, Winter K, Dicker AP, Miller B,
Eifel PJ, Ryu J, Avizonis V, Fromm M, Small W and Greven K:
Efficacy and Patterns of failure for locally advanced cancer of the
cervix treated with celebrex (celecoxib) and chemoradiotherapy in
RTOG 0128. Int J Radiat Oncol Biol Phys. 69:111–117. 2007.
View Article : Google Scholar : PubMed/NCBI
|