Introduction
Hilar cholangiocarcinoma, also termed Klatskin's
tumor, is an uncommon, but not rare, malignancy that involves the
confluence of the hepatic ducts or the right or left main hepatic
ducts. Hilar cholangiocarcinoma accounts for ~60% of all
cholangiocarcinoma cases (1). En bloc
resection of the tumor, including negative histological resection
margins, is the only treatment method that results in the long-term
survival of patients (2). However,
despite hilar cholangiocarcinoma exhibiting slow growth and late
metastasis, tumor invasion of the vital hilar structures often
leads to a low rate of resectability (3). A study published by the Memorial
Sloan-Kettering Cancer Centre revealed that the rate of radical
excision of hilar cholangiocarcinoma is <30% (2). Therefore, a large proportion of patients
have lost the opportunity for curative treatment at the time of the
initial diagnosis, and undergo palliative care to alleviate the
symptoms of the disease and lengthen life.
In the previous two decades, percutaneous biliary
stenting and radiotherapy (RT) were palliative care programs that
were widely used for patients with unresectable hilar
cholangiocarcinoma (4–9). However, the risk of complications of
percutaneous biliary stenting was increased compared with the risks
of endoscopic biliary stenting (10).
By contrast, the bile duct consists of isolated segments and not
all of the bile ducts come together, so only an uncovered metallic
stent may achieve full biliary drainage. However, as a result of
tumor ingrowth or overgrowth, the insertion of an uncovered
metallic stent alone cannot yield a satisfactory stent patency or
patient survival time (11–12).
Consequently, a retrospective review of the cases of
38 patients with unresectable hilar cholangiocarcinoma that
underwent percutaneous biliary uncovered metallic stenting (UMS)
was performed. Out of the 38 patients, 25 patients underwent UMS
combined with RT, termed the UMS+RT group, and 13 patients
underwent UMS alone, termed the UMS group. The purpose of the
present study was to evaluate the safety of percutaneous biliary
stenting and to analyze whether percutaneous biliary stenting
combined with RT prolonged the stent patency and patient survival
time.
Materials and methods
Patients
In total, 106 patients with unresectable hilar
cholangiocarcinoma were treated at the Navy General Hospital
(Beijing, China) between January 2007 and December 2013, and were
identified in the present study using a database maintained by the
Department of Hepatobiliary Surgery and Liver Transplantation
Surgery of the Navy General Hospital. Unresectable hilar
cholangiocarcinoma was diagnosed by cholangioscopy with biopsy,
clinical manifestations and imaging examinations, including
magnetic resonance imaging and computed tomography. The clinical,
imaging and survival data of the patients were retrospectively
analyzed in the present study. The unresectable standard was
defined as the vital hilar structures being infringed by the tumor
and distant lymph node or viscera metastases, which was unable to
be resected using an R0 resection, or patients were unable to
tolerate surgery due to accompanying surgical
contraindications.
In total, 38 patients underwent percutaneous biliary
stenting, 1 of which succumbed within a perioperative period of 30
days, and no patients were lost to follow-up. Endoscopic stenting
was performed in 7 patients, purely external biliary drainage was
performed in 49 patients and supportive care was administered to 12
patients. According to the performance status and economic
conditions for the use of RT, 25 patients were treated using UMS
combined with RT, termed the UMS+RT group, and 13 patients were
treated using UMS alone, termed the UMS group. Percutaneous biliary
stenting was performed at the Department of Radiology of the Navy
General Hospital prior to June 2013, and was subsequently performed
by the Department of Hepatobiliary Surgery Comprehensive Treatment
Room. Informed consent was obtained from each patient and ethical
approval was obtained from the ethics committee of the Navy General
Hospital.
Percutaneous biliary stenting
Subsequent to intravenous administration of
wide-spectrum antibiotics [1.5 g cefotaxime sodium and sulbactam
sodium every 12 h (0.75 g/vial) or 0.4 g moxifloxacin hydrochloride
and sodium chloride every day (0.4 g/bottle)], percutaneous biliary
stenting was performed under moderate sedation and analgesia. Under
ultrasound guidance, the dilated peripheral hepatic biliary tree
was accessed using a 22 gage, 15-cm Neff Percutaneous Access set
(Cook Medical, Inc., Bloomington, IN, USA), and diagnostic
cholangiography was performed. A 6.0-Fr, 20-cm catheter (Cook
Medical, Inc.) was inserted into the distal obstruction, and
contrast agent [100 ml (32 g) ioversol; Covidien Canada,
Pointe-Claire, Quebec, Canada] was injected distal to the biliary
obstruction, which revealed the length of the biliary occlusion.
The guide wire (Merit Medical Systems, Inc., Jordan, UT, USA) was
placed through the malignant strictures, and a Niti-S Biliary
uncovered stent (Taewoong Medical, Gimpo, Gyeonggi, Korea) of an
appropriate length (6 or 8 mm) was advanced across the stricture
under fluoroscopy to facilitate the insertion of uncovered metallic
stents. Biliary cholangiography was then performed to determine
whether the contrast agent flowed through the stent. Once biliary
cholangiography revealed that the contrast agent flowed through the
stent, a 8.5-Fr 25-cm Multipurpose Drainage Catheter (Cook Medical,
Inc.) was inserted into the biliary tract to prevent bile leakage,
and the catheter was removed 2 weeks subsequent to surgery.
Additional percutaneous transhepatic biliary drainage was performed
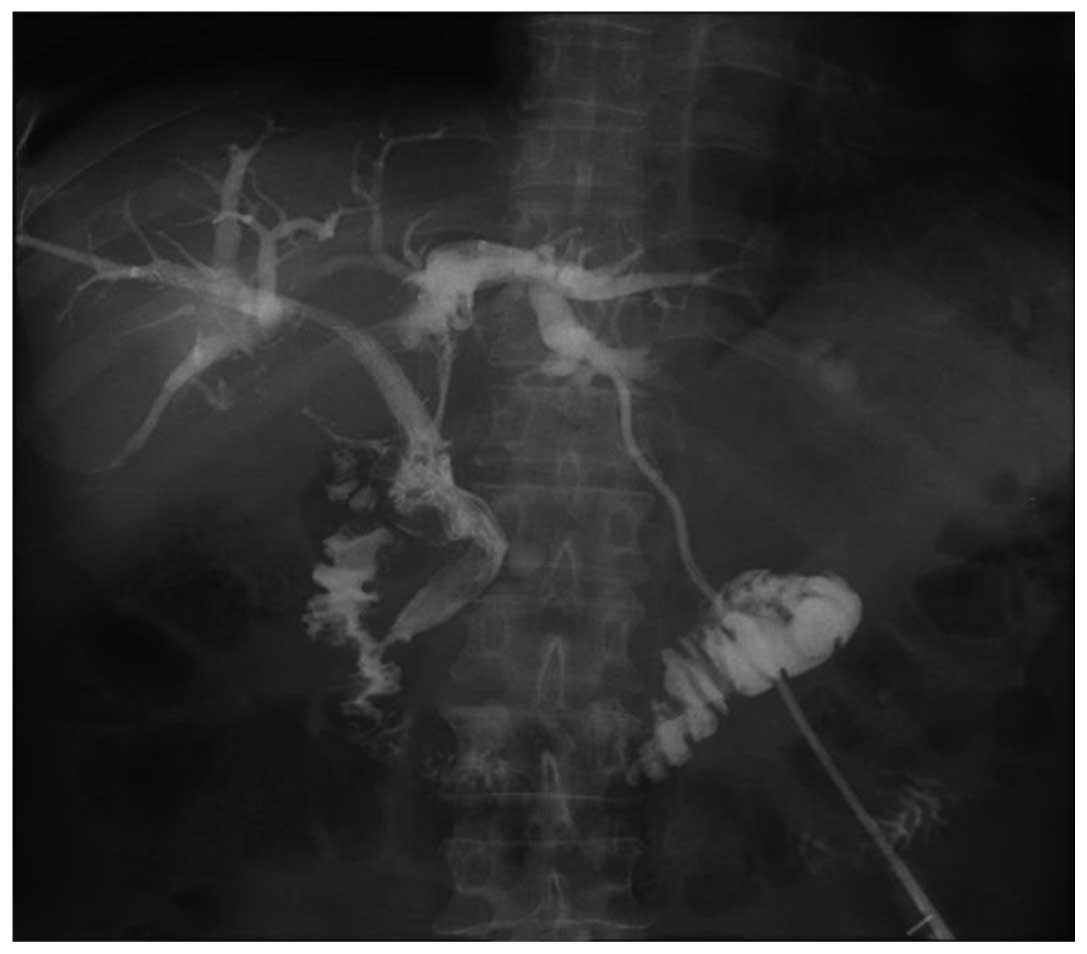
if the biliary obstruction had not improved (Fig. 1).
RT administration
RT was performed following the improvement of
obstructive jaundice subsequent to percutaneous biliary stenting.
All 25 patients received a total dose of 37.0–40.7 Gy external
radiation (3.7 Gy; 10–11 fractions). In addition, 13 patients
received supportive care due to the absence of RT administration.
Blood tests were performed up to the fifth administration of RT in
order to assess bone marrow suppression and to address the
complications rapidly.
Follow-up
The patient symptoms and biochemical test results
were assessed by the outpatient clinic, telephone follow-ups and
hospitalization records. Successful drainage was defined as a
decrease in the serum bilirubin level of >75% within 2 weeks of
stenting. Stent occlusion was defined as an increase in the
activity of hepatic-biliary enzymes, serum bilirubin level and
leucocyte count. Abdominal ultrasonography, computed tomography or
magnetic resonance imaging revealed dilatation of the intrahepatic
bile ducts. The clinical manifestation of dilatation consisted of a
body temperature >38°C and tenderness in the right upper
quadrant.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Quantitative variables were compared using Wilcoxon signed-rank
test. Qualitative variables were compared using Fisher's exact
test. The cumulative stent patency and survival curves were
analyzed by the Kaplan-Meier method and compared using the log-rank
test. The end-point events were stent occlusion and patient
survival time. Stent patency was defined as the time between stent
placement and occlusion, and the patient survival time was defined
as the time to mortality. If neither of the two events occurred by
the end of follow-up, the time interval was recorded as censored
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients
Between January 2007 and December 2013, a total of
38 patients with unresectable hilar cholangiocarcinoma underwent
percutaneous biliary stenting at the Navy General Hospital. The
patients consisted of 25 males and 13 females, with a mean age of
65.1 years (range, 41–80 years). In total, 25 patients underwent
UMS combined with RT and 13 patients underwent UMS alone. The end
of the follow-up was June 2014. The technical success rate was 100%
and successful drainage was achieved in 92.0 and 76.9% of patients
in the UMS+RT and UMS groups, respectively. However, there was no
significant difference between the two groups. In addition, there
was no significant difference in the patient age, patient gender,
Bismuth type, drainage area, pre-operative bilirubin and cancer
antigen 19-9 (CA19-9) levels between the two groups. Overall, 17
and 8 patients developed stent occlusion in the UMS+RT and UMS
groups, respectively (Table I).
 | Table I.General characteristics of enrolled
patients. |
Table I.
General characteristics of enrolled
patients.
|
| Group |
|
|---|
|
|
|
|
|---|
| Characteristics | UMS+RT, n | UMS, n | P-value |
|---|
| Total | 25 | 13 |
|
| Age |
|
|
|
| <60
years | 10 | 2 | 0.158 |
| ≥60
years | 15 | 11 |
|
| Gender |
|
|
|
| Male | 14 | 10 | 0.294 |
|
Female | 11 | 3 |
|
| Bismuth type |
|
|
|
| I–II | 8 | 6 | 0.486 |
|
III–IV | 17 | 7 |
|
| Pre-operative
bilirubin level, mg/dl | 241.4±109.5 | 268.0±131.8 | 0.511 |
| Pre-operative CA19-9
level, U/ml | 243.0±219.6 | 364.3±349.1 | 0.270 |
| Drainage area |
|
|
|
|
Unilateral | 14 | 9 | 0.501 |
|
Bilateral | 11 | 4 |
|
| Successful
drainage |
|
|
|
| Yes | 23 | 10 |
|
| No | 2 | 3 |
|
| Early
complications |
|
|
|
|
Cholangitis | 2 | 1 |
|
|
Hemobilia | 1 | 1 |
|
| Bile
leakage | 0 | 1 |
|
| Late stent
obstruction | 17 | 8 |
|
| Procedure-associated
mortality | 0 | 1 |
|
Early complications within 30
days
In total 2 patients in the UMS+RT group and 1
patient in the UMS group experienced cholangitis. Hemobilia was
experienced by 1 patient each in the UMS+RT and UMS groups. In
addition, 1 patient developed a bile leak in the UMS group, and 1
patient experienced cholangitis and hemobilia simultaneously in the
UMS group. The latter patient succumbed to the complications 26
days subsequent to receiving a stent. The complications were
treated with conservative treatment. The overall complication rate
was 15.8% (6/38) and the procedure-associated mortality rate was
2.6% (1/38) (Table I).
Stent patency and survival time
The stent patency and patient survival time were
compared between the two groups. The median stent patency was 326
days in the UMS+RT group and 196 days in the UMS group. Univariate
analysis indicated that the stent patency was significantly longer
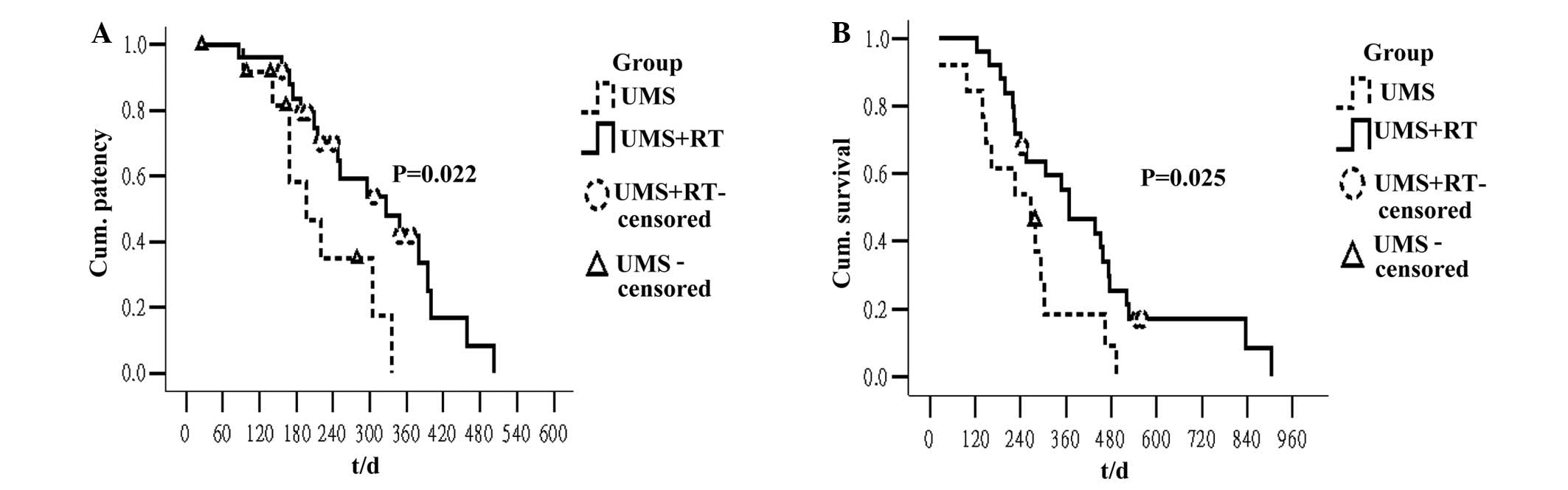
in the UMS+RT group compared with the UMS group (Fig. 2A; P=0.022). Other factors, including
patient age, patient gender, Bismuth type, drainage area, and
pre-operative bilirubin and CA19-9 levels were not associated with
the stent patency. The median survival time was 367 days in the
UMS+RT group and 267 days in the UMS group. Univariate analysis
indicated that the survival time was significantly longer in the
UMS+RT group compared with the UMS group (Fig. 2B; P=0.025). Furthermore, the survival
time was significantly longer in the patients with a CA19-9 level
<150 U/ml compared with the patients with a CA19-9 level ≥150
U/ml. Other factors did not significantly affect the survival time,
according to univariate analysis (Table
II).
 | Table II.Univariate analysis of factors
associated with stent patency and survival time in all
patients. |
Table II.
Univariate analysis of factors
associated with stent patency and survival time in all
patients.
| Variables | Total, n | Median stent patency
(95% CI) | P-value | Median survival time
(95% CI) | P-value |
|---|
| Age |
|
| <60
years | 12 | 305 (141–469) | 0.929 | 279 (0–651) | 0.427 |
| ≥60 years | 26 | 251 (122–380) |
| 302 (205–399) |
|
| Gender |
|
| Male | 24 | 305 (210–400) | 0.511 | 307 (228–386) | 0.854 |
|
Female | 14 | 215 (195–307) |
| 227 (148–306) |
|
| Bismuth type |
|
|
I–II | 14 | 305 (163–447) | 0.919 | 295 (186–404) | 0.941 |
|
III–IV | 24 | 296 (205–387) |
| 302 (225–379) |
|
| Pre-operative
bilirubin level |
|
| <10
mg/dl | 12 | 348 (266–430) | 0.201 | 349 (227–471) | 0.478 |
| ≥10 mg/dl | 26 | 215 (145–285) |
| 267 (174–360) |
|
| Pre-operative
CA19-9 level |
|
| <150
U/ml | 21 | 296 (203–389) | 0.690 | 438 (212–664) | 0.018 |
| >150
U/ml | 17 | 348 (61–635) |
| 241 (112–370) |
|
| Drainage area |
|
|
Unilateral | 23 | 296 (197–395) | 0.576 | 349 (248–450) | 0.660 |
|
Bilateral | 15 | 215 (0–442) |
| 267 (162–372) |
|
| Treatment
program |
|
|
UMS+RT | 25 | 326 (202–450) | 0.022 | 367 (228–506) | 0.025 |
|
UMS | 13 | 196 (124–268) |
| 267 (139–395) |
|
Discussion
En bloc resection of hilar cholangiocarcinoma
tumors, including negative histological resection margins, is the
only method for patients to achieve long-term survival, and the
majority of patients require resection in combination with partial
hepatectomy (2). However, tumors at
the confluence of the hepatic ducts usually involve the
contralateral second-order biliary or portal veins may not be
suitable for resection with a concomitant partial hepatectomy. In
addition, certain patients also demonstrate a surgical
contraindication (5). This results in
a small percentage of patients possessing an opportunity for
complete resection of the tumor with negative histological
resection margins. Jarnagin et al previously reported that
out of 225 patients with hilar cholangiocarcinoma treated at the
Memorial Sloan-Kettering Cancer Centre between 1991 and 2000, 65
patients possessed unresectable disease. In addition, of the 160
patients that underwent exploration with curative intent, only 62
patients underwent radical resection, and the rate of radical
excision was <30% (2). However, it
is important to provide adequate decompression for patients with
unresectable hilar cholangiocarcinoma, since effective biliary
drainage may palliate the symptoms of biliary obstruction and
improve survival times (5,6).
The endoscopic or percutaneous approach to biliary
stenting is a recognized and effective palliative treatment in
patients with malignant obstructive jaundice (13). However, the complexity of the hilar
structures and the frequent involvement of the confluence of the
hepatic ducts results in certain patients being unable to achieve
successful internal drainage through the endoscopic approach
(6). Percutaneous biliary stenting is
technically simple compared with endoscopic biliary stenting,
particularly for palliation using Bismuth type III and IV
strictures (5). Paik et al
indicated that percutaneous biliary stenting may be used as a first
treatment modality for biliary decompression in patients with
advanced type III or IV hilar cholangiocarcinoma, as the success
rate of biliary drainage in the percutaneous biliary stenting group
was increased compared with that of the endoscopic biliary stenting
group (92.7 vs. 77.3%; P=0.049) (6).
This study also revealed that successful drainage prolongs the
survival time of patients compared with the survival time of
patients with failed biliary drainage (8.7 vs. 1.8 months;
P<0.001) (6).
In the present study, it was found that the rate of
successful biliary drainage was 86.8%. However, since the patients
that demonstrated failed biliary drainage underwent percutaneous
transhepatic biliary drainage, the difference in post-operative
survival was not comparable. The risk of complications in patients
treated with percutaneous biliary stenting was increased compared
with the risk in patients treated with endoscopic biliary stenting.
The relevant literature reported that the rate of successful
biliary drainage, early complications and stent obstruction in
malignant hilar biliary obstruction were extremely similar to those
in hilar cholangiocarcinoma (5,14,15). A previous randomized trial compared
the success rate of biliary drainage, complications and overall
median survival between percutaneous and endoscopic stenting for
malignant hilar biliary obstruction (10). In the percutaneous group, the clinical
success was increased (71 vs. 42%; P=0.03), the rate of
complications was higher (61 vs. 35%; P=0.09) and the overall
median survival was significantly longer (3.7 vs. 2 months;
P=0.02). The Cox regression analysis performed in this study
revealed that percutaneous biliary stenting was the only
independent predictor of survival. These results indicated that,
although it exhibited a high rate of complications, percutaneous
biliary stenting is more suitable for patients with malignant hilar
biliary obstruction (10).
In addition, previous studies have reported that the
rate of early complications ranges between 5.7 and 28.0%, the
procedure-associated mortality rate ranges between 0 and 4%, and
the 30-day mortality rate ranges between 9 and 15%. However, the
majority of early complications may be treated conservatively, and
the 30-day mortality rate was usually associated with the
underlying disease (16–19). The findings of the present study
revealed that the rate of early complications was 15.9%, and all
complications were treated conservatively. However, a serious
complication occurred in only 1 patient, who succumbed to hemobilia
and cholangitis. The procedure-associated mortality rate was
therefore 2.6%. Thus, percutaneous biliary stenting may be
considered as a relatively safe palliative method.
The hilar bile duct is comprised of isolated
segments, and not all of the bile ducts come together, so only the
uncovered metallic stent achieves full biliary drainage (5). However, an issue that occurred
subsequent to UMS was stent obstruction, which led to patients
requiring additional intervention during the limited survival time.
Shinchi et al identified that the mean patency duration of
the expandable metallic stent alone was 3.7 months (20). Another study found that the median
stent patency was 10 weeks (21). The
primary cause of stent obstruction in the patients that undergo UMS
has been identified as tumor ingrowth into the stent mesh (13,22). This
has also been reported in a previous study that identified a longer
patency time in covered metallic stents compared with uncovered
metallic stents in the treatment of distal biliary obstruction
(23). Therefore, it is crucial to
prolong the duration of the patency to improve the quality of life
for the patients with limited life expectancy.
Although RT has frequently been recommended as the
main treatment for patients with unresectable hilar
cholangiocarcinoma (8,9,20,24,25, the
use of percutaneous biliary stenting combined with RT to treat
patients with unresectable hilar cholangiocarcinoma is rarely
reported. When the background of patients in the two groups was not
evidently different, the median stent patency was significantly
longer in the UMS+RT group than in the UMS group (326 vs. 196 days;
P=0.022). The outcome was virtually identical to prior rarely
reports (17,19). In addition to extending the stent
patency, RT is also conducive to survival following biliary
stenting. Isayama et al reported that the cumulative
survival rate in the group that underwent stenting combined with RT
was not significantly different from the cumulative survival in the
non-curative resection group (R1 group). However, the survival time
was longer in the UMS+RT group compared with the survival time of
the UMS group (22). Similarly, the
present results indicated that percutaneous biliary stenting
combined with RT may improve the survival time of patients with
unresectable hilar cholangiocarcinoma (median, 367 vs. 267 days;
P=0.025).
In summary, percutaneous biliary stenting offers a
safe and effective method for providing palliative treatment for
patients with unresectable hilar cholangiocarcinoma. Percutaneous
biliary stenting combined with RT may prolong stent patency and
patient survival time. The limitations of the present study are the
retrospective design and small sample size, and consequently, a
prospective multicenter large sample study is required to confirm
the present findings.
References
|
1
|
Rerknimitr R, Angsuwatcharakon P,
RatanachuEk T, Khor CJ, Ponnudurai R, Moon JH, Seo DW,
PantongragBrown L, Sangchan A, Pisespongsa P, et al: Asia-pacific
consensus recommendations for endoscopic and interventional
management of hilar cholangiocarcinoma. J Gastroenterol Hepatol.
28:593–607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M,
Burke EC, Bodniewicz J, Youssef M, Klimstra D and Blumgart LH:
Staging, resectability and outcome in 225 patients with hilar
cholangiocarcinoma. Ann Surg. 234:507–519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rea DJ, MunozJuarez M, Farnell MB, Donohue
JH, Que FG, Crownhart B, Larson D and Nagorney DM: Major hepatic
resection for hilar cholangiocarcinoma: Analysis of 46 patients.
Arch Surg. 139:514–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Delden OM and Laméris JS: Percutaneous
drainage and stenting for palliation of malignant bile duct
obstruction. Eur Radiol. 18:448–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singhal D, van Gulik TM and Gouma DJ:
Palliative management of hilar cholangiocarcinoma. Surg Oncol.
14:59–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paik WH, Park YS, Hwang JH, Lee SH, Yoon
CJ, Kang SG, Lee JK, Ryu JK, Kim YT and Yoon YB: Palliative
treatment with self-expandable metallic stents in patients with
advanced type III or IV hilar cholangiocarcinoma: A percutaneous
versus endoscopic approach. Gastrointest Endosc. 69:55–62. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohnishi H, Asada M, Shichijo Y, Iijima N,
Itobayashi E, Shimura K, Suzuki T, Yoshida S and Mine T: External
radiotherapy for biliary decompression of hilar cholangiocarcinoma.
Hepatogastroenterology. 42:265–268. 1995.PubMed/NCBI
|
|
8
|
Kuvshinoff BW, Armstrong JG, Fong Y,
Schupak K, Getradjman G, Heffernan N and Blumgart LH: Palliation of
irresectable hilar cholangiocarcinoma with biliary drainage and
radiotherapy. Br J Surg. 82:1522–1525. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Golfieri R, Giampalma E, Fusco F, Galuppi
A, Faccioli L, Galaverni C and Frezza G: Unresectable hilar
cholangiocarcinoma: Multimodality treatment with percutaneous and
intraluminal plus external radiotherapy. J Chemother. 16 Suppl
5:55–57. 2004.PubMed/NCBI
|
|
10
|
Piñol V, Castells A, Bordas JM, Real MI,
Llach J, Montañà X, Feu F and Navarro S: Percutaneous
self-expanding metal stents versus endoscopic polyethylene
endoprostheses for treating malignant biliary obstruction:
Randomized clinical trial. Radiology. 225:27–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DePalma GD, Pezzullo A, Rega M, Persico M,
Patrone F, Mastantuono L and Persico G: Unilateral placement of
metallic stents for malignant hilar obstruction: A prospective
study. Gastrointest Endosc. 58:50–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Indar AA, Lobo DN, Gilliam AD, Gregson R,
Davidson I, Whittaker S, Doran J, Rowlands BJ and Beckingham IJ:
Percutaneous biliary metal wall stenting in malignant obstructive
jaundice. Eur J Gastroenterol Hepatol. 15:915–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tapping CR, Byass OR and Cast JE:
Percutaneous transhepatic biliary drainage (PTBD) with or without
stenting-complications, re-stent rate and a new risk stratification
score. Eur Radiol. 21:1948–1955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stoker J, Laméris JS and van Blankenstein
M: Percutaneous metallic self-expandable endoprostheses in
malignant hilar biliary obstruction. Gastrointest Endosc. 39:43–49.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schima W, Prokesch R, Osterreicher C,
Thurnher S, Függer R, Schöfl R, Havelec L and Lammer J: Biliary
Wallstent endoprosthesis in malignant hilar obstruction: Long-term
results with regard to the type of obstruction. Clin Radiol.
52:213–219. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inal M, Akgül E, Aksungur E, Demiryürek H
and Yağmur O: Percutaneous self-expandable uncovered metallic
stents in malignant biliary obstruction. Complications, follow-up
and reintervention in 154 patients. Acta Radiol. 44:139–146. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inal M, Akgül E, Aksungur E and Seydaoğlu
G: Percutaneous placement of biliary metallic stents in patients
with malignant hilar obstruction: Unilobar versus bilobar drainage.
J Vasc Interv Radiol. 14:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi P, Bezzi M, Rossi M, Adam A, Chetty
N, Roddie ME, Iacari V, Cwikiel W, Zollikofer CL and Antonucci F:
Metallic stents in malignant biliary obstruction: Results of a
multicenter European study of 240 patients. J Vasc Interv Radiol.
5:279–285. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inal M, Akgül E, Aksungur E, Demiryürek H
and Yağmur O: Percutaneous self-expandable uncovered metallic
stents in malignant biliary obstruction. Complications, follow-up
and reintervention in 154 patients. Acta Radiol. 44:139–146. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shinchi H, Takao S, Nishida H and Aikou T:
Length and quality of survival following external beam radiotherapy
combined with expandable metallic stent for unresectable hilar
cholangiocarcinoma. J Surg Oncol. 75:89–94. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kose F, Oguzkurt L, Besen A, Sumbul T,
Sezer A, Karadeniz C, Disel U, Mertsoylu H and Ozyilkan O:
Effectiveness of percutaneous metal stent placement in
cholangiocarcinoma patients with midterm follow-up: Single center
experience. Eur J Radiol. 81:1724–1727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isayama H, Tsujino T, Nakai Y, Sasaki T,
Nakagawa K, Yamashita H, Aoki T and Koike K: Clinical benefit of
radiation therapy and metallic stenting for unresectable hilar
cholangiocarcinoma. World J Gastroenterol. 18:2364–2370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isayama H, Komatsu Y, Tsujino T, Sasahira
N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H, et al:
A prospective randomised study of ‘covered’ versus ‘uncovered’
diamond stents for the management of distal malignant biliary
obstruction. Gut. 53:729–734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Válek V, Kysela P, Kala Z, Kiss I, Tomásek
J and Petera J: Brachytherapy and percutaneous stenting in the
treatment of cholangiocarcinoma: A prospective randomised study.
Eur J Radiol. 62:175–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaiser GM, Frühauf NR, Lang H, Sauerwein
W, Sotiropoulos GC, Zöpf T, Grabellus F, Wittig A, Oldhafer KJ,
Malagó M, et al: Impact of intraoperative radiotherapy (IORT) on
survival of patients with unresectable hilar cholangiocarcinoma.
Hepatogastroenterology. 55:1951–1954. 2008.PubMed/NCBI
|