Introduction
Primary extragonadal germ cell tumors (EGCTs) are
rare, accounting for 1–3% of all GCTs (1), of which 60% are seminomas. Seminomas are
common germ cell tumors (2), with a
good prognosis and a mortality rate of 0.1% (3). The typical clinical feature of seminoma
is a progressive increase in testicular size. At present,
treatments include surgery, radiotherapy and chemotherapy (4). EGCTs can occur in the mediastinum,
thymus, retroperitoneal organs and pineal gland, while seminoma
originating in the prostate tissue is extremely rare (5). The clinical symptoms of EGCTs are
nonspecific and vary according to tumor location (2). For example, tumors of the central
nervous system, mediastinum and thymus may cause oppression
symptoms, tumors of the retroperitoneum usually present as a
painless mass (6) and in the
prostate, EGCTs may cause progressive difficulty in urination
(2). The diagnosis of primary
prostate seminoma is difficult based on clinical features alone and
thus, pathological examination is the most effective diagnostic
technique (5). EGCTs have a good
prognosis and a low rate of metastatic potential; even prostate
metastasis is uncommon (7).
The present study describes the case of a
54-year-old male with prostate seminoma who presented with symptoms
that included progressive difficulty in urination, increased
frequency of nocturia and nocturnal incontinence. Ethical approval
was obtained from the of First Affiliated Hospital of Dalian
Medical University (Dalian, China), and the patient provided
informed written consent. The patient was followed up in the First
Affiliated Hospital of Dalian Medical University from 2001
onwards.
Case report
In February 2001, a 54-year-old male patient first
presented to the First Affiliated Hospital of Dalian Medical
University due to progressive difficulty with urination, increased
frequency of nocturia and nocturnal incontinence. Findings from the
digital rectal exam indicated an enlarged prostate, with a rougher
than normal surface, non-palpable nodules and no central sulcus.
The prostate-specific antigen (PSA) level was 3.54 ng/ml (normal
range, 0–4 ng/ml). Ultrasound revealed solid masses within the
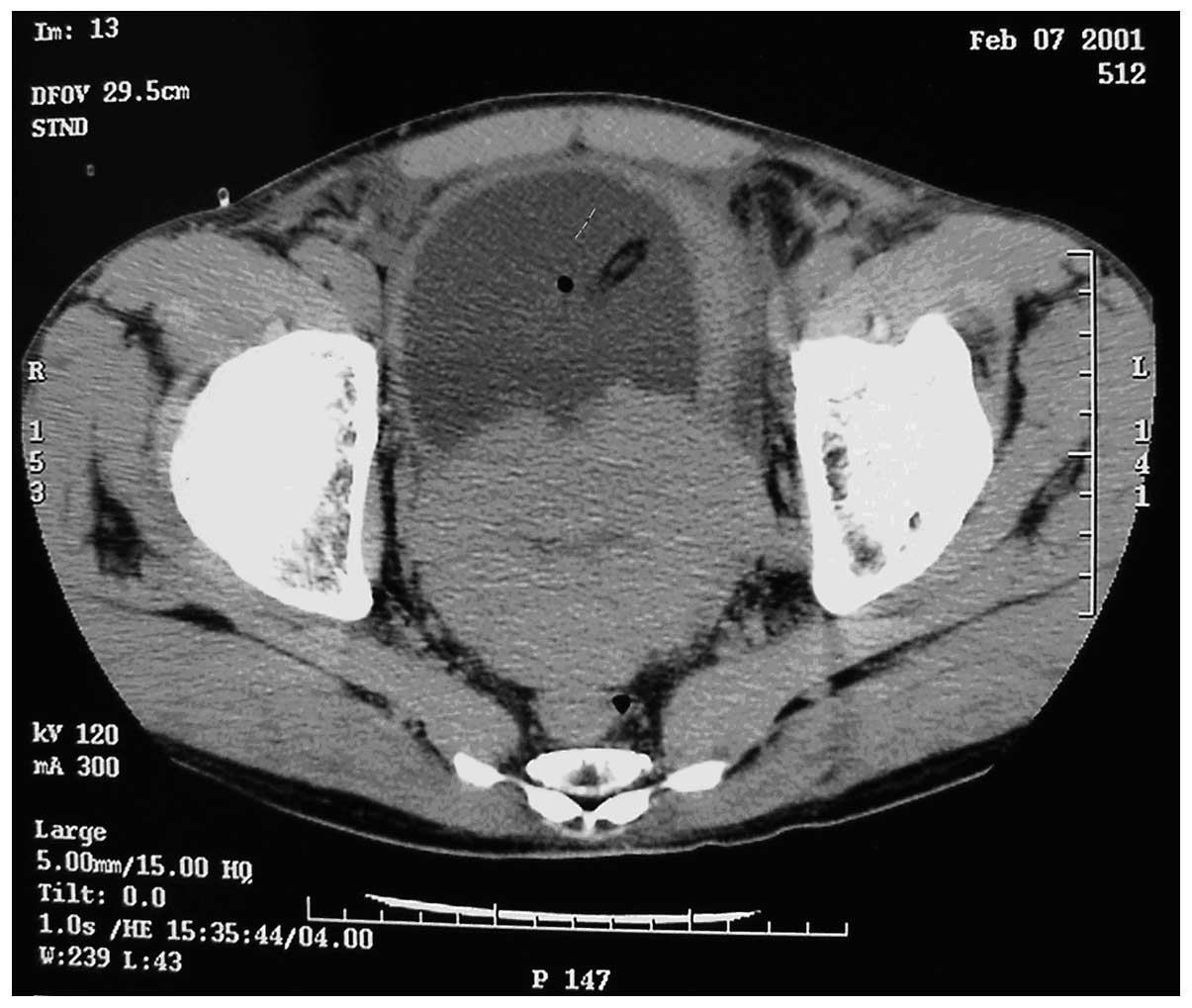
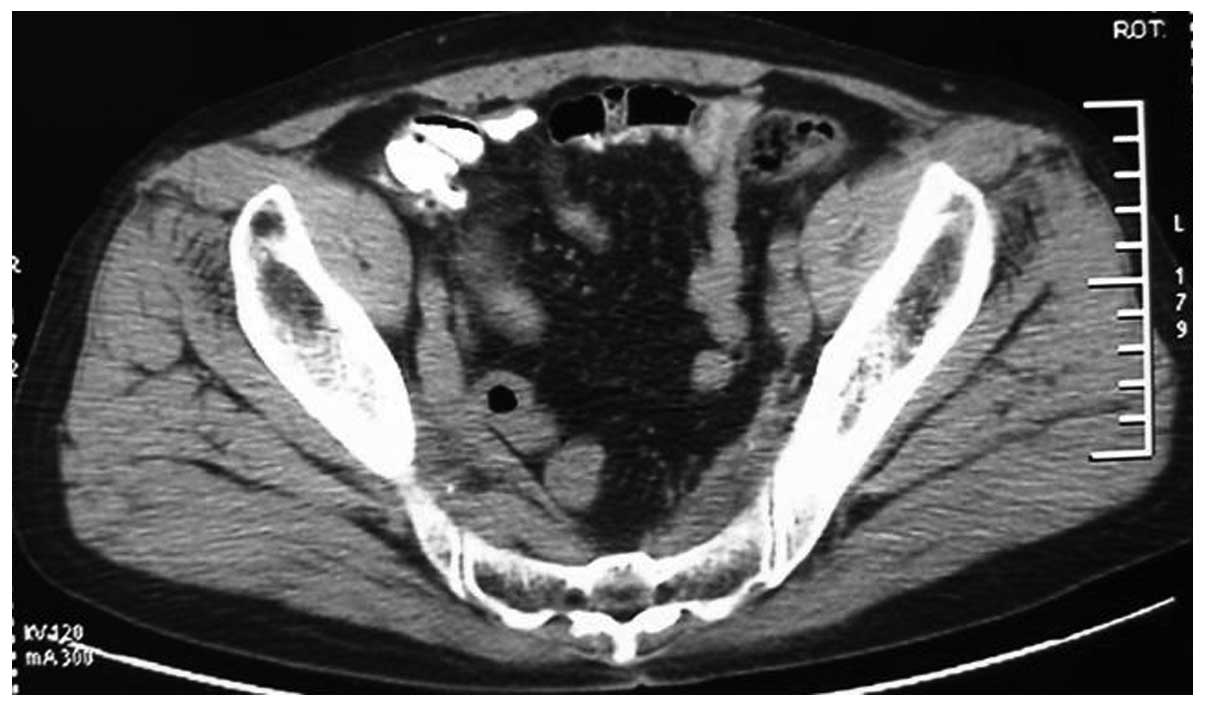
prostate suggestive of prostate cancer. Computed tomography (CT)
scans revealed small sections of low density within the prostate,
suggestive of prostate cancer, invading the bladder (Fig. 1). The tumor was 95×72×65 mm in size.
The diagnostic prostate biopsy confirmed the diagnosis of prostate
cancer; the tumor cells were arranged in tubular and cribriform
structures, and solid nests, with dense nuclear chromatin and one
or more large, irregular, distinct nuclei. On February 28, 2001,
the patient underwent a total resection of the pelvic organs,
pelvic lymph node dissection, continent detenial cecum-ascending
colic bladder and orchidectomy. The surgery was successful.
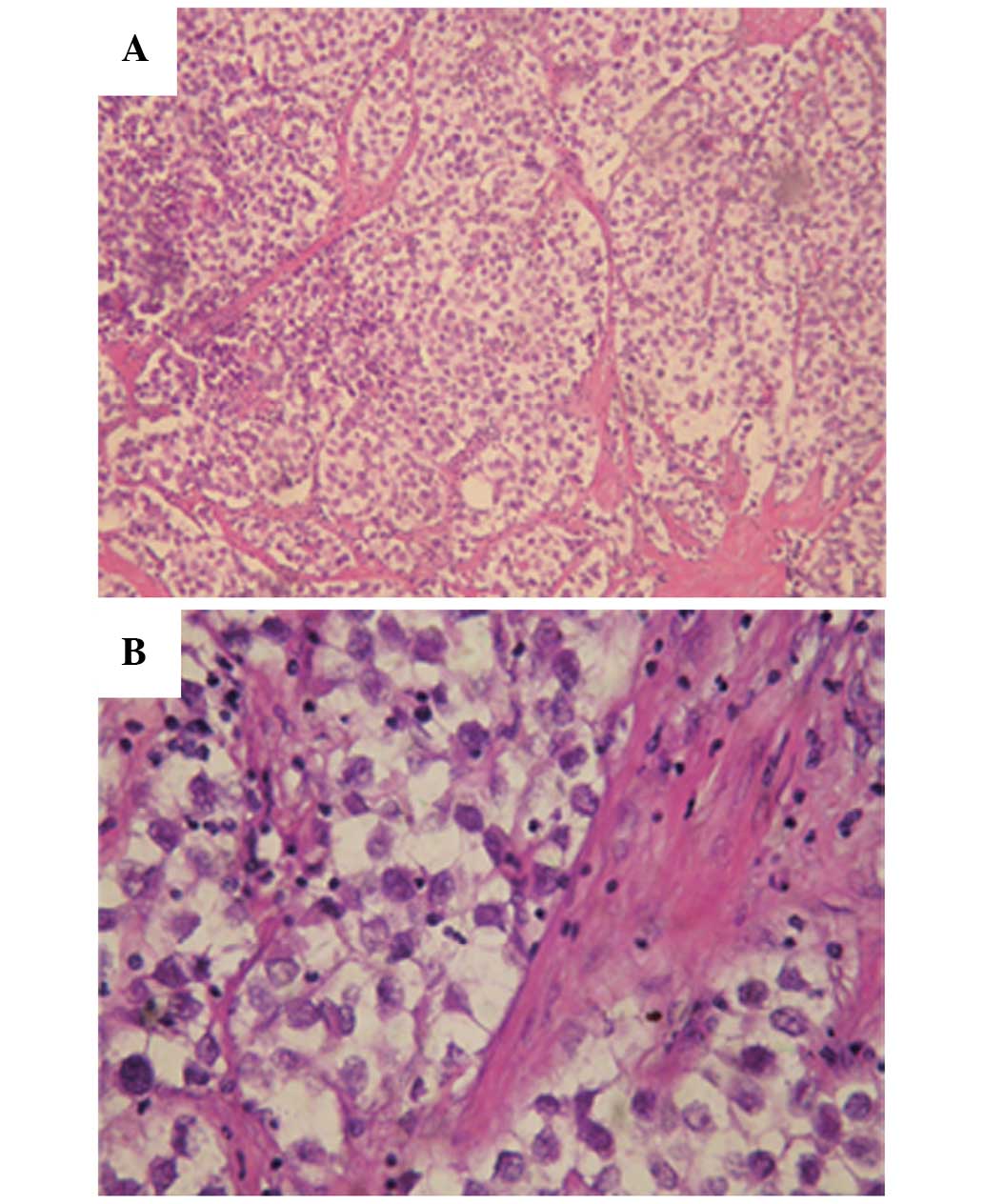
The pathology results revealed that the tumor
structure was in line with a diagnosis of seminoma (Fig. 2); the tumor was composed of large
neoplastic cells with a centrally located nucleus, containing
prominent nucleoli. The nucleus contained 1–2 nucleoli with rich
cytoplasm and stromal lymphocyte infiltration was evident. The
tumor involved the wall of the bladder, while the rectal wall and
the left ureteral stump were not involved. The lymph nodes of the
right and front left iliac vessels and seminal vesicles were
involved. Following the removal of the testicles, the pathology
report indicated that no tumor cells were present in the testicles,
and no evident disorders were identified in the lungs and abdominal
organs according to the imaging studies. Therefore, the patient was
diagnosed with primary prostate seminoma.
The patient was readmitted to the hospital after 6
months to consolidate the efficacy of intravenous chemotherapy. The
initial and consolidation chemotherapy regimens both consisted of 9
cycles of 400 mg cyclophosphamide plus 500 ml normal saline,
together with an intravenous glucose tolerance test, once a day,
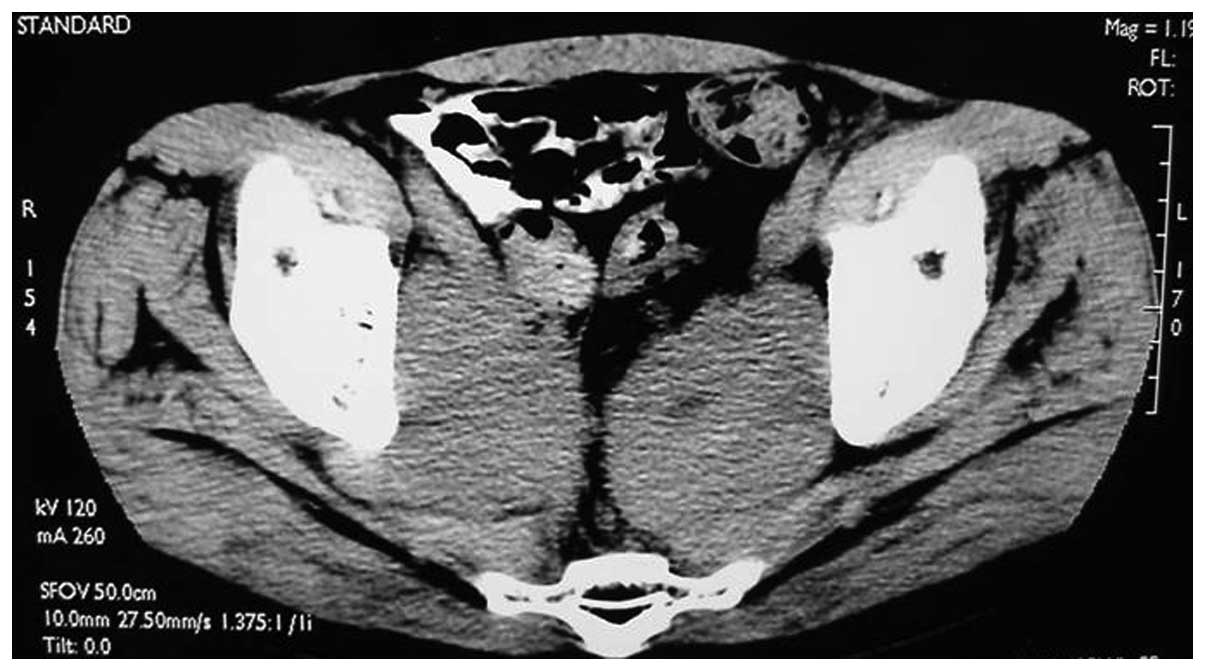
for 1 week. In June 2003, 2 years after the surgery, the pelvic CT
scan showed a pelvic mass suggestive of the metastasis (Fig. 3). The lactate dehydrogenase (LDH)
tumor marker level (745 IU/l; normal range, 100–300 IU/l) was
significantly increased, the total PSA (tPSA) and free PSA (fPSA)
levels were normal, and the β-human chorionic gonadotropin (β-HCG;
4.54 ng/ml) level was mildly raised. Subsequent to the patient
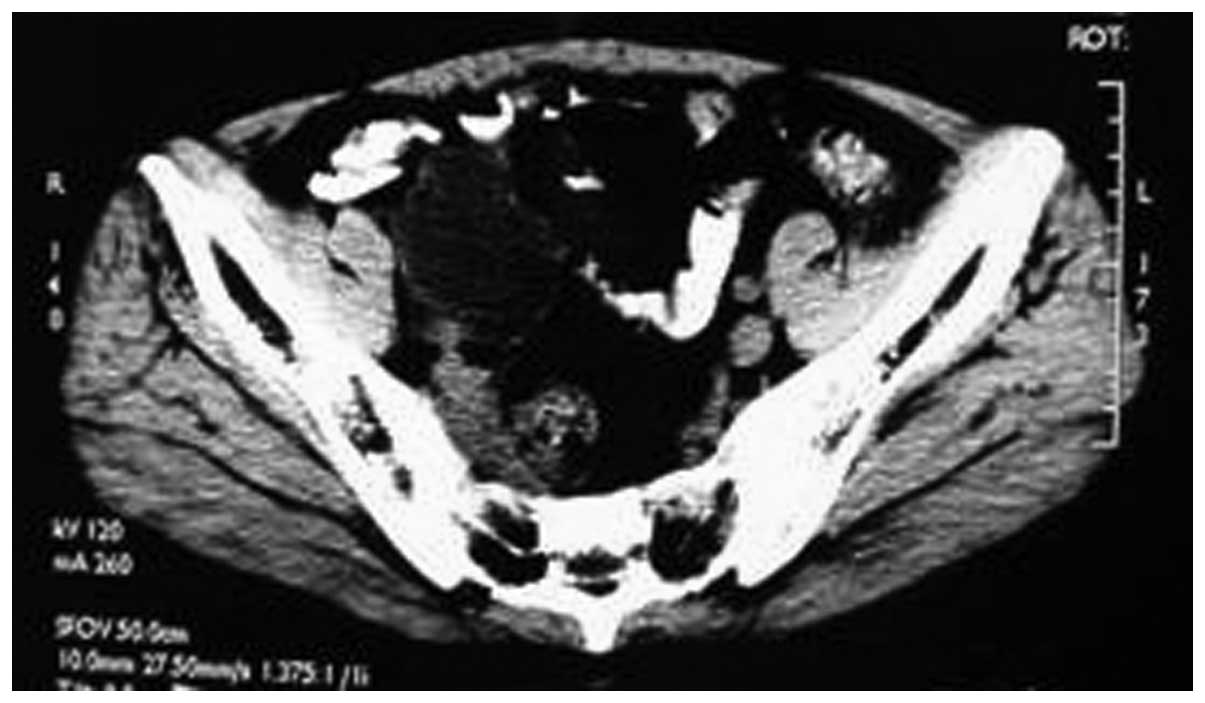
receiving cyclophosphamide chemotherapy for 1 week, the symptoms
significantly improved and a review of the pelvic CT showed a
decrease in tumor size (Fig. 4). The
patient then received a total of nine cycles of chemotherapy
regimen in line with the previous regimen. In July 2012, 11 years
after the surgery, a review of the pelvic CT showed no lumps
(Fig. 5), the chest X-ray showed no
evident abnormalities, the tests of liver and kidney function,
electrolyte levels and coagulation, and the blood routine
examination showed no abnormalities, and the associated tumor
marker levels (β-HCG, α-fetoprotein, tPSA, fPSA and LDH) were
normal. During chemotherapy, the patient occasionally suffered from
gastrointestinal symptoms, including nausea, vomiting and anorexia.
The patient's condition was improved following symptomatic
treatment. Thus far, the follow-up has indicated that the patient
eats well and exhibits no other symptoms, such as constipation.
Discussion
GCTs usually occur in the ovaries and testicles, and
account for only 1% of all malignant tumors, although it is one of
the most common malignancies among males aged 30–50 years old. In
2008, 800 new cases of testicular cancer were reported, including
390 mortalities (8). EGCTs account
for only 1–3% of GCTs. The mechanisms generally considered are as
follows: i) During embryonic development, primordial germ cells
differentiating from the yolk sac endoderm migrate to the
urogenital ridge, then reach the gonads, in which course certain
cells may remain undifferentiated. ii) During the blastocyst stage
of embryonic development, the pluripotent stem cells converting to
the germ cells shift to other organs. The vast majority of
progenitor cells in these two cases degenerate, but a few
non-degenerated cells retain the differentiation potential, and
under the stimulation of certain factors they transform into GCTs
in the future (9). The former
mechanism may explain the cancer incidence in areas such as the
mediastinum, thymus, retroperitoneal organs, pineal gland,
prostate, seminal vesicles and epididymis, through which the
primordial germ cells migrate during the embryonic phase. The
latter mechanism may explain the involvement of the liver, stomach,
lungs and other unrelated sites (9).
The etiology of primary prostate seminoma is
unclear, although there are several possibilities: The origin of
the urinary and reproductive systems is similar and the two are
closely associated with each other. The urinary and reproductive
systems begin to develop between the third and seventh week of
development. From the perspective of embryology, the prostate can
be defined as a subsidiary organ. Prostate gland cells develop from
endoderm cells. One possible inducement of primary prostate
seminoma is the translocation of the yolk sac to the gonadal ridge.
The prostatic germ cells form tumors. Another hypothesis is that
prostatic pluripotent stem cells transform neoplastic cells into
gonadal cells, which results in the formation of tumors (10).
Since cases are rare, the standard of care for
primary prostate seminoma is controversial. Similar to testicular
seminoma, with the exception of surgical treatment, extragonadal
seminoma is sensitive to radiotherapy and chemotherapy, which can
also confer significant results. Currently, common drugs selected
for chemotherapy include cisplatin, etoposide and bleomycin
(11).
The patient in the present study was found to
exhibit bladder and lymph node metastases, therefore, treatment
with radical surgery combined with subsequent chemotherapy was
selected. The surgery involved the total resection of the pelvic
organs, pelvic lymph node dissection, continent detenial
cecum-ascending colic bladder and orchidectomy in order to resect
the primary tumor and metastases completely. Continent detenial
cecum-ascending colic bladder is a safe and reliable surgical
method, with effective urinary diversion, large capacity, internal
pressure, high compliance, good urinary control, few complications
and a simple procedure. This creates an ideal intestinal
neobladder. The patient has experienced considerable quality of
life for >10 years since the surgery and the disease has not
reappeared. Therefore, the selected treatment appears a reasonable
option in the present case.
However, there are certain shortcomings, including
the results of the post-operative pathology, which are inconsistent
with the early prostate biopsy pathology. The present study
highlights that findings should be combined with biochemical tests
such as that for PSA. PSA in patients with prostate cancer is
usually higher than normal (12). If
the disease can be recognized and confirmed initially, the correct
pathological diagnosis can be obtained. The treatment is then
likely to be altered. Chemotherapy is initially used to shrink the
tumor. Subsequently, surgical treatment is performed (13). At this time, the range of the surgical
resection may be smaller and the post-operative quality of life is
improved. This therefore puts a higher demand on the pathological
diagnosis.
Testicular seminoma in situ treated with
chemotherapy post-operatively is similar. Testicular seminoma is
sensitive to radiotherapy and chemotherapy. The simple application
of radiotherapy and chemotherapy can also obtain an apparent
curative effect. The cure rate is considered to be as high as 80%
when using a good combination chemotherapy with cisplatin (11,14).
Although the short-term outcome of combination chemotherapy
containing cisplatin is promising, the long-term effect remains
unclear (15,16). The prognosis is promising, with a
five-year survival rate of 85%, and few recurrences or distant
metastases. The lung, liver and brain are the common sites of
metastasis, and recurrence and metastasis are sensitive to
chemotherapy and radiotherapy (11,14).
Cyclophosphamide was selected as the chemotherapy
drug of choice for the present patient. Cyclophosphamide is a
common antitumor and immunosuppressive agent. The chemical
mechanism mainly has two aspects: The first is the reaction of
alkylating groups with the cell functional groups, such as DNA and
RNA basic groups, or the reaction with protein functional groups,
including mercapto, amino and hydroxyl groups. The alkylating
groups take the place of the hydrogen atom leading to mismatch
between DNA intrastrand and interstrand cross-linking, chain
fractures, termination of replication and loss of protein or
physiological activity, resulting in cell apoptosis. The second is
the activation and regulation of gene expression changes induced by
several signal transduction pathways resulting in interference to
cell growth, proliferation and differentiation (17–19).
It has been confirmed that cyclophosphamide can
cause spermatogenic impairment. The mechanisms behind this are: i)
Damage to the genetic material of the germ cells, inducing the
apoptosis of spermatogenic cells; ii) interference to the function
of A-type spermatogonial cells in self-renewal, proliferation and
differentiation; iii) damage to the spermatogenesis
microenvironment, including the decrease of seminiferous tubule
cell layers, structural disorder, Sertoli cell morphological
changes, germ cell sloughing and spermatogenesis disorder; and iv)
inhibition of the activity of testis 3β-hydroxysteroid
dehydrogenase (3β-HSD) and 17β-HSD, thereby causing a reduction of
the serum testosterone level and the number of germ cells (20).
Due to the chemical mechanism of cyclophosphamide
and its suppression of spermatogenesis, this chemical treatment may
be most suitably applied to seminoma patients who have offspring
(13). In the present study,
cyclophosphamide was used as the chemotherapy drug of choice for
>10 years. The patient exhibited positive treatment effects, and
no relapse or metastasis was identified.
In conclusion, the present study shows a reasonable
and feasible treatment method using total pelvic organ resection,
pelvic lymph node dissection, continent detenial cecum-ascending
colic bladder, testicular resection and post-operative adjuvant
chemotherapy treatment with cyclophosphamide for a patient with
primary prostate seminoma who already exhibited lymph node
metastasis of the surrounding organs. The process of treatment and
the >10-year follow-up record for this patient provides a
valuable reference case and a theoretical basis for the treatment
standard of advanced primary prostate seminoma.
References
|
1
|
Bokemeyer C, Nichols CR, Droz JP, Schmoll
HJ, Horwich A, Gerl A, Fossa SD, Beyer J, Pont J, Kanz L, et al:
Extragonadal germ cell tumors of the mediastinum and
retroperitoneum: results from an international analysis. J Clin
Oncol. 20:1864–1873. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
KragJacobsen G, Barlebo H, Olsen J, et al:
Testicular germ cell tumours in Denmark 1976–1980. Pathology of
1058 consecutive cases. Acta Radiol Oncol. 23:239–247. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raghavan D: Testicular cancer: Maintaining
the high cure rate. Oncology (Williston Park). 17:218–228;
discussion, 228–229. 2003.PubMed/NCBI
|
|
4
|
Majewski W, Majewski S, Maciejewski A, et
al: Adverse effects after radiotherapy for early stage (I, IIa,
IIb) seminoma. Radiother Oncol. 76:257–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto T, Ohori M, Sakamoto N,
Matsubayashi J, Izumi M and Tachibana M: Primary seminoma of the
prostate. Int J Urol. 16:967–970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albany C and Einhorn LH: Extragonadal germ
cell tumors: Clinical presentation and management. Curr Opin Oncol.
25:261–265. 2013.PubMed/NCBI
|
|
7
|
Stein ME, Charas T, Drumea K, Sabo E and
Ben-Yosef R: Spermatocytic variant of classic seminoma: A report of
five cases and a brief review of the literature. Rambam Maimonides
Med J. 5:e00212014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters JA, Beckjord EB, Banda Ryan DR,
Carr AG, Vadaparampil ST, Loud JT, Korde L and Greene MH:
Testicular cancer and genetics knowledge among familial testicular
cancer family members. J Genet Couns. 17:351–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Utz DC and Buscemi MF: Extragonadal
testicular tumors. J Urol. 105:271–274. 1971.PubMed/NCBI
|
|
11
|
Dueland S, Stenwig AE, Heilo A, Høie J,
Ous S and Fosså SD: Treatment and outcome of patients with
extragonadal germ cell tumours-the Norwegian Radium Hospital's
experience 1979–94. Br J Cancer. 77:329–335. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wallner LP, Frencher SK, Hsu JW, Chao CR,
Nichol MB, Loo RK and Jacobsen SJ: Changes in serum
prostate-specific antigen levels and the identification of prostate
cancer in a large managed care population. BJU Int. 111:1245–1252.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alapetite C, Brisse H, Patte C, et al:
Pattern of relapse and outcome of non-metastatic germinoma patients
treated with chemotherapy and limited field radiation: The SFOP
experience. Neuro Oncol. 12:1318–1325. 2010.PubMed/NCBI
|
|
14
|
Gerl A, Clemm C, Lamerz R and Wilmanns W:
Cisplatin-based chemotherapy of primary extragonadal germ cell
tumors. A single institution experience. Cancer. 77:526–532. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Powles T, Robinson D, Shamash J, Moller H,
Tranter N and Oliver T: The long-term risks of adjuvant carboplatin
treatment for stage I seminoma of the testis. Ann Oncol.
19:443–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horwich A: Stage I seminoma and
carboplatin risks. Ann Oncol. 19:407–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Waxman DJ: Glutathione S-transferases:
role in alkylating agent resistance and possible target for
modulation chemotherapy - a review. Cancer Res. 50:6449–6454.
1990.PubMed/NCBI
|
|
18
|
Shulman LN: The biology of
alkylating-agent cellular injury. Hematol Oncol Clin North Am.
7:325–335. 1993.PubMed/NCBI
|
|
19
|
Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC,
Yeo SM, Kim T, Seo HG and Kim JH: Murine male germ cell apoptosis
induced by busulfan treatment correlates with loss of
c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS
Lett. 575:41–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schimenti KJ, Hanneman WH and Schimenti
JC: Evidence for cyclophosphamide-induced gene conversion and
mutation in mouse germ cells. Toxicol Appl Pharmacol. 147:343–350.
1997. View Article : Google Scholar : PubMed/NCBI
|