Introduction
Heterotopic pancreas (HP), also known as ectopic or
aberrant pancreas, is a congenital disorder with an occurrence rate
of 0.5–13% in the general population (1). HP has no any vascular or anatomical
communication with the normal pancreas, yet exhibiting the
histological features of pancreatic acinar formation, the
development of ducts and the presence of islets of Langerhans, with
an independent blood supply (2–4). The
heterotopic organ often remains silent, but may occasionally be
symptomatic, including gastrointestinal bleeding, gastric outlet
obstruction, gastric ulceration, pancreatitis and even malignant
degeneration (5–7).
Appearing as intraluminal protrusions with normal
overlying mucosa, HPs are usually diagnosed as submucosal tumors
(SMTs) at first during routine endoscopy. Despite the advent of
novel diagnostic modalities, including endoscopic ultrasonography
(EUS), computer tomography (CT) and even EUS-guided fine-needle
aspiration (EUS-FNA), the differentiation from a neoplasm remains a
clinical challenge (8–10). Invasive surgery or endoscopic
resection are often inappropriately performed in HP cases due to
tissue sampling errors and the difficulty in forming a
pre-operative diagnosis on imaging (11,12).
However, asymptomatic HP is benign and can be monitored long-term
without further intervention. Therefore, the ability to
pre-operatively distinguish HPs from neoplastic SMTs, such as
gastrointestinal stromal tumors (GISTs), is extremely
important.
The present study describes a case with a lesion
located on the lesser curvature of the gastric body, which was
initially diagnosed as GIST through EUS and CT, until finally being
confirmed as an HP. The study also presents a brief literature
review concerning this relatively rare disorder. Written informed
consent was obtained from the patient for the publication of this
study.
Case report
A 48-year-old female with no past medical history
was referred to Taizhou People's Hospital (Taizhou, Jiangsu, China)
on September 9, 2009, due to recurrent epigastric pain that had
persisted for more than one month. A physical examination revealed
that the patient was in good health without weight loss. The
abdomen was soft and non-tender, with no palpable mass. Moreover,
the patient's vital signs, and respiratory and vascular systems
were normal. Routine blood tests, including amylase levels,
biochemistry, and plain chest and abdominal ultrasonography, were
unremarkable. Tumor marker levels were also normal. Upper
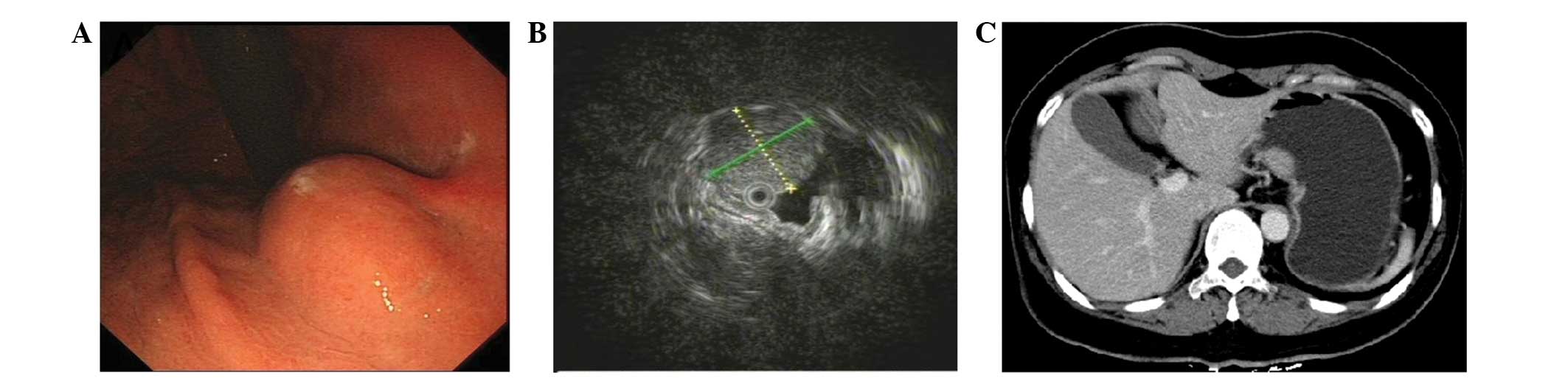
gastrointestinal endoscopy revealed a submucosal tumor-like lesion
~20 mm in diameter located on the lesser curvature of the middle
gastric body, but the mucosa itself was normal (Fig. 1A). EUS was further performed with a
radial scanning 20-MHz catheter probe being passed through the
instrument channel of a one-channel endoscope. EUS examination
demonstrated a hypoechoic lesion, 16×17 mm in size, mostly
originating from the muscularis propria (Fig. 1B). In addition, contrast-enhanced CT
revealed a mild homogeneously-enhanced mass measuring 20×15 mm in
the mid-body of the stomach (Fig.
1C). Based on these findings, the lesion was initially
suspected to be a GIST. Considering that this lesion would
subsequently be removed, either through exploratory laparoscopy or
endoscopic dissection, EUS-FNA was not performed to acquire biopsy
specimens.
The patient opted for endoscopic intervention.
Therefore, after obtaining informed consent, an endoscopic
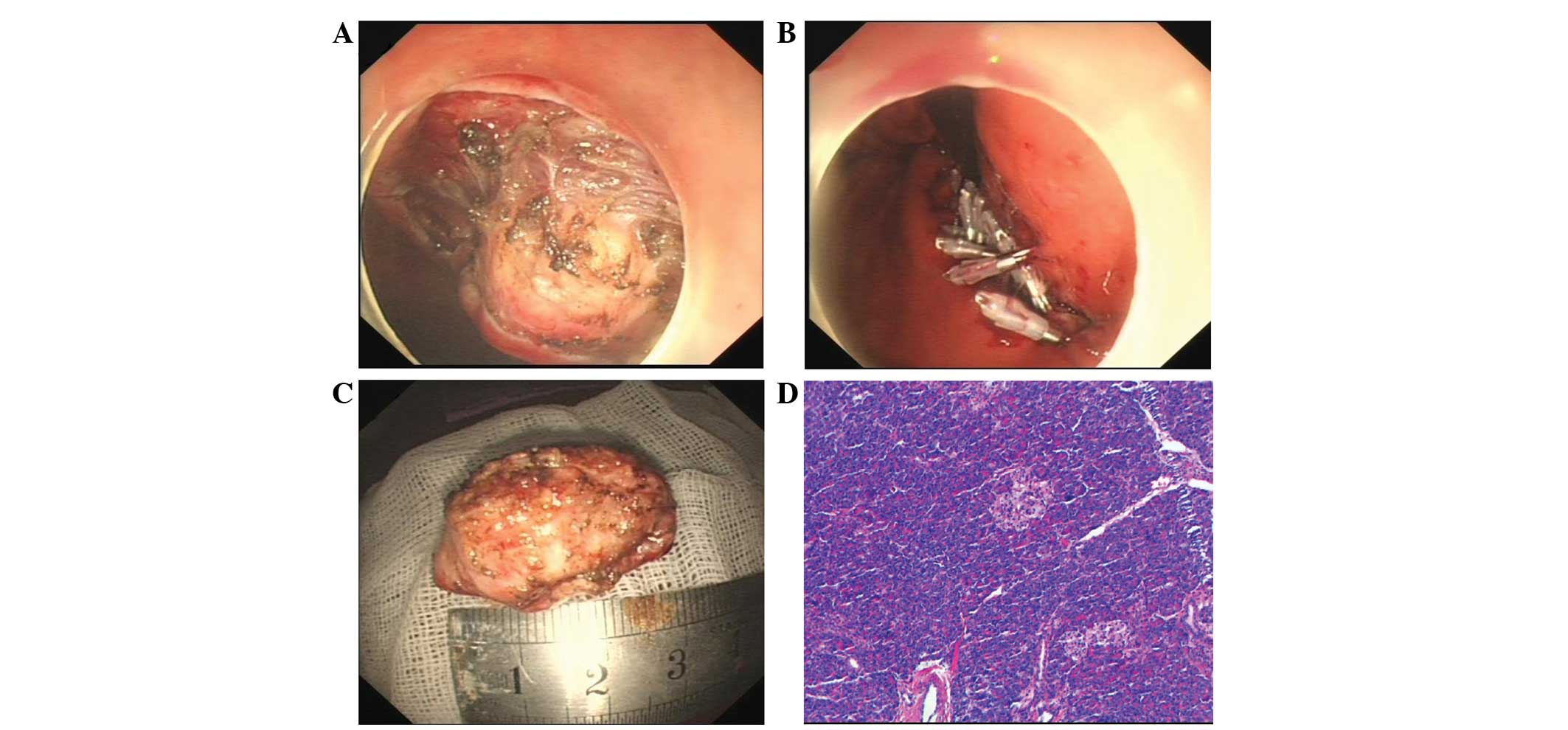
resection was performed. Firstly, the margins of the lesion were
marked by a needle knife, and a submucosal saline injection with a
small amount of epinephrine (0.025 mg/ml) and indigo carmine was
used to lift the lesion. Next, a circumferential incision in the
submucosa and a submucosal dissection around the lesion was
performed with a hook knife (Fig.
2A). Subsequent to removal, the en bloc pathological specimen
was mounted and oriented to facilitate histological examination
(Fig. 2B), and the mucosal defects
and a small perforation was sutured by combining a nylon loop with
the clips (Fig. 2C). The patient
experienced no post-operative complications and was discharged 7
days later. Unexpectedly, the histopathological examination of the
lesion revealed a final diagnosis of a gastric HP, with acinar
tissue architecture characteristic of an ectopic pancreas. The
pancreatic acinar cells were located mainly in the gastric
submucosa and partially in the muscularis propia (Fig. 2D). The overlying gastric mucosa was
normal.
Discussion
First reported by Schultz in 1729 (13), with further histological confirmation
provided by Klob in 1859 (14), HP is
defined as pancreatic tissue without any anatomical or vascular
continuity with the normal pancreas. Although its cause is unclear,
several theories, including the ‘theory of metaplasia,’ the ‘theory
of misplacement’ and the latest addition, the ‘theory of
abnormalities of notch signaling’, have been proposed to explain
the pathogenesis and occurrence of pancreatic heterotopia (15–18). The
most common site of this entity is the stomach, accounting for
25–38% of cases, followed by the duodenum (17–36%) and jejunum
(15–22%). By contrast, an HP is rarely found in the esophagus,
common bile duct, gallbladder, mesentery, spleen, mediastinum or
fallopian tubes (19). In the
stomach, >95% of lesions are found in the antrum, mainly
situated close to the greater curvature (20). However, in the present case, the
ectopic pancreas was located on the lesser curvature of the middle
gastric body, a relatively uncommon location for this rare disorder
in the stomach.
To the best of our knowledge, the pre-operative
diagnosis of an ectopic pancreas remains quite challenging despite
the advancement in diagnostic tools; this has been demonstrated by
the frequent inability to differentiate HP from neoplastic lesions
warranting surgical excision. Imaging techniques such as EUS and CT
are frequently used for gastrointestinal submucosal tumor diagnosis
and may be of use in the diagnosis of gastric HP, but are not
sufficiently specific for differential diagnosis. Endoscopically,
HP in the stomach wall has been described as an elevated
delomorphic submucosal tumor that presents with a normal overlying
mucosa, with characteristic central umbilication (3), which was not featured in the present
case. However, in more than half of recorded cases, the endoscopic
view is not that specific, for example, the central dimpling is
missing, as in the present case, and the tumor may therefore easily
be misinterpreted as another submucosal tumor, such as a GIST, as
occurred in the present case, or as another malignancy on
endoscopic examination (9,10). On EUS, HP is typically hypoechoic and
heterogeneous, with indistinct margins. HP usually arises from the
second, third and/or fourth layers of the gastrointestinal tract,
or from a combination of the three (21). Meanwhile, GISTs are typically
hypoechoic, homogeneous lesions with well-defined margins. However,
these tumors can also occasionally present with ulcerations and
irregular margins. The majority of GISTs originate from within the
muscularis propria. Small lesions may originate from the muscularis
mucosa (22). Although the imaging
features of an ectopic pancreas, including a larger
longest:shortest diameter ratio, a mural growth pattern, an antral
location, third (submucosal) layer disruption, intermediate
echogenicity and irregular margins, can occasionally aid in
distinguishing HP from other submucosal gastrointestinal tumors,
none of these findings, either individually or collectively, are
characteristic of this entity, and no long-term studies have
supported this description. Therefore, a surgical resection is
required to confirm the diagnosis in the majority of circumstances
(8,9).
The definitive diagnosis of HP can be reached by the
histopathological examination of the tissue. However, endoscopic
biopsy performed using standard biopsy forceps most often leads to
unremarkable results. Recently, the use of EUS-FNA has emerged as
an important diagnostic technique for SMT (23). However, as this method exhibits
limited diagnostic accuracy due to the small amount of tissue that
can be collected, an improved method, such as ESD-based
intervention or surgery, is required for tissue sampling (24). Histologically, based on the
classification devised by Von Heinrich et al in 1909 and the
subsequent modification by Gaspar Fuentes et al in 1973,
pancreatic heterotopia is divided into four types (25,26). Type
I shows typical pancreatic tissue with acini, ducts and islet
cells, similar to the normal pancreas. Type II shows only
pancreatic ducts, while type III has only acinar tissue and type IV
(endocrine pancreas) has only islet cells. In the present study,
the lesion was of type III, with acinar cells but no ducts or islet
cells found in the specimen.
The majority of cases of ectopic pancreases,
particularly those that are small, remain asymptomatic during the
human lifespan. However, certain lesions, particularly those
>1.5 cm, can exhibit non-specific symptoms, including abdominal
pain and fullness, nausea and vomiting, weight loss, anorexia,
anemia and melena. In certain cases, ectopic pancreases manifest as
conditions that require emergency treatment, such as
gastrointestinal bleeding, gastric outlet obstruction or
perforation (27–30). These cases require further management
involving surgery or endoscopical intervention.
There is currently an ongoing academic debate as to
whether an ectopic pancreas should be excised. Supporters advocate
the removal of this lesion as early as possible as a means of
prophylaxis due to the fact that it can be a predisposing factor
for all typical pancreatic disorders, from acute and chronic
pancreatitis to neoplastic transformation (2,3). by
contrast, antagonists prefer conservative management, with only
those patients with severe pain, actual disordered function or
emergency presentations treated by surgical intervention (31). At present, selecting the optimal
treatment is a challenge even for experienced clinicians. In our
opinion, a precise pre-operative diagnosis through imaging and
pathology, which may provide useful information, should be
performed prior to deciding on the therapeutic strategy. EUS-FNA is
useful for making an accurate histological diagnosis of the lesion,
although its value requires further assessment and future
improvements. In addition, an ESD-based method, such as that
mentioned in the study by Kobara et al (24), can overcome the flaw of no adequate
tissue samples being obtained from conventional biopsy and EUS-FNA
(32), and can deeply improve the
accuracy of diagnosing this disorder prior to management. As a
result, when diagnosed with benign lesion characteristics through
EUS and pathology, HPs, particularly the relatively small lesions
and those in asymptomatic patients, do not require treatment.
However, patients with symptoms do require further therapy. Three
methods of treatment intervention currently exist: Classic
laparotomy with wedge resection, laparoscopic sleeve resection and
endoscopic submucosal dissection (33–35), the
latter of which has been gaining in popularity over the last
decade. Traditional surgical interventions may be preferred in less
experienced centers due to the incorrect identification of ectopic
pancreases, leading to unnecessary partial or total gastrectomies
in certain patients. In the present case, an laparoscopic wedge
resection was initially planned when considering the GIST features
and relatively rare location. However, after successfully
performing a technique that combined a nylon loop with clips to
suture the mucosal defects and perforation of the stomach on
endoscopy, the experienced endoscopist successfully removed the
lesion. After seven days of follow-up, the patient did not
demonstrate any complications, including abdominal pain or a
fever.
In conclusion, HP should always be considered when
diagnosing extramucosal gastric masses. Precise pre-operative
diagnostics may avoid misleading and unnecessarily excisions.
ESD-based management in ectopic pancreases deserves further
consideration and investigation in the future.
References
|
1
|
DeBord JR, Majarakis JD and Nyhus LM: An
unusual case of heterotopic pancreas of the stomach. Am J Surg.
141:269–273. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang LX, Xu J, Wang XW, Zhou FR, Gao W,
Yu GH, Lv ZC and Zheng HT: Gastric outlet obstruction caused by
heterotopic pancreas: A case report and a quick review. World J
Gastroenterol. 14:6757–6759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Agale SV, Agale VG, Zode RR, Grover S and
Joshi S: Heterotopic pancreas involving stomach and duodenum. J
Assoc Physicians India. 57:653–657. 2009.PubMed/NCBI
|
|
4
|
Yuan Z, Chen J, Zheng Q, Huang XY, Yang Z
and Tang J: Heterotopic pancreas in the gastrointestinal tract.
World J Gastroenterol. 15:3701–3703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armstrong CP, King PM, Dixon JM and
Macleod IB: The clinical significance of heterotopic pancreas in
the gastrointestinal tract. Br J Surg. 68:384–387. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trifan A, Târcoveanu E, Danciu M, Huţanaşu
C, Cojocariu C and Stanciu C: Gastric heterotopic pancreas: An
unusual case and review of the literature. J Gastrointestin Liver
Dis. 21:209–212. 2012.PubMed/NCBI
|
|
7
|
Mehra R, Pujahari AK and Jaiswal SS:
Duodenal heterotopic pancreatic tissue: A case report and
literature review. Gastroenterol Rep Oxf. pii:guo0492014.(Epub
ahead of print).
|
|
8
|
Kim JH, Lim JS, Lee YC, Hyung WJ, Lee JH,
Kim MJ and Chung JB: Endosonographic features of gastric ectopic
pancreases distinguishable from mesenchymal tumors. J Gastroenterol
Hepatol. 23:e301–e307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Lee JM, Kim KW, Park HS, Choi JY,
Kim SH, Kim MA, Lee JY, Han JK and Choi BI: Ectopic pancreas: CT
findings with emphasis on differentiation from small
gastrointestinal stromal tumor and leiomyoma. Radiology.
252:92–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Otani Y, Yoshida M, Saikawa Y, Wada N,
Kubota T, Kumai K, Sugino Y, Mukai M, Kameyama K and Kitajima M:
Discrimination between gastric ectopic pancreas and mesenchymal
tumors, including GIST - from 12 years' surgical experience in one
institute. Aliment Pharmacol Ther. 2:292–296. 2006. View Article : Google Scholar
|
|
11
|
Lai EC and Tompkins RK: Heterotopic
pancreas. Review of a 26 year experience. Am J Surg. 151:697–700.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khashab MA, Cummings OW and DeWitt JM:
Ligation-assisted endoscopic mucosal resection of gastric
heterotopic pancreas. World J Gastroenterol. 15:2805–2808. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hunt VC and Bonsteel HTS: Meckel's
diverticulum containing aberrant pancreas. Arch Surg. 28:425–439.
1934. View Article : Google Scholar
|
|
14
|
Klob J: Pancreas accessorium. J Imperial
Royal Soc Physicians Vienna. 15:7321859.(In German).
|
|
15
|
Chandan VS and Wang W: Pancreatic
heterotopia in the gastric antrum. Arch Pathol Lab Med.
128:111–112. 2004.PubMed/NCBI
|
|
16
|
Armstrong CP, King PM, Dixon JM and
Macleod IB: The clinical significance of heterotopic pancreas in
the gastrointestinal tract. Br J Surg. 68:384–387. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weppner JL, Wilson MR, Ricca R and Lucha
PA Jr: Heterotopic pancreatic tissue obstructing the gallbladder
neck: A case report. J Pancreas. 10:532–534. 2009.
|
|
18
|
ArtavanisTsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Christodoulidis G, Zacharoulis D, Barbanis
S, Katsogridakis E and Hatzitheofilou K: Heterotopic pancreas in
the stomach: A case report and literature review. World J
Gastroenterol. 13:6098–6100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seneviratne SA, Ramanayaka IT and
Samarasekara DN: Heterotopic pancreas in the body of stomach.
Ceylon Med J. 54:57–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen SH, Huang WH, Feng CL, Chou JW, Hsu
CH, Peng CY and Yang MD: Clinical analysis of ectopic pancreas with
endoscopic ultrasonography: An experience in a medical center. J
Gastrointest Surg. 12:877–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chak A, Canto MI, Rösch T, Dittler HJ,
Hawes RH, Tio TL, Lightdale CJ, Boyce HW, Scheiman J, Carpenter SL,
et al: Endosonographic differentiation of benign and malignant
stromal cell tumors. Gastrointest Endosc. 45:468–473. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Philipper M, Hollerbach S, Gabbert HE,
Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T and
Schumacher B: Prospective comparison of endoscopic
ultrasound-guided fine-needle aspiration and surgical histology in
upper gastrointestinal submucosal tumors. Endoscopy. 42:300–305.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobara H, Mori H, Fujihara S, Nishiyama N,
Tsutsui K and Masaki T: Gastric Heterotopic pancreas can be
identified by endoscopic direct imaging with submucosal endoscopy.
J Gastrointestin Liver Dis. 22:345–348. 2013.PubMed/NCBI
|
|
25
|
VonHeinrich H: Ein peitrang zur
histrologie des sogen akzessorischen pancreas. Virchows Arch.
198:392–401. 1909. View Article : Google Scholar
|
|
26
|
GasparFuentes A, Campos Tarrech JM,
Fernández Burgui JL, Castells Tejón E, Ruíz Rossello J, Gómez Pérez
J and Armengol Miró J: Pancreatic ectopias. Rev Esp Enferm Apar
Dig. 39:255–268. 1973.(In Spanish). PubMed/NCBI
|
|
27
|
Ormarsson OT, Gudmundsdottir I and Mårvik
R: Diagnosis and treatment of gastric heterotopic pancreas. World J
Surg. 30:1682–1689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teke Z, Kabay B, Kelten C, Yilmaz M and
Duzcan E: Ectopic pancreas of the gastric antrum contiguous to a
gastrointestinal stromal tumor manifesting as upper
gastrointestinal bleeding: Report of a case. Surg Today. 37:74–77.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang YC, Chen HM, Jan YY, Huang TL and
Chen MF: Ectopic pancreas with gastric outlet obstruction: Report
of two cases and literature review. Chang Gung Med J. 25:485–490.
2002.PubMed/NCBI
|
|
30
|
Gurocak B, Gokturk HS, Kayacetin S and
Bakdik S: A rare case of heterotopic pancreas in the stomach which
caused closed perforation. Neth J Med. 67:285–287. 2009.PubMed/NCBI
|
|
31
|
Sadeghi NR, Godambe A, Shienbaum AJ and
Alloy A: Premalignant gastric heterotopic pancreas. Gastroenterol
Hepatol (NY). 4:218–221. 2008.
|
|
32
|
Kojima T, Takahashi H, ParraBlanco A,
Kohsen K and Fujita R: Diagnosis of submucosal tumor of the upper
GI tract by endoscopic resection. Gastrointest Endosc. 50:516–522.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryu DY, Kim GH, Park DY, Lee BE, Cheong
JH, Kim DU, Woo HY, Heo J and Song GA: Endoscopic removal of
gastric ectopic pancreas: An initial experience with endoscopic
submucosal dissection. World J Gastroenterol. 28(16): 4589–4593.
2010. View Article : Google Scholar
|
|
34
|
Makarewicz W, Bobowicz M, Dubowik M,
Kosinski A, Jastrzebski T and Jaskiewicz J: Endoscopic submucosal
dissection of gastric ectopic pancreas. Wideochir Inne Tech
Maloinwazyjne. 8:249–252. 2013.PubMed/NCBI
|
|
35
|
Faigel DO, Gopal D, Weeks DA and Corless
C: Cap-assisted endoscopic submucosal resection of a pancreatic
rest. Gastrointest Endosc. 54:782–784. 2001. View Article : Google Scholar : PubMed/NCBI
|