Introduction
Oral squamous cell carcinoma (OSCC) is the most
common form of head and neck cancer, accounting for ~3% of
malignancies worldwide and 500,000 newly diagnosed cases annually.
The mechanisms underlying the development of OSCC have not been
fully elucidated; however, tobacco and alcohol use are reported to
increase the risk for developing this type of cancer (1–3).
Additionally, despite the accessibility of the oral cavity for
medical examination, the majority of cases of oral cancer are only
detected at advanced stages; this is one of the reasons for the low
OSCC survival rates (4), which rarely
exceed 50% (5).
Surgery and chemotherapeutic agents, including
ifosfamide, 5-fluorouracil, taxane and methotrexate, constitute the
primary treatment approaches for this malignancy (6). However, patients eventually succumb to
the disease with the development of resistance to such agents.
Tobacco smoke produces various free radicals,
reactive oxygen species (ROS) and reactive nitrogen species (RNS),
leading to the production of hydrogen peroxide, superoxide and
nitric oxide in cells, which causes oxidative/nitrosative damage
and oxidative stress (7). Due to
their ability to induce DNA damage, ROS and RNS are crucial
determinants of the development of OSCC (3). Conversely, antioxidants may exert a
protective effect against the molecular and cellular damage that
results from the interactions of ROS and RNS (2,8).
The most frequently used natural antioxidants are
flavonoids, a group of polyphenolic compounds commonly found in
medicinal plants, vegetables, fruits and a various beverages,
including tea, coffee and wine (9).
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is the most abundant
dietary flavonoid and has been widely used for the prevention and
treatment of cardiovascular diseases and cancer (10). The cancer-preventive effects of
quercetin have been attributed primarily to its antioxidant
activity; it is able to act as a scavenger of radicals and form
complexes with metal ions and DNA (9). In addition, a number of studies have
demonstrated that quercetin may be a plausible agent for overcoming
multidrug resistance, a significant impediment to successful
chemotherapy (11).
Numerous in vitro studies have demonstrated
consistent anticancer effects of quercetin in various cell lines
and tumors, including oral cavity cancer (12,13).
Similarly, studies investigating the chemopreventive effects of
quercetin in vivo have revealed that its oral administration
may inhibit the induction of carcinogenesis, particularly in the
colon (14). When administered in the
diet, quercetin is reportedly able to prevent the initiation,
growth and/or dissemination of induced tumors in animal models
(15). However, these results are
controversial, as both a lack of an effect and inhibition of tumor
growth have been reported by independent studies (13,16).
Experimental carcinogenesis induced by
4-nitroquinoline 1-oxide (4-NQO) in mice is one of the most
frequently used animal models for the study of oral cancer
(17). In this system, the clinical,
histological and molecular changes of the oral mucosa are similar
to those observed in humans during oral carcinogenesis (18,19).
The current study aimed to examine the effect of
orally administered quercetin in 4-NQO-treated mice. The survival
rate of the treated animals, plasmatic levels of reduced
glutathione (GSH) as measure for systemic oxidative status, and the
type and severity of lesions were assessed. In addition, the
organization of the extracellular matrix (ECM) was analyzed using
carbohydrate and collagen histochemistry, and the expression of the
tumor markers proliferating cell nuclear antigen (PCNA) and mutated
p53 were assessed using immunohistochemistry.
Materials and methods
Animals and experimental design
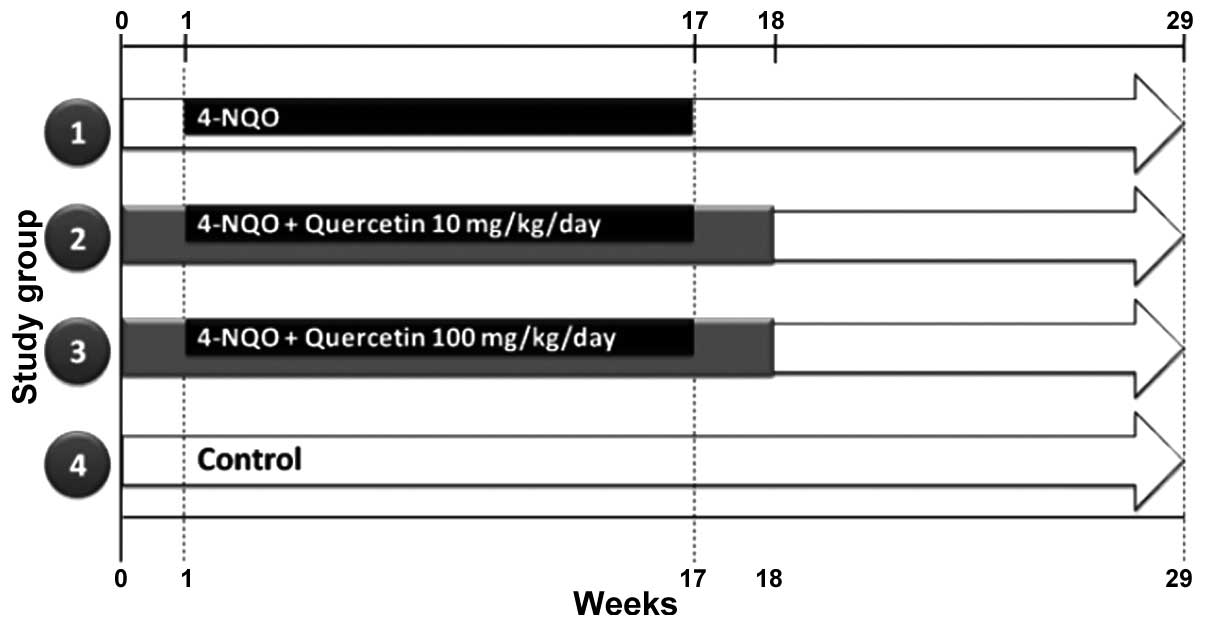
A total of 70 six-week-old, male, CF-1 mice,
obtained from the Public Health Institute of Chile (Santiago,
Chile), were maintained under controlled conditions (access to food
and water ad libitum, 12/12 h light/dark cycle, 22°C) in the
Animal Facility of the University of Talca (Talca, Chile). The mice
were randomly divided into four groups: Group 1, 4-NQO (n=20);
group 2, 4-NQO + quercetin (10 mg/kg/day) (n=20); group 3, 4-NQO +
quercetin (100 mg/kg/day) (n=20); and group 4, untreated controls
(n=10) (Fig. 1). OSCC was induced as
described previously (16,17). Briefly, mice were treated with a
solution of 100 µg/ml propylene glycol/4-NQO (Sigma-Aldrich, St.
Louis, MO, USA) in drinking water for 16 weeks. Quercetin
(Sigma-Aldrich), at a dose of 10 or 100 mg/kg/day, was administered
orally over the course of 18 weeks [1 week prior to (week 0),
during, and 1 week after the 4-NQO treatment]. At week 29 (Fig. 1), the animals were sacrificed by
cervical dislocation, their tongues were dissected, and their blood
was collected and processed for further analysis. The survival of
the animals was recorded daily.
This experimental protocol was approved by the
Bioethics Committee of the University of Talca.
Histological and histochemical
techniques
The tongues of the mice were processed for
conventional histology. Sections were stained with hematoxylin and
eosin for routine histopathological analysis, Picro Sirius
Red-hematoxylin (cat no. 365548; Sigma-Aldrich) for collagen
histochemistry (20), and periodic
acid-Schiff (PAS) for carbohydrate-containing tissue elements
(Sigma-Aldrich kit 395B). As a control for Picro Sirius Red and
PAS, 5 µm-thick sections were incubated, respectively, with a
solution of collagenase (2 µg/ml; cat no. 10103586001; Roche,
Basel, Switzerland) or α-amylase (4 µg/ml; cat no. A6380;
Sigma-Aldrich) in phosphate-buffered saline (PBS; pH 6.0), for 30
min at 37°C, prior to the histochemical reaction. A reduction in
the intensity of the Picro Sirius Red reaction or PAS staining
following enzyme treatment was considered as evidence of the
presence of carbohydrates or collagen (data not shown). In ten
randomly selected fields, the intensity of the histochemical
reaction was analyzed by two independent observers using polarized
light microscopy (Leitz Orthoplan microscope; Leica Camera AG,
Wetzlar, Germany) and the signal intensity was scored as follows:
−, absent; +, weak; ++, moderate; +++, high (21).
Diagnosis of OSCC and pre-neoplastic lesions was
performed by an oral pathologist (Mr. Daniel Droguett). The
severity of the lesions was determined according to the World
Health Organization (WHO) International Histological Classification
of Tumors and Histological Malignancy Grading System for the
Invasive Tumor Front (ITF) (22). The
resulting scores were grouped by assigning each score a value of
1–3, according to the method developed by Tumuluri et al
(23)and the ITF morphological
characteristics were compared separately, as proposed by Bryne's
Multifactorial Grading System (24).
Immunohistochemistry
Tongues were processed using standard
immunoperoxidase techniques (21) to
label PCNA (rabbit anti-mouse polyclonal IgG antibody; #sc-7907;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA; dilution, 1:100
v/v) and mutated p53 (Novocastra™ rabbit anti-mouse polyclonal
antibody (CM5); #P53-CM5P; Leica Biosystems Nussloch GmbH,
Nussloch, Germany; dilution, 1:100 v/v). The primary antibodies
were applied individually to each section for 60 min at 37°C.
Immunostaining was performed using a horseradish peroxidase-labeled
streptavidin biotin kit (R.T.U. Vectastain® Universal ABC Kit;
Vector Laboratories, Inc., Burlingame, CA, USA) following the
manufacturer's protocol, with diaminobenzidine as the chromogen.
Sections were counterstained with Mayer's hematoxylin (ScyTek
Laboratories, Inc., Logan, UT, USA) and mounted with Entellan
(Merck Millipore, Darmstadt, Germany). Immunohistochemical controls
were processed by substituting the primary antibodies with PBS, and
all of the controls were negative. For analysis of staining, ten
fields were randomly selected, the localization and intensity of
the immunoreactivity was analyzed by two independent observers
using light microscopy (BA310; Motic, Hong Kong, China) and the
signal intensity was scored as follows: −, absent; +, weak; ++,
moderate; +++, high (21).
GSH levels
At week 29 of the experimental phase, GSH levels
were measured using the method described by Beutler et al
(25), with
5,5′-dithiobis-(2-nitrobenzoic acid) (Sigma-Aldrich)as the
sulfhydryl reagent. The optical density at 432 nm was measured in a
spectrophotometer (Spectronic GENESYS™ 10S UV–Vis; Thermo Fisher
Scientific, Waltham, MA, USA). GSH levels were determined using the
molar absorption coefficient of GSH at 432 nm (1.36×104
l/mol/cm) and expressed as mg/dl.
Statistical analysis
Analysis of survival in the different groups was
performed using the Kaplan-Meier method followed by comparison of
the groups by log rank test. Quantitative data were expressed as
the mean ± standard deviation and analyzed using analysis of
variance followed by Tukey's post-test. Qualitative data were
analyzed by Fisher's exact test. SPSS software (version 14.0; SPSS,
Inc., Chicago, IL, USA) was used for all calculations and Prism
software (version 5.0; GraphPad, San Diego, CA, USA) was used for
all graphics. A statistical significance threshold of P≤0.05 was
used for all results.
Results
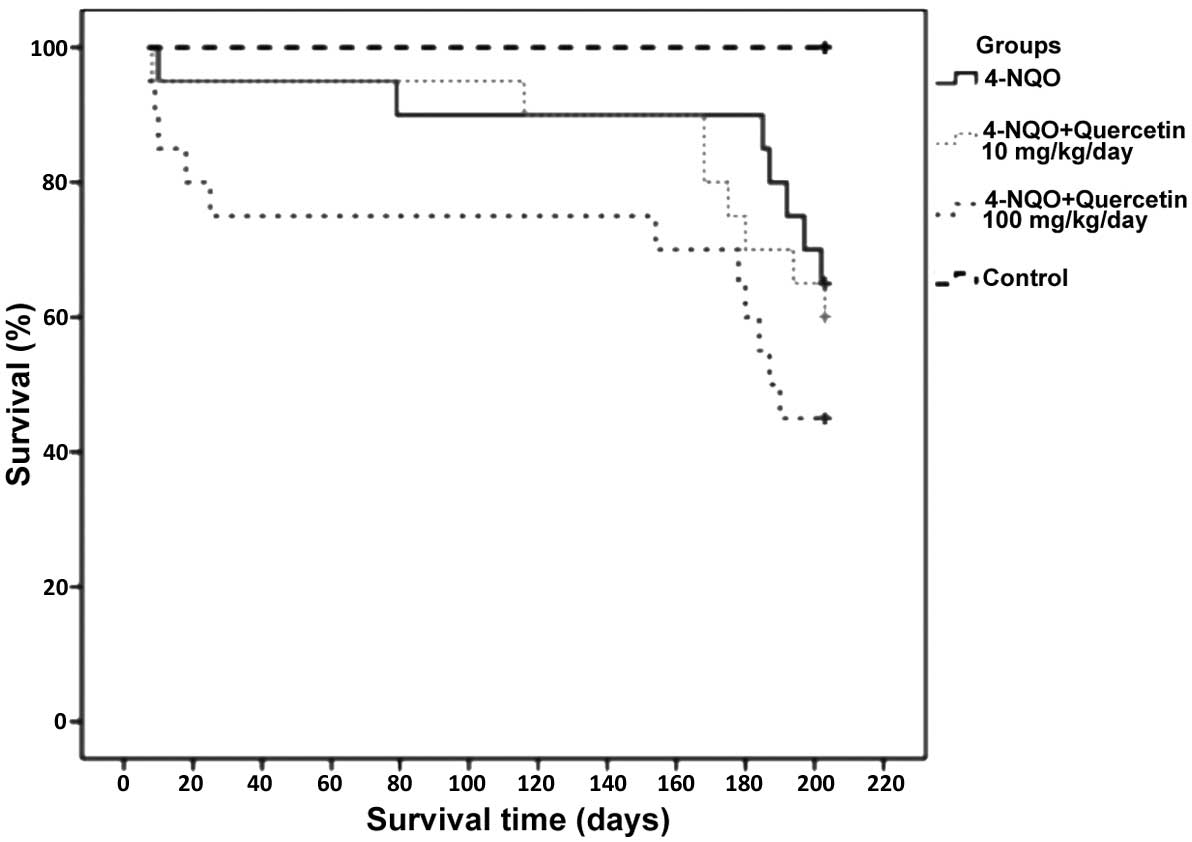
Quercetin does not improve survival in
mice treated with 4-NQO
Mice treated with 4-NQO alone or in combination with
10 or 100 mg/kg/day quercetin exhibited survival rates of 65%
(n=14), 60% (n=13) and 45% (n=9), respectively. No statistically
significant difference was identified between these groups (groups
1, 2 and 3). Notably, however, the group treated with the higher
dose of quercetin (100 mg/kg/day) had the poorest survival rate
(45%). The only statistically significant difference observed was
between the healthy, untreated control group (group 4) and each of
groups 1–3 (P≤0.05); the animals in group 4 had a survival rate of
100% (Fig. 2).
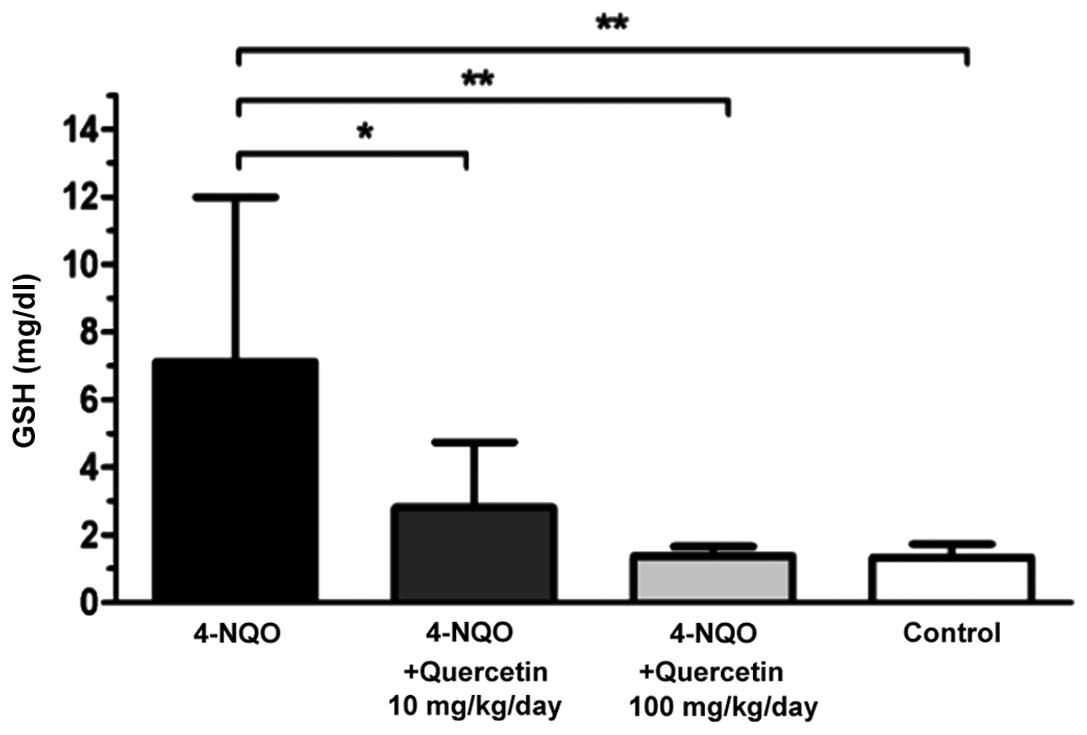
Quercetin decreases plasmatic GSH
levels in 4-NQO-treated mice
Plasmatic GSH levels were determined at the end of
the experimental phase (week 29). Healthy, untreated control mice
and animals treated with 4-NQO in combination with one of the two
doses of quercetin exhibited significantly lower levels of
plasmatic GSH compared with mice treated only with the carcinogen
(Fig. 3). Mice in the 4-NQO-treated
group (Fig. 3, black bar) exhibited a
mean plasmatic GSH level of 7.1±4.9 mg/dl, while animals treated
with 4-NQO plus 10 mg/kg/day (Fig. 3,
dark grey bar) or 100 mg/kg/day (Fig.
3, light grey bar) quercetin had a mean GSH level of 2.8±1.9
mg/dl (P≤0.05 vs. 4-NQO-only group) and 1.4±0.3 mg/dl (P≤0.01 vs.
4-NQO-only group), respectively. In the latter group, GSH levels
were similar to those of the healthy, untreated control mice
(1.3±0.4 mg/dl; Fig. 3, white
bar).
Quercetin does not decrease the
severity of pre-neoplastic lesions and OSCC
In the current study, tongues from 46 surviving
animals were analyzed. The type and severity of the lesions were
determined according to the WHO International Histological
Classification of Tumors and Histological Malignancy Grading System
for the ITF (as described by Bryne et al) (24).
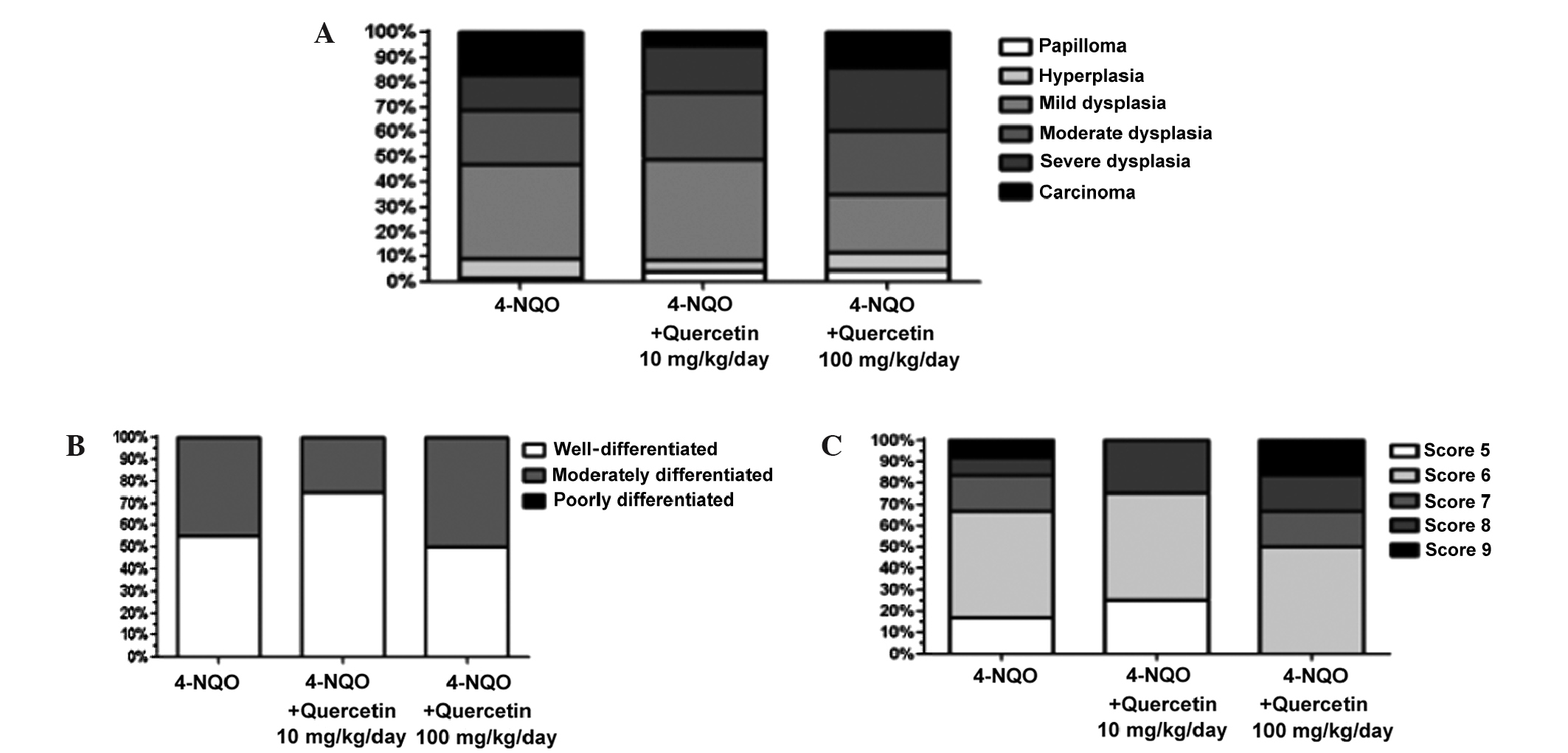
Mice that were treated with 4-NQO alone and in
combination with the different doses of quercetin (10 and 100
mg/kg/day) exhibited various pre-neoplastic lesions, including
papilloma, hyperplasia and different degrees of dysplasia, as well
as OSCC (Fig. 4A). No statistically
significant difference in the relative frequencies of these lesions
was identified between the experimental groups (P=0.339). In
healthy, untreated control mice, only healthy mucosa was observed
(data not shown).
A total of 21 lesions (Fig. 4B–C) were diagnosed as OSCC and
classified using the aforementioned classification systems. None of
the samples analyzed was diagnosed as poorly differentiated OSCC.
Additionally, there was no statistically significant difference in
the relative frequencies of moderately and well-differentiated
lesions between animals treated with 4-NQO alone or with the
carcinogen plus quercetin (P=0.724, Fig.
4B), despite a higher percentage of well-differentiated lesions
in animals treated with the lower dose of quercetin.
The OSCC scores ranged from 5–9 in the Histological
Malignancy Grading System for the ITF; these numbers are considered
standard for well- and moderately differentiated lesions.
Qualitative analysis and Fisher's exact test, performed using the
mean of the data, revealed no statistically significant difference
(P=1) between animals treated with 4-NQO alone or with the
carcinogen plus quercetin (Fig.
4C).
A histochemical analysis of the ECM in peritumoral
tissue was also performed. As expected, OSCCs, independently of the
treatment used, exhibited a lower PAS reactivity (Fig. 5B and C), particularly in the basal
lamina, and a marked disorganization of collagen I (Fig. 5E and F) relative to healthy control
mucosa (Fig. 5A and D). However,
there were no statistically significant differences [P=0.054 (PAS)
and P=0.346 (Picro Sirius Red)] between animals treated with 4-NQO
alone or with the carcinogen plus quercetin (Fig. 5C and F).
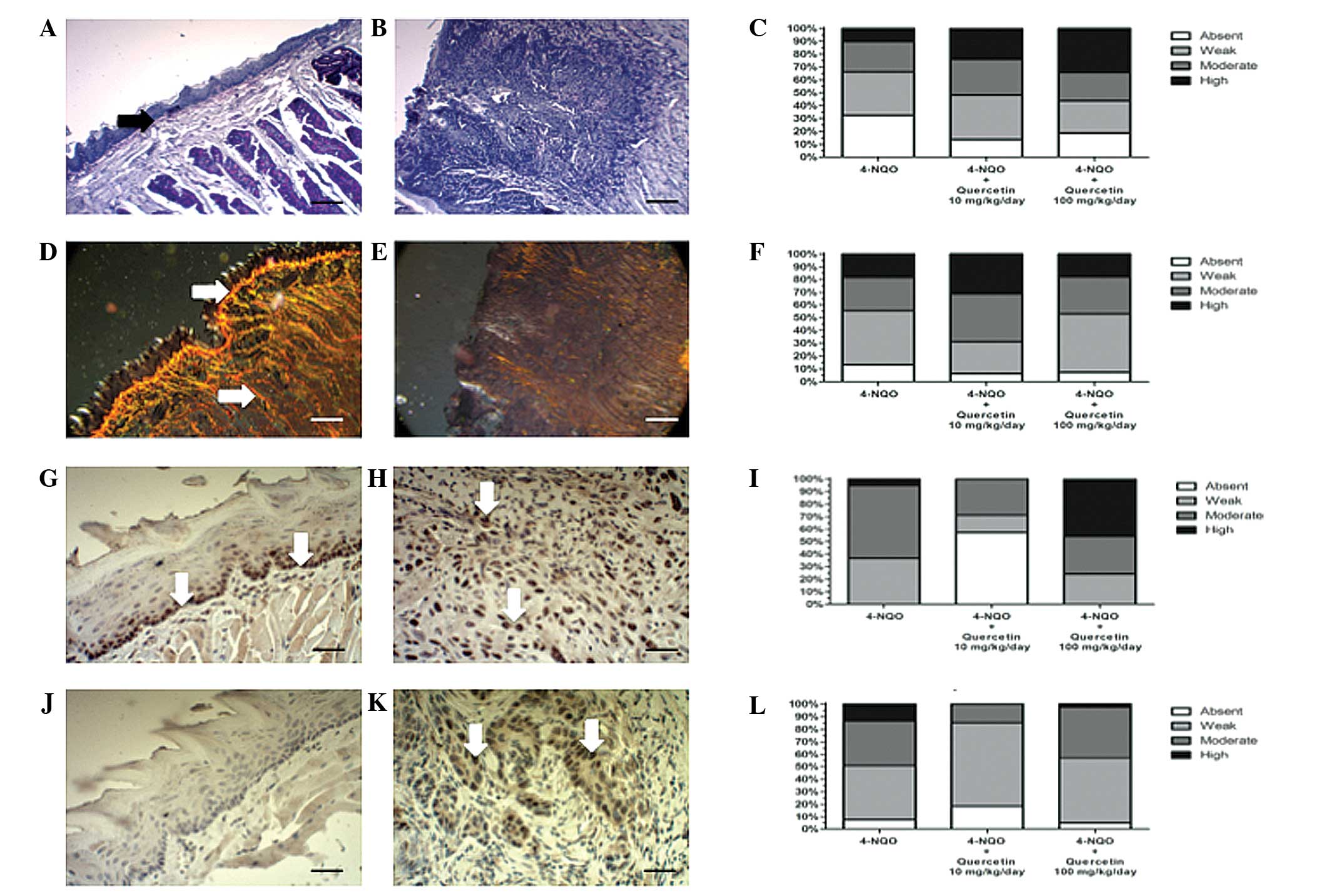 | Figure 5.Quercetin does not ameliorate changes
in the peritumoral extracellular matrix of OSCC; however, treatment
with quercetin at a low dose decreases the immunoreactivity of
tumor markers. Mice were treated with 4-NQO alone or in combination
with 10 mg/kg/day or 100 mg/kg/day quercetin. OSCCs and healthy
mucosa samples were processed for routine histochemistry and
stained with (A-C) PAS to detect glycosylated components, or with
(D-F) Picro Sirius Red for collagen histochemistry (scale bar,
25µm). Additionally, processed samples were immunohistochemically
stained for (G-I) PCNA, or (J-L) mutated p53, and counterstained
with Mayer's hematoxylin (scale bar, 25 µm). (A, D, G and J)
Representative images from healthy oral mucosa; (B, E, H and K)
representative images from moderately differentiated OSCC treated
with 4-NQO alone. In healthy tissue, (A) strong PAS reactivity is
evident, particularly at the basal lamina (black arrow) and (D)
strong collagen I staining can be observed (white arrows). In
OSCCs, the (B) PAS and (E) collagen I reactivity observed is
notably decreased. PCNA immunoreactivity in (G) healthy mucosa is
limited to the basal epithelial layer (white arrows), while in (H)
OSCC tissue, high immunoreactivity is evident throughout the entire
tumor (white arrows). (J) No immunoreactivity for mutated p53 is
evident in healthy tissue; however, in (K) OSCC tissue, p53
immunoreactivity is increased and evident throughout the entire
tumor. Graphs indicate the analysis of the intensity of
histochemical or immunohistochemical reactions from the different
experimental conditions: (C) PAS staining, (F) Picro Sirius Red
staining (collagen), (I) PCNA staining, and (L) p53 staining. No
statistically significant differences in the PAS and Picro Sirius
Red histochemical analyses were observed between the groups: Data
were analyzed using Fisher's exact test (P=0.064 and P=0.346 for
PAS and Picro Sirius Red histochemistry, respectively). Analysis of
immunohistochemistry revealed that animals treated with 4-NQO plus
the low dose of quercetin exhibited a statistically significant
decrease in the immunoreactivity of PCNA and p53 tumor markers,
relative to the other experimental conditions (P<0.001). OSCC,
oral squamous cell carcinoma; 4-NQO, 4-nitroquinoline 1-oxide; PAS,
periodic acid-Schiff; PCNA, proliferating cell nuclear antigen. |
Treatment with low-dose quercetin
decreases the immunoreactivity of tumor markers
OSCCs were analyzed for the presence of the
proliferation marker PCNA, which increases in proliferating cells
and is involved in the induction of DNA repair at the S phase cell
cycle checkpoint (26). OSCCs from
animals treated with the lower dose of quercetin (10 mg/kg/day)
displayed significantly reduced PCNA immunoreactivity compared with
animals that had received 4-NQO alone or the carcinogen plus the
higher dose of quercetin (Fig. 5I;
P≤0.001). PCNA immunoreactivity was limited to the basal layer of
the tongue epithelia in the healthy mucosa (Fig. 5G, arrows) whilst, in OSCC tissues, the
proliferation marker was observable throughout the entire lesion
(Fig. 5H, arrows).
Another important tumor marker is the mutant variant
of p53, a tumor suppressor protein that is normally expressed in
the presence of DNA damage (27).
Immunohistochemical analysis of mutated p53 in OSCC revealed
increased immunoreactivity in all groups treated with 4-NQO,
including with and without quercetin (Fig. 5J), compared with that of healthy
tongue mucosa, where the expression of this protein was absent
(Fig. 5K). OSCCs from animals that
received the lower dose of quercetin exhibited significantly less
intense immunoreactivity of p53 compared with those treated with
the carcinogen alone or with the carcinogen plus the higher dose of
quercetin (P≤0.05; Fig. 5L).
Discussion
The high incidence and mortality of OSCC, as well as
the limited treatment modalities currently available for this
cancer, increases the urgency to develop novel therapies for these
patients (1). Cancer chemoprevention,
defined as long-term intervention with natural or synthetic
molecules to prevent, inhibit or reverse carcinogenesis, is
increasing in importance, with a number of clinical trails
conducted thus far (28) and may
provide complementary therapeutic strategies. The flavonoid
quercetin is considered to be the prototypical naturally occurring
chemopreventive agent, as its biological activities
(anti-proliferative, anti-inflammatory, antioxidant, proapoptotic
and anti-angiogenic) may act at various stages of carcinogenesis,
from initiation to invasion and metastasis. Additionally, this
molecule may affect various genetic, biochemical and immunological
factors that underlie the development and maintenance of tumors
(10).
There are a number of promising studies regarding
the anticancer effect of quercetin in oral carcinoma cell lines
(29) and animal models (16,30,31).
However, studies of in vivo models of different types of
OSCCs are controversial (15).
The use of 4-NQO is a valuable technique for
inducing OSCC, and induces carcinogenesis in animal models in a
manner similar to the natural progression of OSCC in humans
(17,18). 4-NQO is a water-soluble quinoline
derivative and is known to form DNA adducts. Additionally, it is
able to induce oxidative DNA damage and chromosomal breakage
(32).
During carcinogenesis, cells acquire various
properties that have been designated as the ‘hallmarks of cancer’
(33). The acquisition of these
properties is a consequence of changes in biochemical signal
transduction pathways resulting from the activation of oncogenes
and the inactivation of tumor suppressor genes (33). It has been hypothesized that quercetin
is able to interfere with different aspects of the ‘hallmarks of
cancer’, and this drug has therefore been proposed to be a
potential multi-target inhibitor with pleiotropic and synergistic
effects in tumor cells (10).
The cancer-preventive effects of quercetin have been
attributed primarily to its antioxidant activity, a property that
is facilitated by its chemical structure. Quercetin contains a high
number of hydroxyl groups and conjugated π orbitals, by which this
flavonoid can donate electrons or hydrogen, as well as scavenge
hydrogen peroxide and superoxide anions. The reaction of quercetin
with superoxide anions leads to the generation of semiquinone
radicals and hydrogen peroxide. Quercetin also reacts with hydrogen
peroxide in the presence of peroxidases, thus decreasing hydrogen
peroxide levels and protecting cells against oxidative damage
(28). However, in the present study,
quercetin was not found to exert significant positive effects
against 4-NQO-induced OSCC in mice. Mice treated with the
carcinogen alone or in combination with either dose of quercetin
exhibited similar mortality rates and severity of pre-neoplastic
lesions and OSCC. It is important to note that quercetin, as a
potent antioxidant, becomes oxidized to generate quercetin-quinone
with its tautomeric forms. Similarly to other semiquinone radicals
and quinones, quercetin-quinone is toxic due to its ability to
arylate protein thiols (34).
Protection against quercetin-quinone may be provided by GSH, which
forms transient adducts (6-glutathionyl-quercetin) that possess an
extremely short half-life and which rapidly dissociate into GSH and
quercetin-quinone (10). This
observation suggests that, with a low GSH concentration,
quercetin-quinone trapping may be inefficient, and
quercetin-quinones may therefore freely react with other thiol
groups, such as protein sulfhydryls (10), or DNA. GSH levels are decreased
following prolonged exposure to quercetin, suggesting an inability
of quercetin to cope with ROS for extended periods. As a
consequence, the pro-oxidant effect of quercetin may be greater
than its antioxidant effect (28). In
the current study, the administration of quercetin over 16 weeks
was confirmed to decrease plasmatic levels of GSH in a
dose-dependent manner. Notably, the decrease in GSH was observed 18
weeks after final administration of quercetin; therefore, it is
more probable that quercetin acts as a pro-oxidant in the current
model of experimental carcinogenesis.
However, the antioxidant activity of quercetin is
not its only mechanism of action; quercetin also interacts with
different proteins (e.g. Bcl-2 proteins and caspases), directly or
indirectly, to inhibit survival signaling cascades, including
phosphoinositide 3-kinase/Akt, mitogen-activated protein kinases
and protein kinase C pathways. This promotes the release of
cytochrome c and the activation of caspases, thereby
triggering apoptotic cell death. Additionally, quercetin is able to
interact with cell cycle regulatory proteins and trigger G2/M phase
cell cycle arrest in vitro; in human cervical cancer (HeLa)
cells, this effect appears to be mediated through the activation of
p53 (13). In the present study, the
proliferation markers PCNA and p53 displayed significantly
decreased immunoreactivity in the OSCC tissues of mice treated with
the lower dose of quercetin.
Another proposed mechanism of action of quercetin
involves the entry of the flavonoid into epithelial cells and its
concentration in the mitochondria and nucleus. In the cytosol,
quercetin disrupts the actin cytoskeleton and inhibits cellular
proliferation and migration. In the nucleus, the transcription of
various genes associated with different cellular processes may be
modified by quercetin; such processes include cell motility, cell
cycle regulation, xenobiotic metabolism, immune-related factors and
transcription (35). However, as
mentioned previously, only the immunoreactivity of PCNA and p53 was
diminished, and Histological Malignancy Grading System for the ITF
scores and changes in tumor-adjacent ECM were not improved in
quercetin-treated mice compared with 4-NQO-only-treated mice. This
result may indirectly indicate that cell motility and invasion
capacity were not affected by quercetin.
An additional explanation of the failure of
quercetin in the current model may be related to its
bioavailability. Many phytochemicals are poorly absorbed, and the
unabsorbed fraction typically undergoes metabolism and rapid
excretion (36). Quercetin molecules
are differentially glycosylated in food sources, and the adsorption
of quercetin glycosides is almost double that of its corresponding
aglycon (10), which was administered
in the present study. However, as quercetin induced a
dose-dependent decrease of plasmatic GSH levels in this study
(Fig. 3), it may be assumed that the
animals absorbed a sufficient quantity of the flavonoid.
The current results suggest that, despite a
promising effect of the flavonoid reported in previous studies
(in vitro or in vivo), quercetin, at the doses
assayed, is ineffective as a chemopreventive agent. However,
interpretation of these results must take account of the fact that
4-NQO does not induce poorly differentiated OSCC. Therefore,
considering the effect of the low dose of this flavonoid on tumor
marker expression, it is important to investigate the effect of
quercetin at lower doses, as well as on more severe lesions.
Acknowledgements
This study was supported by National Fund for
Scientific and Technological Development grants no. 1120230 (to Dr
Ulrike Kemmerling), no. 1120096 (to Dr Guillermo
Schmeda-Hirschmann), and no. 1110054 (to Mrs. Cristina Theoduloz),
and by the ‘Fondo para la Realización de Tesis del Programa de
Magister en Ciencias Biomédicas de la Facultad de Ciencias de la
Salud de la Universidad de Talca’.
References
|
1
|
Bai LY, Weng JR, Hu JL, Wang D, Sargeant
AM and Chiu CF: G15, a GPR30 antagonist, induces apoptosis and
autophagy in human oral squamous carcinoma cells. Chem Biol
Interact. 206:375–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Zha L, Li B, Zhang L, Yu T and Li
L: Correlation between superoxide dismutase 1 and 2 polymorphisms
and susceptibility to oral squamous cell carcinoma. Exp Ther Med.
7:171–178. 2014.PubMed/NCBI
|
|
3
|
Lin WJ, Jiang RS, Wu SH, Chen FJ and Liu
SA: Smoking, alcohol and betel quid and oral cancer: A prospective
cohort study. J Oncol. 2011:5259762011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hollows P, McAndrew PG and Perini MG:
Delays in the referral and treatment of oral squamous cell
carcinoma. Br Dent J. 188:262–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taghavi N and Yazdi I: Prognostic factors
of survival rate in oral squamous cell carcinoma: Clinical,
histologic, genetic and molecular concepts. Arch Iran Med.
18:314–319. 2015.PubMed/NCBI
|
|
6
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ames BN and Gold LS: Endogenous mutagens
and the causes of aging and cancer. Mutat Res. 250:3–16. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korde SD, Basak A, Chaudhary M, Goyal M
and Vagga A: Enhanced nitrosative and oxidative stress with
decreased total antioxidant capacity in patients with oral
precancer and oral squamous cell carcinoma. Oncology. 80:382–389.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dolatabadi JE: Molecular aspects on the
interaction of quercetin and its metal complexes with DNA. Int J
Biol Macromol. 48:227–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Russo M, Spagnuolo C, Tedesco I, Bilotto S
and Russo GL: The flavonoid quercetin in disease prevention and
therapy: Facts and fancies. Biochem Pharmacol. 83:6–15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borska S, Chmielewska M, Wysocka T,
DragZalesinska M, Zabel M and Dziegiel P: In vitro effect of
quercetin on human gastric carcinoma: Targeting cancer cells death
and MDR. Food Chem Toxicol. 50:3375–3383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang JW, Kim JH, Song K, Kim SH, Yoon JH
and Kim KS: Kaempferol and quercetin, components of Ginkgo biloba
extract (EGb 761), induce caspase-3-dependent apoptosis in oral
cavity cancer cells. Phytother Res. 24 Suppl 1:S77–S82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dajas F: Life or death: Neuroprotective
and anticancer effects of quercetin. J Ethnopharmacol. 143:383–396.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami A, Ashida H and Terao J:
Multitargeted cancer prevention by quercetin. Cancer Lett.
269:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harwood M, DanielewskaNikiel B, Borzelleca
JF, Flamm GW, Williams GM and Lines TC: A critical review of the
data related to the safety of quercetin and lack of evidence of
in vivo toxicity, including lack of genotoxic/carcinogenic
properties. Food Chem Toxicol. 45:2179–2205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dayan D, Hirshberg A, Kaplan I, Rotem N
and Bodner L: Experimental tongue cancer in desalivated rats. Oral
Oncol. 33:105–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rivera CA, Droguett DA, Kemmerling U and
Venegas BA: Chronic restraint stress in oral squamous cell
carcinoma. J Dent Res. 90:799–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang XH, Knudsen B, Bemis D, Tickoo S and
Gudas LJ: Oral cavity and esophageal carcinogenesis modeled in
carcinogen-treated mice. Clin Cancer Res. 10:301–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Junqueira LC, Bignolas G and Brentani RR:
Picrosirius staining plus polarization microscopy, a specific
method for collagen detection in tissue sections. Histochem J.
11:447–455. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castillo C, López-Muñoz R, Duaso J,
Galanti N, Jaña F, Ferreira J, Cabrera G, Maya JD and Kemmerling U:
Role of matrix metalloproteinases 2 and 9 in ex vivo Trypanosoma
cruzi infection of human placental chorionic villi. Placenta.
33:991–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pindborg JJ, Reichert PA, Smith CJ and van
de Waal I: Histological typing of cancer and precancer of the oral
mucosaWorld Health Organization Histological Classification of
Tumours. 2nd. Springer; Berlin: pp. 11–12. 1997
|
|
23
|
Tumuluri V, Thomas GA and Fraser IS:
Analysis of the Ki-67 antigen at the invasive tumour front of human
oral squamous cell carcinoma. J Oral Pathol Med. 31:598–604. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bryne M, Boysen M, Alfsen CG, Abeler VM,
Sudbø J, Nesland JM, Kristensen GB, Piffko J and Bankfalvi A: The
invasive front of carcinomas. The most important area for tumour
prognosis? Anticancer Res. 18:4757–4764. 1998.PubMed/NCBI
|
|
25
|
Beutler E, Duron O and Kelly BM: Improved
method for the determination of blood glutathione. J Lab Clin Med.
61:882–888. 1963.PubMed/NCBI
|
|
26
|
Myoung H, Kim MJ, Lee JH, Ok YJ, Paeng JY
and Yun PY: Correlation of proliferative markers (Ki-67 and PCNA)
with survival and lymph node metastasis in oral squamous cell
carcinoma: A clinical and histopathological analysis of 113
patients. Int J Oral Maxillofac Surg. 35:1005–1010. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Fukumoto M and Liu D: Prognostic
significance of p53 immunoexpression in the survival of oral
squamous cell carcinoma patients treated with surgery and
neoadjuvant chemotherapy. Oncol Lett. 6:1611–1615. 2013.PubMed/NCBI
|
|
28
|
Gibellini L, Pinti M, Nasi M, Montagna JP,
De Biasi S, Roat E, Bertoncelli L, Cooper EL and Cossarizza A:
Quercetin and cancer chemoprevention. Evid Based Complement
Alternat Med. 2011:5913562011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SF, Nien S, Wu CH, Liu CL, Chang YC
and Lin YS: Reappraisal of the anticancer efficacy of quercetin in
oral cancer cells. J Chin Med Assoc. 76:146–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makita H, Tanaka T, Fujitsuka H, Tatematsu
N, Satoh K, Hara A and Mori H: Chemoprevention of 4-nitroquinoline
1-oxide-induced rat oral carcinogenesis by the dietary flavonoids
chalcone, 2-hydroxychalcone and quercetin. Cancer Res.
56:4904–4909. 1996.PubMed/NCBI
|
|
31
|
Priyadarsini RV, Vinothini G, Murugan RS,
Manikandan P and Nagini S: The flavonoid quercetin modulates the
hallmark capabilities of hamster buccal pouch tumors. Nutr Cancer.
63:218–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ribeiro FA, de Moura CF, Gollucke AP,
Ferreira MS, Catharino RR, Aguiar O Jr, Spadari RC, Barbisan LF and
Ribeiro DA: Chemopreventive activity of apple extract following
medium-term oral carcinogenesis assay induced by
4-nitroquinoline-1-oxide. Arch Oral Biol. 59:815–821. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gliszczyńska-Świglo A and Oszmianśki J:
Antioxidant and prooxidant activity of food componentsFood Oxidants
and Antioxidants: Chemical, Biological, and Functional Properties.
Bartosz G: CRC Press; Boca Raton, FL: pp. 375–432. 2013
|
|
35
|
Notas G, Nifli AP, Kampa M, Pelekanou V,
Alexaki VI, Theodoropoulos P, Vercauteren J and Castanas E:
Quercetin accumulates in nuclear structures and triggers specific
gene expression in epithelial cells. J Nutr Biochem. 23:656–666.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnson IT: Phytochemicals and cancer.
Proc Nutr Soc. 66:207–215. 2007. View Article : Google Scholar : PubMed/NCBI
|