Introduction
Lung cancer is the primary cause of
cancer-associated mortality worldwide (1,2). The most
prominent etiology of lung cancer is smoking, which is responsible
for 80% of cases (3). In addition,
non-small cell lung cancer (NSCLC) accounts for ~85% of lung cancer
cases (2). The disease is usually
diagnosed at advanced stage, resulting in poor overall survival
rates. Treatment options for lung cancer include surgery, radiation
therapy and chemotherapy (4).
Although chemotherapy remains an important form of treatment, novel
drug development has focused on molecular targeted therapies, which
may enable the use of specific treatments based on a tumor's
genetic alterations. The most common somatic mutations in NSCLC are
located in the epidermal growth factor receptor
(EGFR) and Kirsten-rat sarcoma oncogene homolog
(KRAS) genes (5,6).
One of the first molecules successfully used as a
target for molecular therapies was EGFR. The application of
first-generation tyrosine kinase (TK) inhibitors (TKI) Gefitinib and
Erlotinib was demonstrated to confer improved response and survival
outcomes in patients with mutations in the TK domain of the
EGFR gene (exons 18–21) (7–10).
Numerous clinicopathological factors have been
associated with EGFR and KRAS mutations, including
gender, smoking history and histology (11,12). In
addition, it was reported that EGFR mutation frequency in
NSCLC patients was ethnicity-dependent, with an incidence rate of
~30% in Asian populations and ~15% in Caucasian populations.
However, limited data has been reported on intra-ethnic differences
throughout Europe.
KRAS mutations are also present in a high
percentage of NSCLC patients and are associated with poorer
prognosis and resistance to EGFR-TKIs. However, the extent to which
this may influence treatment selection remains to be elucidated
(13–15). In addition, KRAS mutation
frequency and mutation spectrum have been suggested to be
influenced by smoking habits (16).
Current guidelines recommend testing all patients
with metastatic NSCLC adenocarcinomas for the presence of
activating EGFR mutations; in addition, these guidelines
suggest the use of EGFR-TKIs as first-line therapy in patients with
adenocarcinoma and a known EGFR mutation (17). Thus, accurate mutation detection is
crucial for appropriate treatment selection. The most commonly used
method for EGFR mutation testing was considered to be Sanger
sequencing (18,19). However, this method has various
disadvantages, since it is considered a laborious technique with
limited sensitivity. Thus, this method may lead to false negative
results when the mutation percentage or the tumor cell content in
the material used is low.
In order to resolve these issues, a variety of
methods are currently available for EGFR mutational testing.
These methods include quantitative polymerase chain reaction
(PCR)-based assays, pyrosequencing, high-resolution melting curve
(HRM) analysis and peptide nucleic acid-PCR clamp, denaturing
high-performance liquid chromatography and next-generation
sequencing (NGS) assays (18). These
methods all have different advantages and disadvantages; therefore,
the use of multiple techniques for EGFR mutation testing may
increase EGFR testing accuracy. In addition, when biased
results are obtained from one method, the use of an alternative
method may be useful in order to confirm the presence of a
mutation. The aim of this study was to determine the frequency and
spectrum of EGFR mutations in a group of Greek NSCLC
patients. Additionally, KRAS mutation analysis was performed
in patients with known smoking history to determine the correlation
of type and mutation frequency with smoking.
Materials and methods
Patients
A total of 1,472 tumors from Greek patients with
newly diagnosed NSCLC were analyzed for mutations in EGFR exons 18,
19, 20 and 21. All available clinical factors, including age,
gender, histology and smoking history, were evaluated. The age of
diagnosis was known for 1,046 patients, pathological reports were
available for 497 patients and smoking history was available for
561 patients. Based on their smoking status, patients were
categorized as non-smokers (<100 cigarettes in their lifetime),
ex-smokers (quit ≥5 year ago) or smokers (quit <1 year ago). For
the 561 with known smoking history, KRAS exon 2 analysis was also
performed. Informed consent was obtained from all patients prior to
testing. This study was approved by the ethics committee of ‘Agii
Anargiri’ Cancer Hospital (Athens, Greece).
DNA extraction and mutation
analysis
DNA extraction was performed using 10-µm-thick
sections of formalin-fixed and paraffin-embedded (FFPE) tissue
samples. For all samples, pathological review and macro-dissection
were performed in order to confirm a tumor cell content of >75%.
The tumor area was determined through comparison with the
corresponding hematoxylin and eosin stained slide. A NucleoSpin
Tissue kit (Macherey-Nagel, Düren, Germany) was used for DNA
extraction according to the manufacturer's instructions.
EGFR exons 18, 19, 20 and 21, as well as
KRAS exon 2 mutation analysis were performed using HRM
analysis. HRM is a sensitive scanning method used for rapid and
reliable mutation screening in human cancers. PCR cycling and HRM
analysis were performed on the Rotor-Gene 6000™ (Corbett Research,
Mortlake, Australia). The intercalating dye used was SYTO 9
(Invitrogen Life Technologies, Carlsbad, CA, USA). In brief, PCR
assays were performed in a 25-µl reaction volume containing 100 ng
genomic DNA, 1X PCR buffer (Qiagen Inc., Valencia, CA, USA), 2.5
mmol/l MgCl2 (Qiagen Inc.), 200 nmol/l each primer
(Invitrogen Life Technologies), 200 µmol/l each deoxynucleotide
(New England Biolabs, Inc., Ipswich, MA, USA), 5 µmol/l SYTO 9
(Invitrogen Life Technologies), 1.25 Units HotStarTaq (5 U/µl;
Qiagen Inc.) and PCR grade water (Invitrogen Life
Technologies).
Primers for all exons were previously described
(19,20) except for the reverse primer of the
EGFR exon 20, which was designed using the primer-BLAST
software (http:www.ncbi.nlm.nih.gov/tools/primer-blast). The PCR
conditions were as follows: Initial denaturation at 95°C for 15
min, followed by 40 cycles of 15 sec at 95°C, 10 sec at 68–58°C
(decrease of 1°C/cycle for the first 10 cycles) and 30 sec at 72°C.
For the HRM melting profile, samples were denatured with an initial
hold at 95°C for 1 sec and a melting profile from 72–95°C rising by
0.2°C every 1 sec. All HRM reactions were performed in
triplicate.
Sequencing analysis
In order to perform the Sanger sequencing reaction,
a NucleoFast® 96 PCR Clean-up kit (Macherey-Nagel GmbH and Co.,
Düren, Germany) was used, according to the manufacturer's
instructions, to purify the PCR amplification products.
Subsequently, 7 µl purified product was used for each sequencing
reaction, which was performed using the BigDye® Terminator v1.1
Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA).
Sequencing reaction products were purified prior to electrophoresis
using the Montage™ SEQ96 Sequencing Reaction kit (EMD
Millipore Corp., Billerica, MA, USA). Sequencing analysis was
performed on an Applied Biosystems 3130 Genetic Analyzer (Applied
Biosystems).
Targeted NGS assay
TruSeq Custom Amplicon Library Preparation
(Illumina, Inc., San Diego, CA, USA) allows targeted sequencing of
the genomic regions spanning upwards of 600 kb with up to 1,536
amplicons in a single multiplex reaction.
A pool of custom upstream and downstream primers
were designed using the web-based sequencing assay design tool
Design Studio (Illumina, Inc.) (http:designstudio.illumina.com/). The oligos were
specific for amplification of specific regions involved in somatic
mutations in different types of cancer (Table I). In total, 17 targets were
amplified, using 42 amplicons, according to the manufacturer's
protocol.
 | Table I.Targets for next generation
sequencing assay, chromosomal location, length of the amplified
regions and number of amplicons per target. |
Table I.
Targets for next generation
sequencing assay, chromosomal location, length of the amplified
regions and number of amplicons per target.
| Target | Chromosome:
start-stop | Length (bp) | Amplicons |
|---|
|
NRAS_Exon_2258071±0 | 1:115, 256,
459–115, 256, 546 | 88 | 1/1 |
|
NRAS_Exon_2255597±0 | 1:115, 258,
671–115, 258, 798 | 128 | 1/1 |
|
NRAS_Exon_2255265±0 | 1:115, 252,
190–115, 252, 349 | 160 | 2/2 |
|
KRAS_Exon_2084598±0 | 12:25, 398, 208–25,
398, 329 | 122 | 2/2 |
|
KRAS_Exon_2081588±0 | 12:25, 380, 168–25,
380, 346 | 179 | 3/3 |
|
KRAS_Exon_2081272±0 | 12:25, 378, 548–25,
378, 707 | 160 | 2/2 |
|
KIT_Exon_1948841±0 | 4:55, 592, 023–55,
592, 216 | 194 | 3/3 |
|
KIT_Exon_1925438±0 | 4:55, 593, 582–55,
593, 708 | 127 | 2/2 |
|
KIT_Exon_1923956±0 | 4:55, 594, 177-55,
594, 287 | 111 | 1/1 |
|
KIT_Exon_1923106±0 | 4:55, 599, 236–55,
599, 358 | 123 | 2/2 |
|
HRAS_Exon_1850745±0 | 11:533, 441–534,
375 | 935 | 11/11 |
|
EGFR_Exon_2086565±0 | 7:55, 242, 415–55,
242, 513 | 99 | 1/1 |
|
EGFR_Exon_2085900±0 | 7:55, 241, 614–55,
241, 736 | 123 | 2/2 |
|
EGFR_Exon_2085577±0 | 7:55, 248, 986–55,
249, 171 | 186 | 3/3 |
|
EGFR_Exon_2084815±0 | 7:55, 259, 412–55,
259, 567 | 156 | 3/3 |
|
BRAF_Exon_2290211±0 | 7:140, 481,
376–140, 481, 493 | 118 | 2/2 |
|
BRAF_Exon_2290058±0 | 7:140, 453,
075–140, 453, 193 | 119 | 1/1 |
In brief, the custom oligos pool was hybridized to
genomic DNA samples. The excess of unbound oligos was removed from
genomic DNA using a filter suitable for size selection (Illumina,
Inc.). Three additional wash steps ensured complete removal of
unbound oligos and prepared samples for the extension-ligation
step. During the extension-ligation step the hybridized upstream
and downstream oligos were connected. This was achieved using a DNA
polymerase (Illumina, Inc.) that extended from the upstream oligo
through the targeted region, followed by ligation to the 5′ end of
the downstream oligo using a DNA ligase (Illumina, Inc.). The
extension-ligation resulted in the formation of products containing
the targeted regions of interest flanked by sequences required for
amplification. The extension-ligation products were amplified using
primers that add sample multiplexing index sequences (i5 and i7) as
well as common adapters required for cluster generation (P5 and P7)
(Illumina, Inc.).
Subsequently, the PCR products were purified from
the other reaction components using Agencourt AMPure XP PCR
purification system (Beckman Coulter, Inc., Brea, CA, USA) and the
quantity of each library was normalized using normalization
additives, beads and wash solution (Illumina, Inc.) to ensure more
equal library representation in the pooled sample. Finally, equal
volumes of normalized library were combined, diluted in
hybridization buffer (Illumina, Inc.) and heat denatured at 96°C
for 2 min prior to sequencing on the MiSeq sequencer (Illumina,
Inc.). NGS data analysis was performed using the genomics computing
environment VariantStudio version 2.2 (Illumina, Inc.).
Sensitivity
The sensitivity test was performed using genomic DNA
reference standards with defined allelic frequencies (Horizon
Diagnostics, Cambridge, UK).
DNAs heterozygous (allele frequency, 50%) for
EGFR mutations p.L858R (exon 21) and A746-E750del (exon 19)
were diluted with wild-type DNA in order to obtain a mutant to
wild-type allelic ratio of 50, 12.5, 7.5 and 5%. These samples were
used to determine the sensitivity of the HRM and sequencing
methods. Calculation of NGS sensitivity, was performed using two
EGFR Multiplex Reference Standards (Horizon Diagnostics)
that cover mutations at codons 719 (p.G719S), 746–750
(A746-E750del), 790 (p.T790M), 858 (p.L858R) and 861 (p.L861Q)
spanning exons 19, 20 and 21. These standards were manufactured
using five engineered EGFR mutant cell lines (Horizon
Diagnostics) and mixed to generate 5 and 1% mutant EGFR
allelic frequencies. Additionally, a third Quantitative Multiplex
FFPE Reference Standard (Horizon Diagnostics) was used. This
standard covered mutations at codons 719 (p.G719S), 746–750
(A746-E750del), 790 (p.T790M), 858 (p.L858R), with mutant
EGFR allelic frequencies of 24.5, 2, 1 and 3%,
respectively.
Statistical analysis
Statistical analysis was performed using Fisher's
exact or χ2 tests. P<0.05 was considered to indicate
a statistically significant difference between values. Statistical
analysis was performed with the MedCalc software v.12.7.2 (MedCalc
Software bvba, Ostend, Belgium).
Results
Sensitivity tests
Using HRM, it was determined that the mutant EGFR
A746-E750del allele frequency in wild-type DNA was 5% and mutant
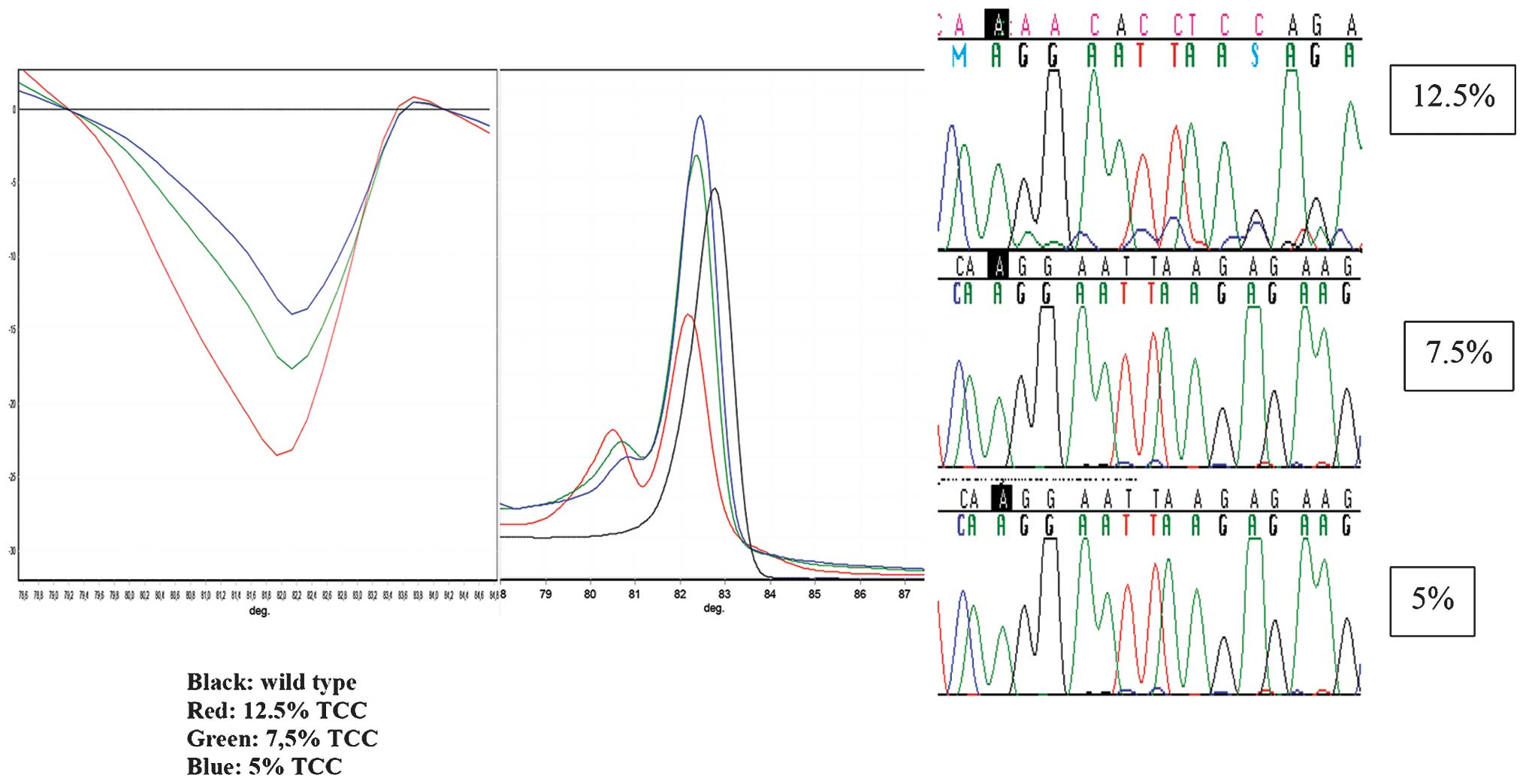
p.L858R allele frequency in wild-type DNA was 7.5% (Table II). Using the Sanger sequencing
method for the same mutations, 12.5% mutant alleles was detected in
wild-type DNA (Fig. 1). The NGS
methodology used detected the A746-E750del mutation with a
sensitivity of 2%, the p.L858R mutation with a sensitivity of 3%,
while for p.L861Q, p.T790M and p.G719S, a mutant allele frequency
of 5% was detectable (Table II).
 | Table II.Comparison of Sanger sequencing, HRM
and NGS methods used for mutation detection. |
Table II.
Comparison of Sanger sequencing, HRM
and NGS methods used for mutation detection.
|
| Detection
method |
|---|
|
|
|
|---|
| Parameters | Sanger
sequencing | HRM | NGS |
|---|
| Limit of detection,
%a | 12.50 | 5.0–7.50 | 2.0–5.0 |
| Specificity, %
(true negative) | 100
(1239/1239) | 100
(1239/1239) | 100 (30/30) |
| Missed mutations, n
(%) | 3/233 (1.29) | 0/233 (0.00) | 0/30 (0.00) |
| Total samples
tested, n | 1472 | 1472 | 60 |
EGFR mutation detection methods
A mutation in exons 18, 19, 20 or 21 of the EGFR
gene was detected in 15.83% (233/1,472) of the patients. In 1,239
patients (of the 1,472 tested), no mutation in the EGFR gene
was detected using both HRM and sequencing (Table II). Of note, there was a 99.8%
concordance between the HRM method and Sanger sequencing. HRM
technology has been reported to be more sensitive than sequencing
(19); however, this method may only
be used for mutation screening, not for mutation characterization,
thus an alternative NGS method was developed and validated. The
accuracy of the NGS technology was determined through analyzing 30
samples with known EGFR mutations and 30 samples normal for
the EGFR gene, which carried KRAS exon 2 mutations. The samples
with an EGFR mutation included 20 samples with EGFR exon 19
deletions, 1 sample with an exon 20 insertion, 1 sample with the
T790M mutation in exon 20, 2 samples with the G719S mutation in
exon 18, 4 samples carrying the point mutation L858R in exon 21 and
2 samples with the L861Q exon 21 mutation. The results revealed a
100% concordance with the HRM method.
In 3 cases, an abnormal melting profile was observed
using HRM, while no mutation was detected using Sanger sequencing
analysis. These cases all concerned exon 19 amplicons. Since the
sensitivity of HRM was found to be superior compared to sequencing,
it was proposed that these samples contained a low percentage of
mutant alleles, which could not be detected by sequencing. To test
this hypothesis NGS was used as an alternative method for mutation
detection. The results confirmed the presence of a deletion
mutation in exon 19 of the EGFR gene in all 3 samples. All
three detection methods used exhibited a high specificity, as no
false positive samples were detected in the 1,239 normal samples
tested. However, Sanger sequencing did not detect 3/233 positive
samples (1.29%), indicating that this method exhibits a decreased
level of sensitivity compared with the HRM and NGS methods
(Table II).
EGFR mutation distribution and patient
characteristics
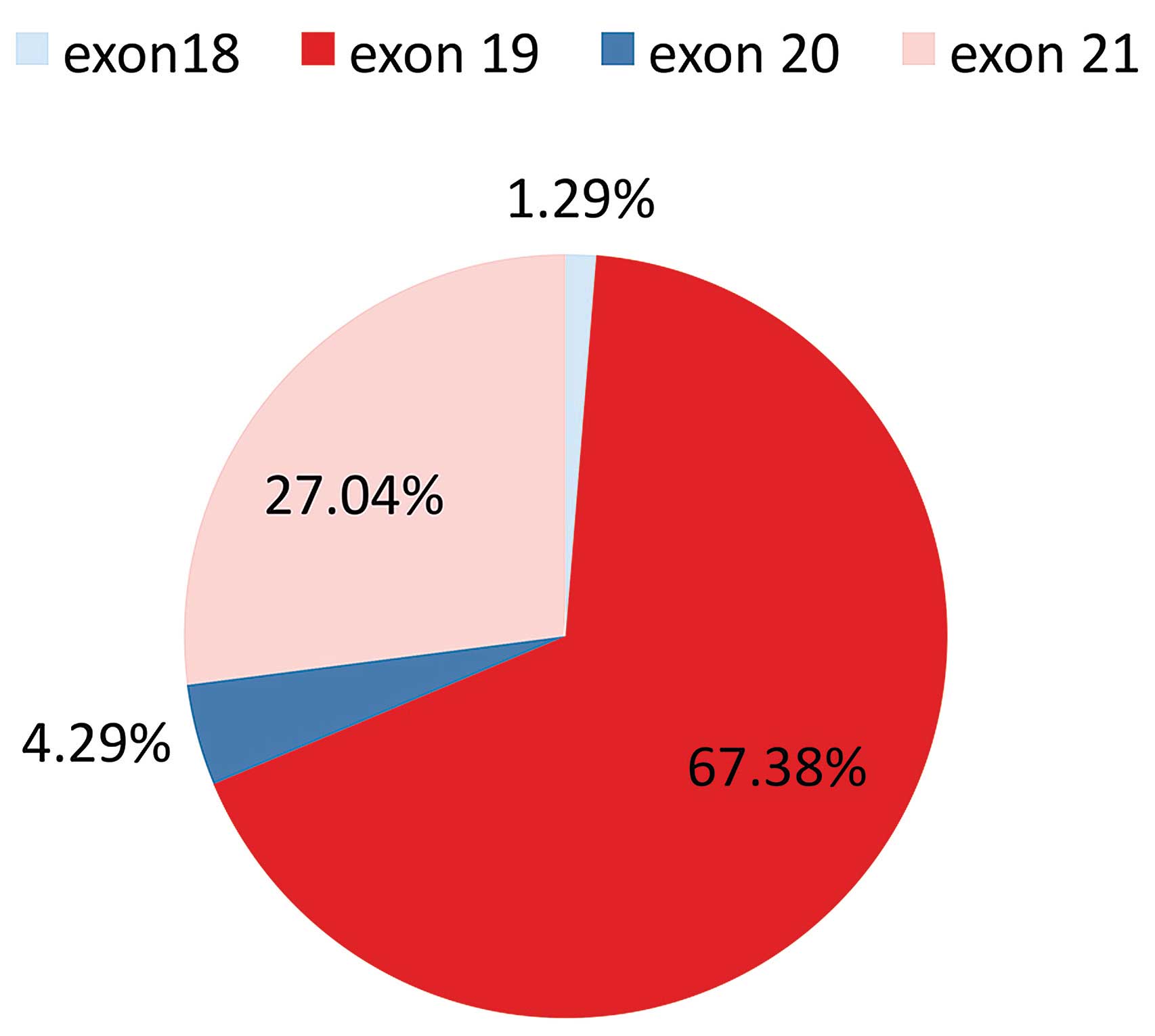
In this population of Greek patients it was
demonstrated that the EGFR mutation distribution was 3 in
exon 18 (1.29%), 157 in exon 19 (67.38%), 10 in exon 20 (4.29%) and
63 in exon 21 (27.04%) (Fig. 2). All
mutations detected in exon 19 were deletions. The most common
mutation was the A746-E750del in exon 19 (76.43% of exon 19
mutations). The p.L858R mutation was the dominant mutation in exon
21, accounting for 90% of the mutations detected.
Out of the 1472 Greek patients, 1,077 (73%) were
male and 395 (27%) were female. The mutation percentages were 11.96
(126/1,077) and 27.09% (107/395) for males and females,
respectively. The mean age of diagnosis was 63 years. The majority
of tumors with known histology were adenomas (82.49%). In addition,
~73% (409/561) of patients with known smoking status were smokers
or ex-smokers (78% of males and 54% of females) (Table III).
 | Table III.Patient demographics. |
Table III.
Patient demographics.
| Variables | No. of
patients | % |
|---|
| Gender
(n=1,472) |
|
|
|
Male | 1,077 | 73.00 |
|
Female |
358 | 27.00 |
| Age (n=1,046) |
|
|
|
<40 |
24 |
2.29 |
|
40–60 |
384 | 36.71 |
|
>60 |
638 | 60.99 |
| Histology
(n=497) |
|
|
|
Adenomas |
410 | 82.49 |
|
Squamous |
62 | 12.47 |
|
Adenosquamous |
14 |
2.82 |
|
Large-cell |
11 |
2.21 |
| Smoking status
(n=561) |
|
|
|
Smokers |
334 | 59.54 |
|
Ex-smokers |
75 | 13.37 |
| Non
smokers |
152 | 27.09 |
Association of patient characteristics
with EGFR mutation frequency
There were notable differences in EGFR mutation
frequency between male and female patients. In male patients the
mutation frequency was lower than in female, indicating a
gender-associated EGFR mutation frequency (P<0.001) (Table IV).
 | Table IV.Incidence of epidermal growth
factor receptor mutation per clinical factor in Greek
non-small-cell lung cancer patients. |
Table IV.
Incidence of epidermal growth
factor receptor mutation per clinical factor in Greek
non-small-cell lung cancer patients.
| Clinical
factor | Male (%) | Female (%) | Total (%) |
|---|
| Histology |
|
|
|
|
Adenomas | 28/286 (9.70) | 40/124 (32.26) | 68/410 (16.58) |
|
Squamous | 4/54 (7.41) | 0/8 (0.00) | 4/62 (6.45) |
|
Adeno-squamous | 2/10 (20.00) | 3/4 (75.00) | 5/14 (35.71) |
| Large
cell | 1/9 (11.11) | 0/2 (0.00) | 1/11 (9.09) |
|
P-value | 0.6707 | 0.0424 | 0.313 |
| Smoking status |
|
|
|
|
Smokers | 27/258 (10.46) | 10/76 (13.16) | 37/334 (11.08) |
|
Ex-smokers | 7/63 (11.11) | 3/12 (25.00) | 10/75 (13.33) |
|
Non-smokers | 9/90 (10.00) | 30/62 (48.39) | 39/152 (25.66) |
|
P-value | 0.9759 | <0.0001 | 0.0002 |
| Age, years |
|
|
|
|
23–40 | 3/14 (21.43) | 2/10 (20.00) | 5/24 (20.83) |
|
40–60 | 28/274 (10.22) | 29/110 (26.36) | 57/384 (14.84) |
|
60+ | 52/468 (11.11) | 49/170 (28.82) | 101/638
(15.83) |
|
P-value | 0.4201 | 0.7785 | 0.7074 |
|
None | 126/1,077
(11.69) | 107/395
(27.09) | 233/1,472
(15.83) |
EGFR mutation rate was more prevalent in the
non-smoker group compared with the ex-smoker and smoker groups
(25.66% vs. 13.33 and 11.08%, respectively; P=0.0002). However,
these values were almost equivalent for males (10.00 vs. 11.11 and
10.46%, respectively; P=0.9759), while a significant difference in
the mutation percentage was observed between female non-smokers and
ex-smoker or smokers (48.39 vs. 25.00 and 13.16% respectively;
P<0.0001) (Table IV).
These results indicated that Greek female
non-smokers were more likely to present with an EGFR
mutation, while the mutation percentage in males was substantially
lower and independent of cigarette smoking.
Histology of the tumors was observed to be
associated with mutation rates (P=0.6707, males; P=0.0424, females;
P=0.0313, both genders). The greatest mutation percentage was
observed in adenosquamous tumors (35.71%) (Table IV); however, the low number of
samples with this type of tumor does not allow for conclusions to
be drawn. By contrast, squamous NSCLC were associated with reduced
mutation rates in males and females.
EGFR mutation frequency was comparable in
patients of age range 40–60 and 60+, while mutation rates were
higher in those under the age of 40 (Table IV). Comparable results were observed
between males and females <40 years (Table IV); however the low number of younger
patients with NSCLC does not allow for conclusions to be drawn.
KRAS exon 2 mutation frequency
A KRAS exon 2 mutation was observed in 18.89%
(106/561) of all tumors analyzed. The majority of mutations were
observed in codon 12 (90/106, 84.90%). No difference in the
mutation frequency was observed in the mutation frequencies between
smokers, ex-smokers or non-smokers; this was the case for both
genders (Table V).
 | Table V.Kirsten-rat sarcoma oncogene
homolog exon 2 mutation frequency according to gender and
smoking history. |
Table V.
Kirsten-rat sarcoma oncogene
homolog exon 2 mutation frequency according to gender and
smoking history.
|
| Smoking status |
|
|---|
|
|
|
|
|---|
| Gender | Smokers (%) | Ex-smokers (%) | Non smokers
(%) | P-value |
|---|
| Male | 50/258 (19.38) | 11/63 (17.46) | 18/90 (20) | 0.9207 |
| Female | 15/76 (19.73) | 2/12 (16.67) | 10/62 (16.12) | 0.8535 |
| Total | 65/334 (19.46) | 13/75 (17.33) | 28/152 (18.42) | 0.8997 |
The distribution of different KRAS mutations
among the different groups is represented in Table VI. Of note, the majority of
KRAS mutations detected in the smoker and ex-smoker groups
(81.54 and 100%, respectively) were transversion mutations
(substitution of a purine for a pyrimidine or conversely, G→T or
G→C), which are known to be smoking-associated (21,22). By
contrast, the percentages of transversion and transition mutations
(substitution of a purine for a purine, e.g., G→A or a pyrimidine
for a pyrimidine, C→T) were equally distrubted in the non-smoker
group (57.14% transversion mutations; 42.86% transition
mutations).
 | Table VI.Distribution of KRAS mutations
according to smoking history. |
Table VI.
Distribution of KRAS mutations
according to smoking history.
| Type of KRAS
mutation | Smokers (%) | Ex-smokers (%) | Non-smokers
(%) |
|---|
| Transversion
mutation |
|
|
|
|
c.35G>T (p.G12V) | 26/65 (40) | 6/13 (46.15) | 10/28 (35.71) |
|
c.34G>T (p.G12C) | 18/65 (27.69) | 5/13 (38.46) | 5/28 (17.86) |
|
c.37G>T (p.G13C) | 9/65 (13.85) | 2/13 (15.38) | 1/28 (3.57) |
|
Total | 53/65 (81.54) | 13/13 (100) | 16/28 (57.14) |
| Transition
mutation |
|
|
|
|
c.35G>A (p.G12D) | 10/65 (15.38) | 0/13/(0) | 9/28 (32.14) |
|
c.38G>A (p.G13D) | 2/65 (3.08) | 0/13/(0) | 2/28 (7.14) |
|
c.34G>A (p.G12S) | 0/65 (0) | 0/13/(0) | 1/28 (3.57) |
|
Total | 12/65 (18.46) | 0/13 (0) | 12/28 (42.86) |
Discussion
In the present study, HRM analysis was performed in
order to produce specific melting profiles for distinguishing
between wild-type and mutated EGFR and KRAS genes in
patient samples. All mutations detected through Sanger sequencing
were also analyzed using HRM. The present study confirmed that the
HRM was highly sensitive, as mutant/wild-type allele detection was
achieved between 5 and 7.5%, dependent on the mutation type and
amplicon; whereas Sanger sequencing analysis had a sensitivity of
12–15%. It was previously reported that Sanger sequencing was a
less sensitive method compared with HRM analysis; however, HRM
analysis has also been demonstrated to produce false positive
results due to bad DNA quality, this most commonly occurs when the
starting material is FFPE tissue (19,20). In
addition, although the HRM method may be used as a screening method
for mutation detection, it cannot characterize the mutation
detected. In the present study, 3 cases that concerned the
EGFR exon 19 amplicon were found to be positive for a
mutation using HRM, whereas the Sanger sequencing found these cases
to be negative. Therefore, despite the higher sensitivity of HRM
analysis compared with that of the Sanger sequencing method, an
alternative NGS method was used to confirm the presence of a
mutation. The NGS method is able to detect 2–5% of mutant alleles,
as it was proved for p.L861Q, 746–750del, p.L858R, p.T790M and
p.G719S mutations in the present study. In addition, NGS is a
rapid, sensitive and accurate method for somatic mutation
detection. It requires minimum manual input and is able to detect
different somatic mutations simultaneously. However, it requires
DNA of very good quality and quantity (>10 ng/µl), which is
sometimes difficult to obtain when using FFPE tissues (23,24).
Furthermore, NGS is more expensive compared with HRM and Sanger
sequencing when a small amount of samples (<30) is processed in
a single experiment. Thus, it is a superior method whenever the
analysis of >1 gene is required and/or >50 samples are
processed in the same experiment.
Another important factor affecting the sensitivity
of mutation detection is appropriate tissue selection. Therefore,
the present study considered the existence of pathological review
crucial for all samples, in order to ensure a tumor cell content of
>75%.
In the present study, 1,472 patients were subjected
to EGFR mutation screening. The EGFR mutation rates
have previously been reported to differ largely among different
populations (12). In the present
Greek population the overall EGFR mutation frequency was
15.83%.
The EGFR mutation frequency in Caucasian
NSCLC patients was reported to be 10–15% (12). A recent study reviewed EGFR
mutation incidence in European countries (25). EGFR mutation frequency in
Europe ranged from 6% (Switzerland) to a maximum of 37.5%
(Germany), depending on ethnicity and patient's characteristics. In
a previous study, a low percentage of EGFR mutations (8.2%)
was observed in a Greek population; however, in the present study,
a higher percentage of EGFR mutation frequency was observed.
This may be due to the larger number of patients analyzed in the
present study or to different clinicopathological characteristics
of this cohort. For example, 82.49% of the patients analyzed in the
present study were diagnosed with adenocarcinomas, which are known
to present greater percentage of EGFR mutation rates
compared to other types of NSCLC.
Of the 1,472 patients tested in the present study,
73% were males and 27% were females. As expected, differences in
EGFR mutation frequency were observed between male and
female patients. In male patients the mutation frequency was lower
compared with females, indicating a gender-associated EGFR
mutation frequency (P<0.001).
In the present study, >72% of patients with known
smoking status were smokers or ex-smokers (78% of males and 54% of
females). This indicated that as previously reported, smoking is an
important factor in NSCLC etiology (3,4).
A previous study investigating the impact of
cigarette smoking on cancer risk in the European population
indicated that the hazard ratio of developing lung cancer for
smokers was 23.30 for men and 7.53 for women (2). This indicates that the risk of NSCLC is
slightly higher for men compared with women. This may be due to
different male-female habits or differences in the physiology
between the two genders. In European countries, almost 1 in every 5
cancers is caused by cigarette smoking (3). Using data on cancer incidence for 2008,
it was estimated that in Greece 7,653 novel cancer diagnoses per
year were attributed to cigarette smoking (2).
In the present study, when smoking habits are taken
in consideration it was revealed that, in females the mutation
frequency differs substantially between non-smokers, ex-smokers and
smokers (P=0.001). By contrast, EGFR mutation frequency was
comparable between non-smoking and smoking males. Female smokers
demonstrated a comparable mutation frequency to that of males. This
indicated that the mechanism of tumor development differs
substantially in females depending on their smoking habits.
Additionally, the present study demonstrated that
EGFR mutation rates were higher in adenoma and adenosquamous
NSCLC compared with squamous NSCLC (P=0.001). EGFR mutation
frequency was comparable in patients aged 40–60 years and >60
years, while mutation rates were higher in those aged <40 years.
Comparable results were observed between males and females <40
years; however the low number of younger NSCLC patients does not
allow for conclusions to be drawn.
Concerning KRAS mutation frequency, numerous
studies have suggested that it is significant lower in non-smokers
compared to smokers (16,21,22).
However, these conclusions were not observed in the present study
population. In accordance with previous studies, a different
mutation distribution was observed between non-smokers, ex-smokers
and smoker groups (16,22). The prevalence of transversion
mutations in ex-smokers and smokers is in accordance with previous
studies and suggested the association of these mutations with
smoking (16,22). Whereas KRAS transitions
mutations were more common in lung adenocarcinomas from patients
without any smoking history.
In conclusion, to the best of our knowledge, the
present study was the largest study reporting EGFR mutation
spectrum and frequencies in a cohort of Greek NSCLC patients.
Sensitive mutation detection techniques were used in routine
diagnostic practice in order to obtain an overall mutation
frequency of 15.83%. In addition, these results demonstrated that
female non-smokers had a high prevalence of EGFR mutations.
Furthermore, KRAS mutation analysis in patients with known
smoking history revealed no difference in mutation frequency
according to smoking status, although a higher prevalence of
transversion mutations in the ex-smoker and smoker groups was
observed. This study highlights the importance of sensitive
molecular techniques, such as NGS for EGFR and KRAS
mutation analysis. Furthermore, this technique may be used in
future studies for simultaneous mutation analysis of a number of
genes that are prognostic and/or predictive markers in lung cancer
patients.
Acknowledgements
The authors would like to thank all patients that
participated in this study as well as the support service team of
GeneKor, in particular Mrs. Anna Kokotaki, who was responsible for
obtaining written informed consent from patients and pathology
reports.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, SteliarovaFoucher E,
LortetTieulent J, Rosso S, Coebergh JW, Comber H, Forman D and Bray
F: Cancer incidence and mortality patterns in Europe: Estimates for
40 countries in 2012. Eur J Cancer. 49:1374–1403. 2011. View Article : Google Scholar
|
|
3
|
Agudo A, Bonet C, Travier N, González CA,
Vineis P, Bueno-de-Mesquita HB, Trichopoulos D, Boffetta P,
Clavel-Chapelon F, Boutron-Ruault MC, et al: Impact of cigarette
smoking on cancer risk in the European prospective investigation
into cancer and nutrition study. J Clin Oncol. 30:4550–4557. 2011.
View Article : Google Scholar
|
|
4
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suda K, Tomizawa K and Mitsudomi T:
Biological and clinical significance of KRAS mutations in
lung cancer: An oncogenic driver that contrasts with EGFR
mutation. Cancer Metastasis Rev. 29:49–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MunfusMcCray D, Harada S, Adams C, Askin
F, Clark D, Gabrielson E and Li QK: EGFR and KRAS
mutations in metastatic lung adenocarcinomas. Hum Pathol.
42:1447–1453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Sciences. 304:1497–1500. 2004.
View Article : Google Scholar
|
|
9
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gazdar AF: Activating and resistance
mutations of EGFR in non-small-cell lung cancer: Role in
clinical response to EGFR tyrosine kinase inhibitors.
Oncogene. 28 Suppl 1:S24–S31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: Biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chougule A, Prabhash K, Noronha V, Joshi
A, Thavamani A, Chandrani P, Upadhyay P, Utture S, Desai S,
Jambhekar N, et al: Frequency of EGFR mutations in 907 lung
adenocarcioma patients of Indian ethnicity. PLoS One. 8:2013.
View Article : Google Scholar
|
|
13
|
Roberts PJ, Stinchcombe TE, Der CJ and
Socinski MA: Personalized medicine in non-small-cell lung cancer:
Is KRAS a useful marker in selecting patients for epidermal
growth factor receptor-targeted therapy? J Clin Oncol.
28:4769–4777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pao W, Wang TY, Riely GJ, Miller VA, Pan
Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG and Varmus HE:
KRAS mutations and primary resistance of lung
adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2:e172005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eberhard DA, Johnson BE, Amler LC, Goddard
AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson
DH, et al: Mutations in the epidermal growth factor receptor and in
KRAS are predictive and prognostic indicators in patients
with non-small-cell lung cancer treated with chemotherapy alone and
in combination with erlotinib. J Clin Oncol. 23:5900–5909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riely GJ, Kris MG, Rosenbaum D, Marks J,
Li A, Chitale DA, Nafa K, Riedel ER, Hsu M, Pao W, et al: Frequency
and distinctive spectrum of KRAS mutations in never smokers
with lung adenocarcinoma. Clin Cancer Res. 14:5731–5734. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS,
Squire J, et al: Molecular testing guideline for selection of lung
cancer patients for EGFR and ALK tyrosine kinase inhibitors:
Guideline from the college of American pathologists, international
association for the study of lung cancer and association for
molecular pathology. Arch Pathol Lab Med. 137:828–860. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellison G, Zhu G, Moulis A, et al:
EGFR mutation testing in lung cancer: A review of available
methods and their use for analysis of tumour tissue and cytology
samples. J Clin Pathol. 66:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Do H, Krypuy M, Mitchell PL, et al: High
resolution melting analysis for rapid and sensitive EGFR and
KRAS mutation detection in formalin fixed paraffin embedded
biopsies. BMC Cancer. 8:1422008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krypuy M, Newnham GM, Thomas DM, et al:
High resolution melting analysis for the rapid and sensitive
detection of mutations in clinical samples: KRAS codon 12
and 13 mutations in non-small cell lung cancer. BMC Cancer.
6:2952006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pham D, Kris MG, Riely GJ, et al: Use of
cigarette smoking history to estimate the likelihood of mutations
in epidermal growth factor receptor gene exons 19 and 21 in lung
adenocarcinomas. J Clin Oncol. 24:1700–1704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahrendt SA, Decker PA, Alawi EA, et al:
Cigarette smoking is strongly associated with mutation of the K-ras
gene in patients with primary adenocarcinoma of the lung. Cancer.
92:1525–1530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong SQ, Li J, Tan AY, et al: CANCER 2015
Cohort: Sequence artefacts in a prospective series of
formalin-fixed tumours tested for mutations in hotspot regions by
massively parallel sequencing. BMC Med Genomics. 7:232014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meldrum C, Doyle MA and Tothill RW:
Next-generation sequencing for cancer diagnostics: A practical
perspective. Clin Biochem Rev. 32:177–195. 2011.PubMed/NCBI
|
|
25
|
Szumera-Ciećkiewicz A, Olszewski WT,
Tysarowski A, Kowalski DM, Głogowski M, Krzakowski M, Siedlecki JA,
Wągrodzki M and Prochorec-Sobieszek M: EGFR mutation testing
on cytological and histological samples in non-small cell lung
cancer: A Polish, single institution study and systematic review of
European incidence. Int J Clin Exp Pathol. 6:2800–2812.
2013.PubMed/NCBI
|