Introduction
Esophageal carcinoma is one of the most common types
of malignant tumors in China (1). Due
to the lack of specificity of symptoms, early-stage esophageal
carcinoma is difficult to detect and some patients have already
entered into the advanced stages of the disease at the time of
diagnosis. Chemoradiotherapy is currently the main treatment
strategy (2–5) for patients with advanced-stage
esophageal carcinoma. However, the adverse effects associated with
chemoradiotherapy undermine the effectiveness of treatment and the
quality of life of patients.
In recent years, the continuous development and
study of traditional Chinese medicine herbal treatments has
obtained fruitful results. The main components of compound Radix
Sophorae Flavescentis injection are Radix Sophorae Flavescentis and
Heterosmilacis Japonicae extract, which can clear away heat,
promote diuresis, cool blood, expel miasma, remove stasis and
relieve pain (6). It has been
demonstrated that CKI suppresses tumor cell growth by inducing
apoptosis (7) and inhibits the
migration, invasion and adhesion capacity of tumor cells by
downregulating the protein expression of CD44v6 (8). Few studies to date have focused on the
efficacy of Kushen alkaloids using animal models and performing
clinical trials before 1992, when Kushen alkaloids were first
approved. Studies have proven that the compound Radix Sophorae
Flavescentis may be used to improve the efficacy of
chemoradiotherapy and may also improve the quality of life of
patients (6,9). Clinical studies have reported that
Kushen alkaloids are efficacious in the treatment of various types
of solid tumors (including tumors of the lung, liver and
gastrointestinal tract). The treatment responses were comparable
to, or better than, those of chemotherapy drug-treated patients
(10). Kushen alkaloids demonstrate a
good safety profile in cancer patients, such as reduced toxicity in
bone marrow when used in combination with chemotherapeutic agents
(11). Long-term survival data for
Kushen alkaloids-treated cancer patients remain to be demonstrated
with well-controlled clinical studies and large patient cohorts.
However, studies on the mechanisms of action of this compound are
limited. Thus, in this study, we analyzed the anti-neoplastic
effects of compound Radix Sophorae Flavescentis, as well as its
mechanisms of action in in vitro (using esophageal carcinoma
TE-8 cells), with the aim of providing a theoretical basis for the
clinical treatment of esophageal carcinoma.
Materials and methods
Experimental materials
Cell culture
Human esophageal squamous cell carcinoma TE-8 cells
were provided by the Laboratory of Reverse Transcriptase Molecular
Biology, Xinxiang Medical College, Xinxiang, China. The cells were
cultured in RPMI-1640 medium supplemented with 100 U/ml penicillin,
100 µg/ml streptomycin and 10% (V/V) fetal bovine serum (FBS) in an
incubator at 37°C with 5% CO2. The medium was changed
every 24 h and the cells were passaged once in every 48 h.
Primary reagents
The compound Radix Sophorae Flavescentis was
provided by Shanxi Zhendong Pharmaceutical Co., Ltd., Changzhi,
China; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was obtained from Jingke Biotechnology Co., Ltd., Beijing,
China; the apoptosis assay kit was from the Beyotime Institute of
Biotechnology, Haimen, China; terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) reagent was
purchased from Roche Diagnostics, Mannheim, Germany; the reverse
transcription kit was obtained from Nanjing Vazyme Biotech Co.,
Ltd. (Nanjing, China); SYBR-Green universal qPCR Master Mix was
from Roche Diagnostics; the real-time PCR system was purchased from
Bio-Rad (Hercules, CA, USA); and the inverted fluorescence
microscope was obtained from Leica Microsystems (Mannheim,
Germany).
Treatment groups
Esophageal carcinoma TE-8 cells were inoculated in
96-well plates at a density of 5×104 cells/ml, and kept
in an incubator at 37°C with 5% CO2 for overnight
culture. The following day, the adherent cells (60–70% adherence)
were placed in RPMI-1640 medium supplemented with 0.2% FBS, and the
supernatant was extracted after 24 h. The compound Radix Sophorae
Flavescentis injection was then added to the medium at various
concentrations (final concentrations: 0, 0.0125, 0.025, 0.05, 0.1,
0.2, 0.4 and 0.8 mg/ml) with a total of 8 gradients, followed by
culture for 24 h. There were 4 different treatment groups: the
control group (untreated cells), the low-dose group (0.05 mg/ml of
the compound), the medium-dose group (0.2 mg/ml of the compound)
and the high-dose group (0.8 ng/ml) of the compound. Cell
proliferation was then examined by MTT assay. The plate was
removed, abd 20 µl MTT solution (5 mg/ml) were added to each well.
The cells were then incubated at 37°C for 4 h. The culture medium
was then removed, and the cells were washed with PBS, followed by
the addition of DMSO. The mixture was then vortexted for 10 min,
and the light absorption (OD value) in each well was measured at
492 nm and the readings were recorded. The experiment was repeated
3 times, and the mean measurements were calculated from
sextuplicate samples. The cell growth inhibition rate (%) was
calculated as 1 − (mean OD value in treatment group/OD value in
control group) x100.
Reverse transcription-quantitative PCR
(RT-qPCR) for the determination of the expression of
apoptosis-related genes
The cells treated with various doses of the compound
(low dose, medium dose and high dose (0.05, 0.2 and 0.8 mg/ml,
respectively) were treated with 1 ml of TRIzol reagent (Beyotime
Institute of Biotechnology) and placed in a 1.5 ml centrifuge tube.
Subsequently, 200 µl chloroform was added, and the tube was shaken
vigorously (for mixing) and then place on ice for 15 min to layer,
followed by centrifugation at 12,000 rpm/min for 15 min. When the
liquid appeared as the layer, the supernatant was placed in 500 µl
isopropanol, on ice, after mixing until the RNA precipitated out
and was then centrifuged at 12,000 rpm/min for 10 min. The
precipitate was washed with 75% pre cooling anhydrous ethanol
twice; it was then dissolve in double-distilled water without RNA
enzyme. Following the detection of the concentration of sample, the
RNA was reverse transcribed into cDNA using the reverse
transcription kit. The primers used for PCR were as follows:
caspase-3 forward, 5′-GGTATTGAGACAGACAGTGG-3 and reverse, 5′-CATG
GGATCTGTTTCTTTGC-3; β-actin forward, 5′-GCGGGAA ATCGTGCGTGAC-3′ and
reverse, CGTCATACTCCT GCTTGCTG-3′; Bax forward, 5′-TCCACCAAGAAGCT
GAGCGAG-3′ and reverse, 5′-GTCCAGCCCATGATG GTTCT-3′; Bcl-2 forward,
5′-TTCTTTGAGTTCGGTGG GGTC-3′ and reverse,
5′-TGCATATTTGTTTGGGGCAGG-3′. The reaction mixture was prepared
using the following reaction system: 2X SYBR-Green universal 10 µl
qPCR Master Mix, 1 µ upstream/downstream primer (10
µmmolxl−1), 1 µl cDNA and 20 µl double-distilled water.
The following reaction conditions were used for PCR: initial
denaturation, 95°C, 30 sec; degeneration, 95°C, 3 sec; annealing
and extension, 60°C, 30 sec; building of solubility curve. Finally,
the data were analyzed using LightCycler software.
Calculation of apoptotic index TUNEL
assay
The TE-8 cells were digested and kept in monoplast
suspension, and inoculated in a culture dish containing a glass
slide with at 1×105 cells/ml; the groups and treatment
conditions were the same as those described above. The medium was
removed after 48 h, the glass slide was removed and TUNEL assay was
carried out according to the instructions provided by the
manufacturer. The slide was removed, washed with PBS for 3 times,
fixed in 4% paraformaldehyde for 40 min, and washed with PBS for
3×3 min. The slide was blocked at room temperature for 10 min,
washed with PBS for 3×3 min, placed at 4°C for 3 min, washed with
cold PBS for 2×5 min, followed by the addition of labeling mixture.
It was then incubated in a dark and wet environment at 37°C for 60
min, washed with PBS for 3×5 min, followed by the addition of 50 µl
transformation solution. It was then incubated in a dark and wet
environment at 37°C for 30 min, and washed with PBS for 3×5 min
again. DAB was then added and the slide was observed under a
microscope. The slide was restained with haematoxylin, dehydrated,
transparentized and mounted on a microscope for observation.
TUNEL-positive cells were indicated by a brown nucleus. The cells
were then observed under a microscope at at 5–10 fields of vision
on a high-power lens (x400 magnification) so that each field of
vision included 100–200 cells. The total number of cells was
approximately 500–1,000 and the proportion of cell apoptosis was
calculated as the apoptotic rate.
Statistical analysis
All the data was analyzed using statistical software
SPSS 17.0, and the data are expressed as the means ± SD.
Comparisons among groups were made by one-way analysis of variance
(ANOVA), and pairwise comparisons were made using the LSD method.
The survival curve was estimated using the Kaplan-Meier method and
the log-rank test. A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of compound Radix Sophorae
Flavescentis injection on the proliferation of esophageal carcinoma
TE-8 cells
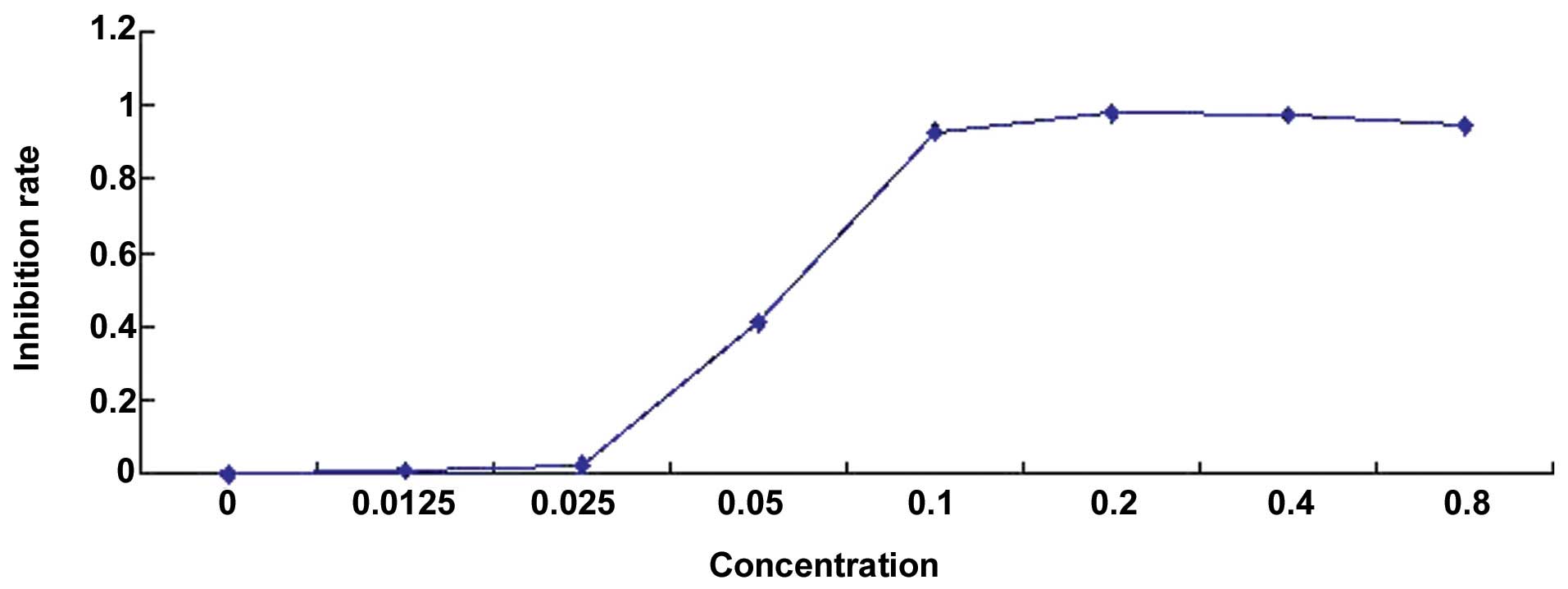
As shown in Fig. 1,
compared with untreated control group, cell proliferation was
significantly inhibited in the cells treated with the compound in a
dose-dependent manner (P<0.05).
Effect of compound Radix Sophorae
Flavescentis injection on the expression of apoptosis-related genes
in esophageal carcinoma TE-8 cells
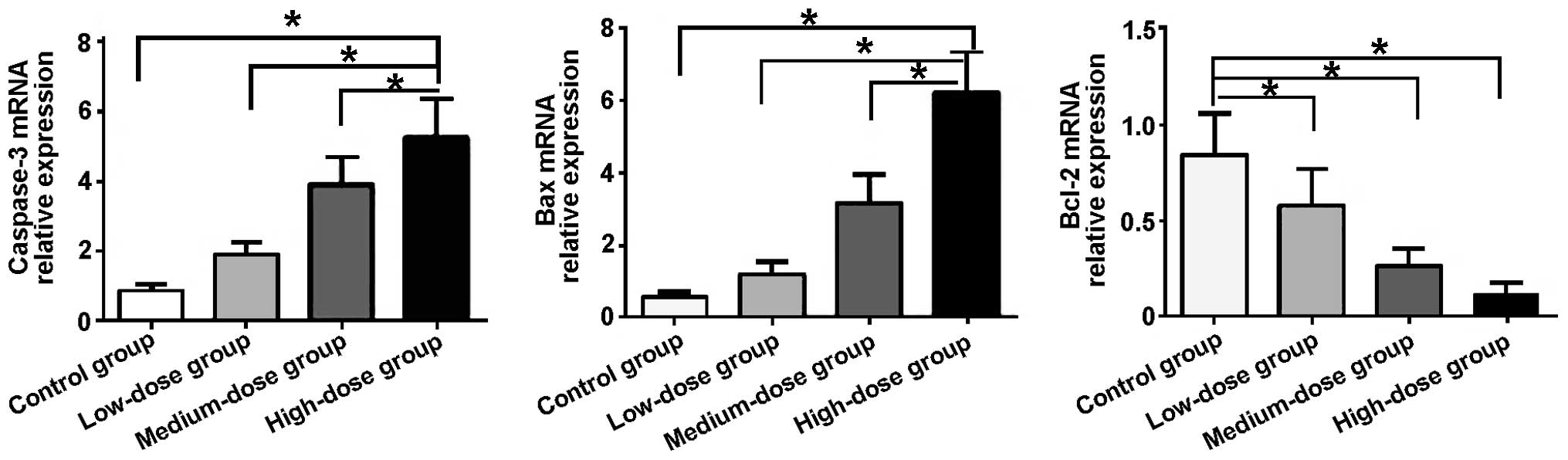
After the cancer cells were treated with various
concentrations of the compound Radix Sophorae Flavescentis, the
mRNA expression levels of the apoptosis-related genes, caspase-3,
Bcl-2 and Bax, were measured by RT-qPCR and the results are shown
in Fig. 2. Compared with the control
group, the mRNA expression levels of caspase-3 and Bax
significantly increased in the cells in the low-dose, medium-dose
and high-dose groups, whereas the mRNA expression level of Bcl-2
markedly decreased (P<0.05). The increase in the mRNA expression
of caspase-3 and Bax in the different treatment groups was as
follows (in the order of lowest to highest): low-dose
group<medium-dose group<high-dose group. The decrease in the
mRNA expression of Bcl-2 in the different treatment groups was as
follows (in the order of highest to lowest): low-dose
group>medium-dose group>high-dose group.
Effect of compound Radix Sophorae
Flavescentis injection on the apoptotic index in esophageal
carcinoma TE-8 cells
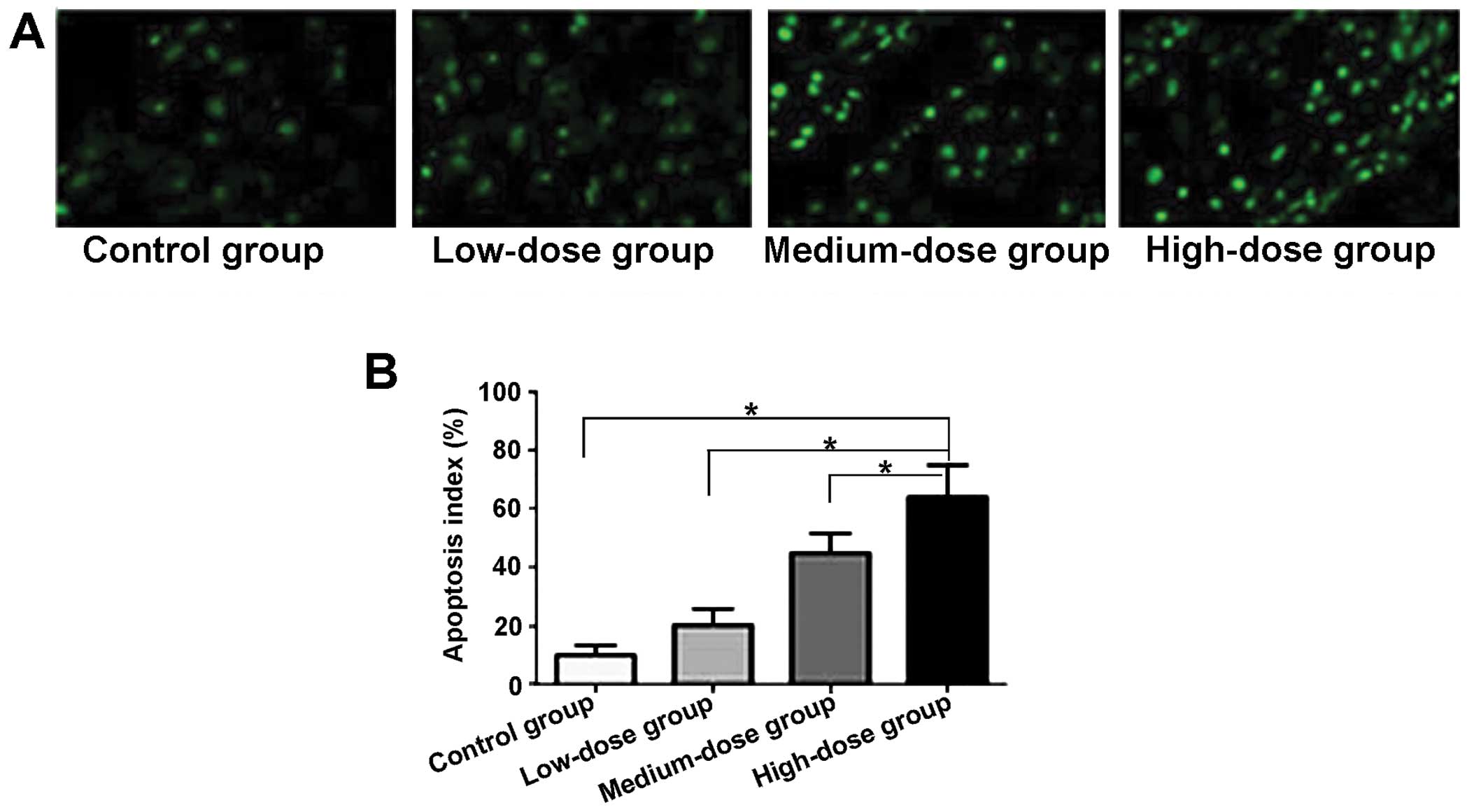
The apoptotic index in the cancer cells was
calculated using TUNEL asay and the results are presented in
Fig. 3. The number of apoptotic the
cells in the control group was low; however, following treatment
with increasing doses of the compound, cell apoptosis gradually
increased. The quantitative analysis of the results of cell
apoptosis is shown in Fig. 3B.
Compared with the control group, the apoptotic index of the cancer
cells in the low-dose, medium-dose and high-dose groups increased
significantly and the difference was statistically significant
(P<0.05). The apoptotic index in the different gropus ranged as
follows (in the order of highest to lowest): high-dose
group>medium-dose group>low-dose group.
Discussion
Esophageal carcinoma is one of the most common
gastrointestinal malignancies within the category of ‘hiccough’ and
‘phrenic’ in traditional Chinese medicine and its pathogenesis is
‘qi, phlegm, blood stasis and heat’ (12). Thus, the treatment of esophageal
carcinoma should aim to clear heat, transform phlegm and soften
hardness. The main components of the compound Radix Sophorae
Flavescentis injection are Radix Sophorae Flavescentis and
Heterosmilacis Japonicae extract, which can clear heat, promote
diuresis, cool blood, expel miasma, remove stasis and relieve pain
(13).
It has been demonstrated that the compound Radix
Sophorae Flavescentis injection exerts antitumor effects and can
alleviaate pain, bleeding, fatigue and other discomforts (14). It has also been reported that
(15) that matrine can kill malignant
tumor cells and promotes cancer cell apoptosis. In this study, we
investigated the antitumor effects of the compound Radix Sophorae
Flavescentis injection by treating esophageal carcinoma TE-8 cells
with different doses of the compound and found that the compound
significantly inhibited the proliferation of the cancer cells in a
dose-dependent manner. Thus, the compound Radix Sophorae
Flavescentis injection inhibits the proliferation of esophageal
carcinoma cells.
In this study, we also measured the mRNA expression
of apoptosis-related genes in esophageal carcinoma TE-8 cells and
the results revealed that the mRNA expression of caspase-3 and Bax
was significantly higher in the cells treated with a high dose of
the compound (0.8 mg/ml) compared with the control group, whereas
the mRNA expression of Bcl-2 markedly decreased in the cells
treated with the compound compared with control group. Caspase-3 is
a common molecule of the cell apoptosis pathway, which plays an
important role in the process mediating apoptosis (16). An increase in its expression promotes
apoptosis. In our study, treatment with the compound Radix Sophorae
Flavescentis injection induced an increase in the expression of
caspase-3, resulting in the apoptosis of the esophageal carcinoma
cells. The balance of Bcl-2 and Bax also plays an important role in
the process of apoptosis in cancer cells, with the increase in
Bcl-2 expression indicating the inhibition of apoptosis, and the
decrease in Bax expression maintaining the proliferation of cancer
cells (17–19). In our study, following treatment of
the cancer cells with the compound Radix Sophorae Flavescentis
injection, Bcl-2 expression markedly decreased and Bax expression
significantly increased, indicating an increase in apoptosis. The
induction of apoptosis may be one of the mechanisms through which
the compound Radix Sophorae Flavescentis injection inhibits the
growth of tumors. Cancer cell apoptosis caused by compound Radix
Sophorae Flavescentis injection was further verified by TUNEL
assay.
In conclusion, our study demonstrated that compound
Radix Sophorae Flavescentis injection significantly inhibited the
proliferation of esophageal carcinoma TE-8 cells and induced
apoptosis. Thus, compound Radix Sophorae Flavescentis injection may
prove to be a valuable and effective therapeutic agent for the
treatment of esophageal carcinoma.
References
|
1
|
Dongxin L: Esophageal cancer molecular
epidemiology research in China. Zhonghua Liu Xing Bing Xue Za Zhi.
24:939–943. 2003.(In Chinese).
|
|
2
|
Chen J, Zhu J, Pan J, Zhu K, Zheng X, Chen
M, Wang J and Liao Z: Postoperative radiotherapy improved survival
of poor prognostic squamous cell carcinoma esophagus. Ann Thorac
Surg. 90:435–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mariette C, Piessen G and Triboulet JP:
Therapeutic strategies in oesophageal carcinoma: Role of surgery
and other modalities. Lancet Oncol. 8:545–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, et al:
Japan Clinical Oncology Group: Surgery plus chemotherapy compared
with surgery alone for localized squamous cell carcinoma of the
thoracic esophagus: A Japan Clinical Oncology Group Study -
JCOG9204. J Clin Oncol. 21:4592–4596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rice TW, Adelstein DJ, Chidel MA, Rybicki
LA, DeCamp MM, Murthy SC and Blackstone EH: Benefit of
postoperative adjuvant chemoradiotherapy in locoregionally advanced
esophageal carcinoma. J Thorac Cardiovasc Surg. 126:1590–1596.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi L, Zhang J and Zhang Z: Determination
of four alkaloids in compound Kushen Injection by high performance
liquid chromatography with ionic liquid as mobile phase additive.
Se Pu. 31:249–253. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Dai ZJ, Gao J, Wang XJ, Ji ZZ, Wu WY, Liu
XX, Kang HF, Guan HT and Ren HT: Apoptotic mechanism of gastric
carcinoma cells induced by matrine injection. Zhonghua Wei Chang
Wai Ke Za Zhi. 11:261–265. 2008.(In Chinese). PubMed/NCBI
|
|
8
|
Dai ZJ, Gao J, Wu WY, Wang XJ, Li ZF, Kang
HF, Liu XX and Ma XB: Effect of matrine injections on invasion and
metastasis of gastric carcinoma SGC-7901 cells in vitro. Zhong Yao
Cai. 30:815–819. 2007.(In Chinese). PubMed/NCBI
|
|
9
|
Sun Q, Ma W, Gao Y, Zheng W, Zhang B and
Peng Y: Meta-analysis: therapeutic effect of transcatheter arterial
chemoembolization combined with compound kushen injection in
hepatocellular carcinoma. Afr J Tradit Complement Altern Med.
9:178–188. 2012.PubMed/NCBI
|
|
10
|
Zhu JL and Chen JZ: Short-term observation
on the efficacy of matrine injection in treating 79 cases of late
stage cancers. Shandong Med J. 43:article 40. 2003.
|
|
11
|
Willert K, Brown JD, Danenberg E, Duncan
AW, Weissman IL, Reya T, Yates JR III and Nusse R: Wnt proteins are
lipid-modified and can act as stem cell growth factors. Nature.
423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H and Huang GH: Cognition and
treatment research of traditional Chinese medicine for esophagus
cancer. Liaoning Zhong Yi Yao Da Xue Xue Bao. 2:212–215. 2012.(In
Chinese).
|
|
13
|
Hai LN, Zhang ZW and Wang JH: Effects of
compound Kushen injection on alalgesis, hemostasis and anti-stress
in mice. Zhongguo Shi Yan Fang Ji Xue Za Zhi. 50:199–202. 2012.(In
Chinese).
|
|
14
|
Ma Y, Zhang QW, Wang ZM and Gao HM:
Advance in study on compound Kushen injection. Zhongguo Shi Yan
Fang Ji Xue Za Zhi. 18:23–24. 2012.
|
|
15
|
Tuo XL and Bai M: Expression and
significance of human mismatch repair gene (Smsh2) and
proliferating cell nuclear antigen (PCNA) in lung cancer. China J
Modem Med. 15:6912005.
|
|
16
|
Simpson KL, Cawthorne C, Zhou C,
Hodgkinson CL, Walker MJ, Trapani F, Kadirvel M, Brown G, Dawson
MJ, MacFarlane M, et al: A caspase-3 ‘death-switch’ in colorectal
cancer cells for induced and synchronous tumor apoptosis in vitro
and in vivo facilitates the development of minimally invasive cell
death biomarkers. Cell Death Dis. 4:e6132013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salakou S, Kardamakis D, Tsamandas AC,
Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos
DS, Papapetropoulos T, Petsas T, et al: Increased Bax/Bcl-2 ratio
up-regulates caspase-3 and increases apoptosis in the thymus of
patients with myasthenia gravis. In Vivo. 21:123–132.
2007.PubMed/NCBI
|
|
19
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|