Introduction
Vasoactive intestinal peptide-secreting tumors
(VIPomas) are one of the least common types of pancreatic
functioning islet cell tumors. The annual incidence of these tumors
is estimated to ~1 per 10 million individuals in the general
population (1). The definitive
diagnosis of VIPoma is established by the laboratory evaluation of
plasma VIP level (2). Imaging studies
also serve important roles in determining size, location of islet
cell tumors, optimal therapy, shortening operative time, and
avoiding unnecessary resection of the pancreas. However, only a
limited number of previous studies have reported computed
tomography (CT) findings of pancreatic VIPoma (3–5). The
radiological manifestations of multiple-phase spiral computed
tomography (MPSCT) have not previously been described. The present
case study reports a rare case of pancreatic VIPoma with liver
metastases and focusses on the imaging features of the tumor,
particularly the MPSCT findings, in a 50-year-old patient, and the
current literature is reviewed. Written informed consent was
obtained from the patient and the study was approved by the ethics
committee of The Second Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, China).
Case report
A 50-year-old woman was admitted to The Second
Affiliated Hospital of Zhejiang University School of Medicine on
March 1, 2013 following a ten-month history of fatigue, weakness
and diarrhea. The patient did not present with abdominal pains or
fever. No positive findings, such as yellow sclera and skin,
abdominal and rebound tenderness, or general superficial lymph node
enlargement, were observed upon physical examination. Laboratory
tests demonstrated marked hypokalemia (2.4 mmol/l, normal range:
3.5–5.5 mmol/l) and hyperglycemia (7.7 mmol/l, normal range:
5.0–5.6 mmol/l). The other hormone levels of the patient included
normal glucagon, gastrin, and insulin levels. In addition, the
expression levels of the tumor markers CEA and CA19-9 were normal.
Transabdominal ultrasound detected a 2.0 cm round lesion with
uniform low echo located at the uncus of the pancreas.
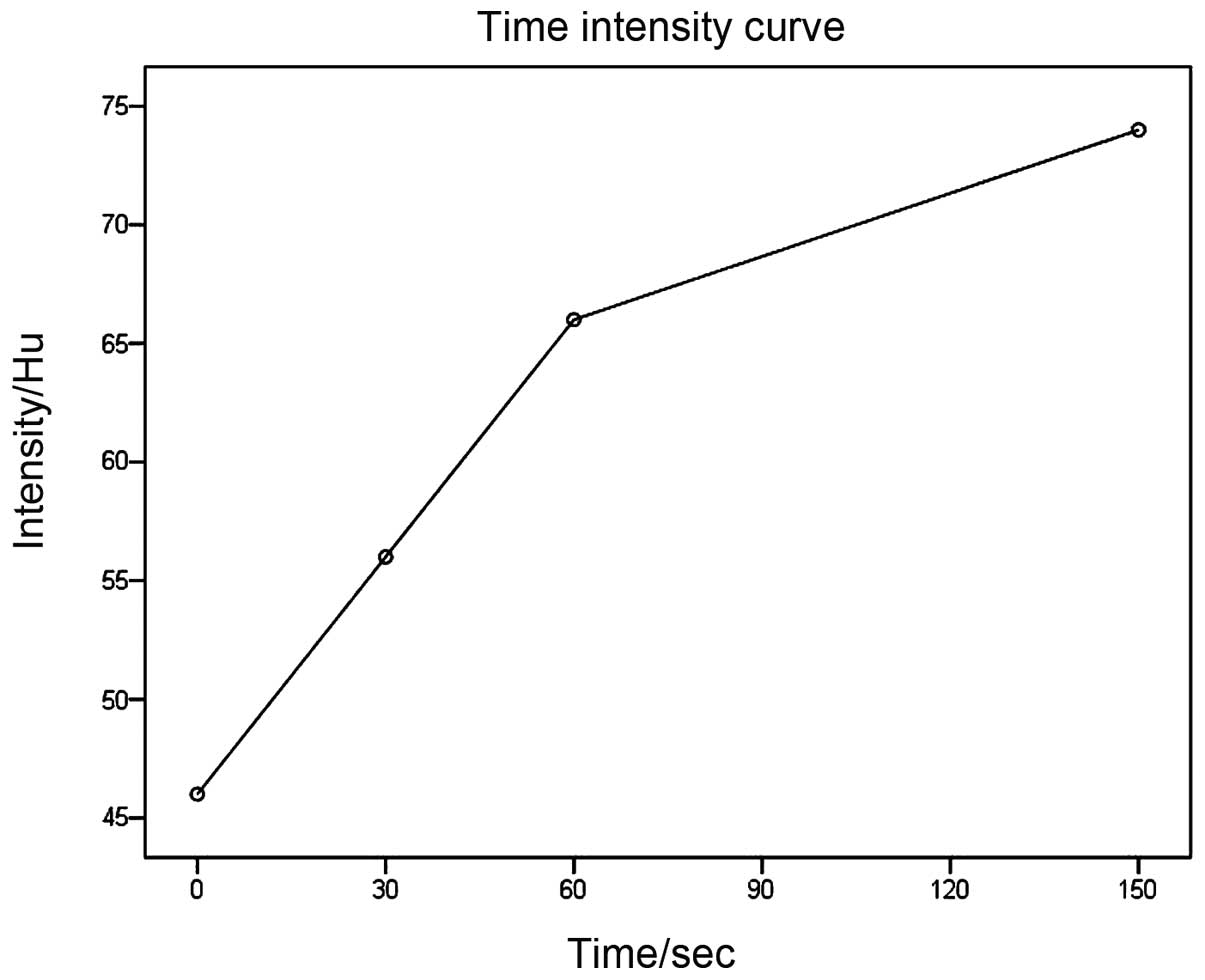
Spiral CT (Somatom Sensation 16, Siemens, Munich,
Germany) images were obtained prior to and at 30 s for the hepatic
artery phase, 60 s for the portal venous phase, and 150 s for
hepatic parenchymal phase after intravenous administration of
contrast material (Omnipaque, GE health care, Fairfield, CT, USA;
total of 80 ml, 3 ml/s, via cubital vein with a mechanical power
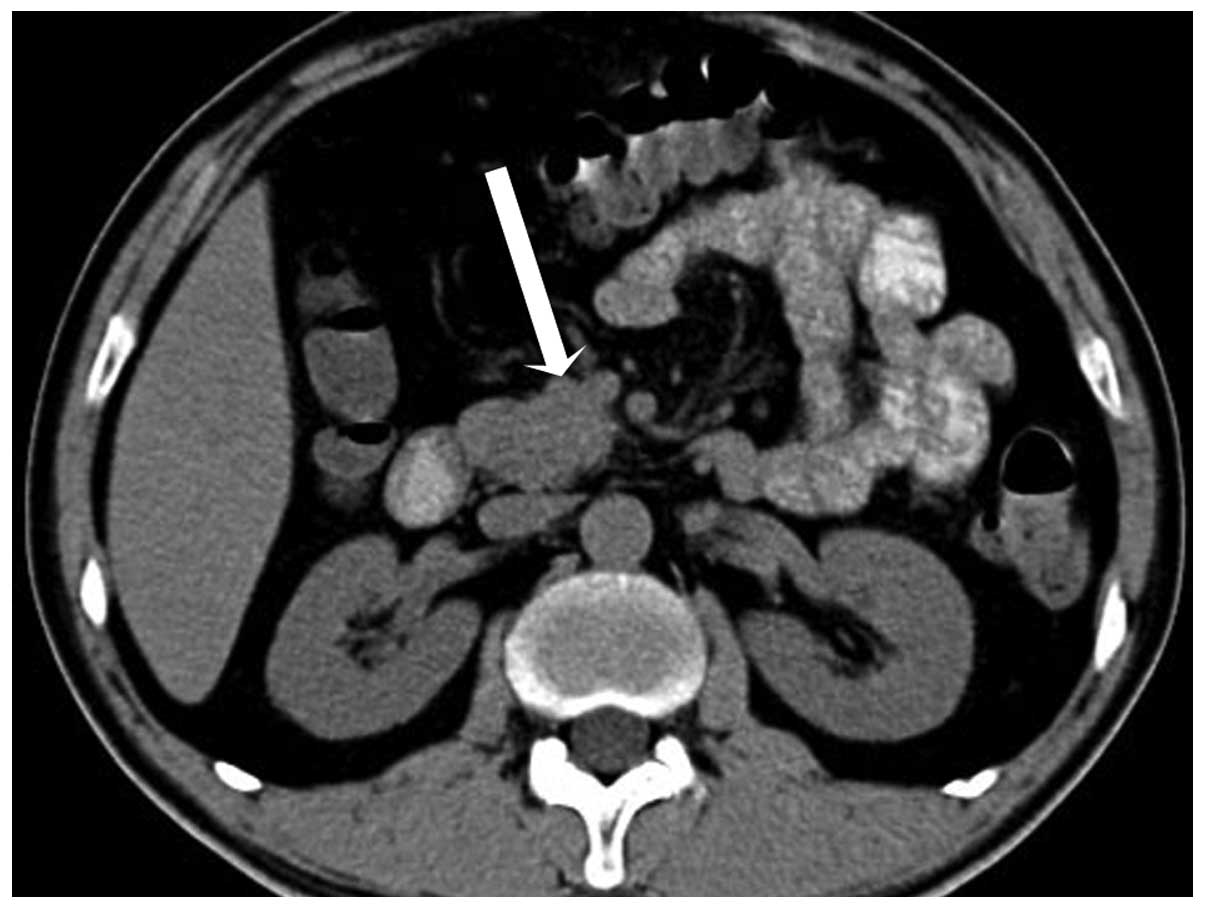
injector). Spiral CT revealed a 2.2 cm round mass in the uncus of
the pancreas, which was isodense compared with the pancreatic
parenchyma, with the mean CT attenuation values being 46 HU
(Fig. 1). During hepatic artery phase
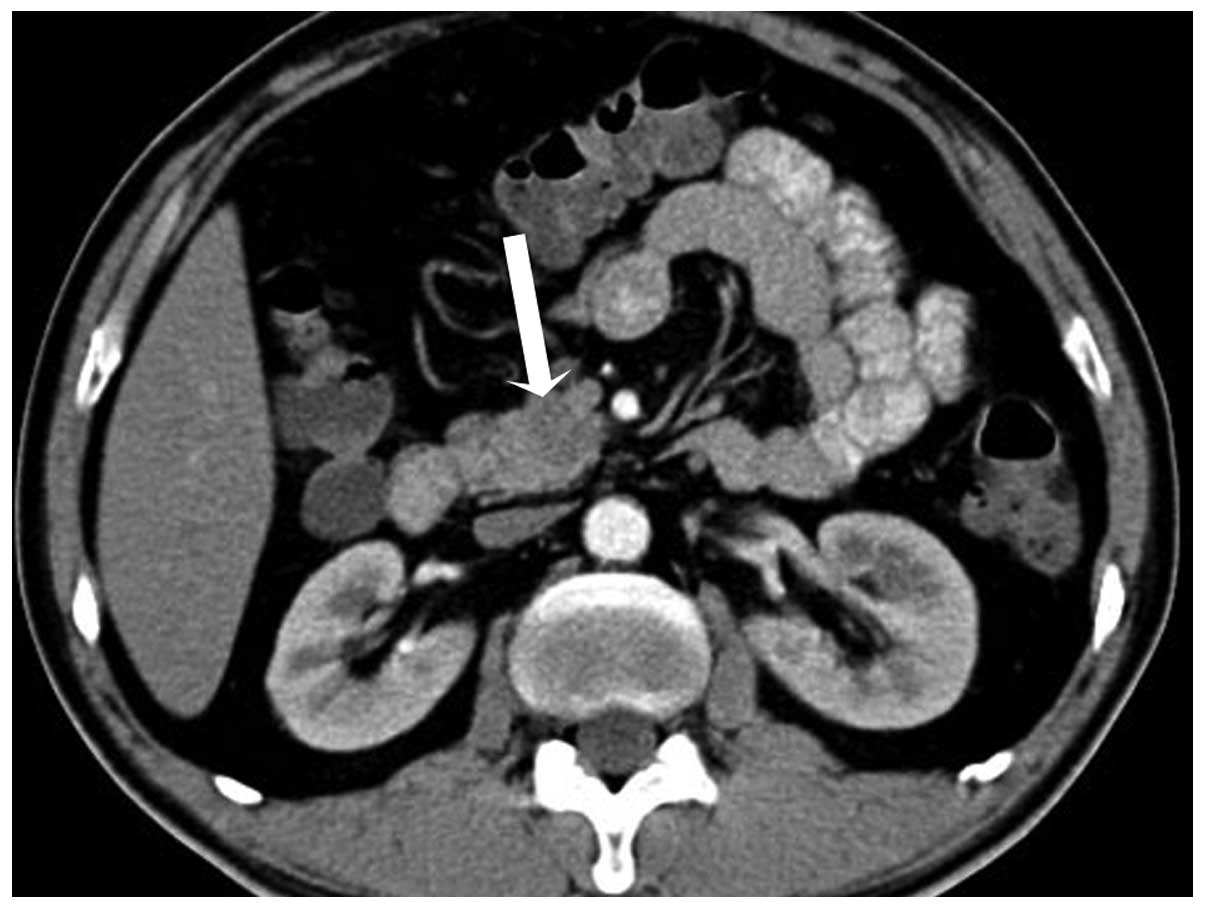
and portal venous phase, the mass was hypodense compared with the
enhanced pancreas, with the mean CT attenuation values being 56 HU
and 66 HU, respectively (Fig. 2).
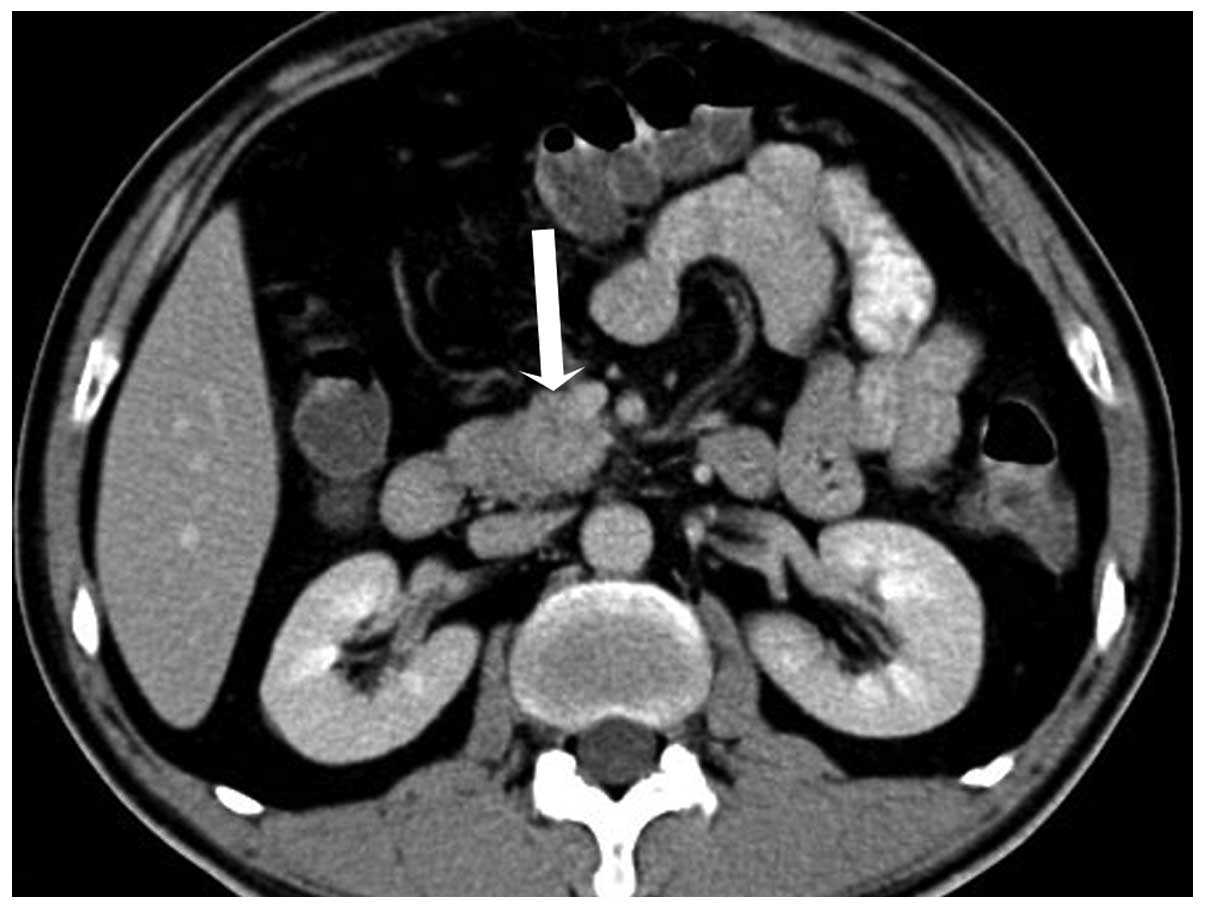
During the hepatic parenchymal phase, it became hyperdense with the
mean CT attenuation values being 74 HU (Fig. 3). The process of dynamic
contrast-enhanced CT demonstrated a hypervascular
progressive-strengthening modality (Fig.
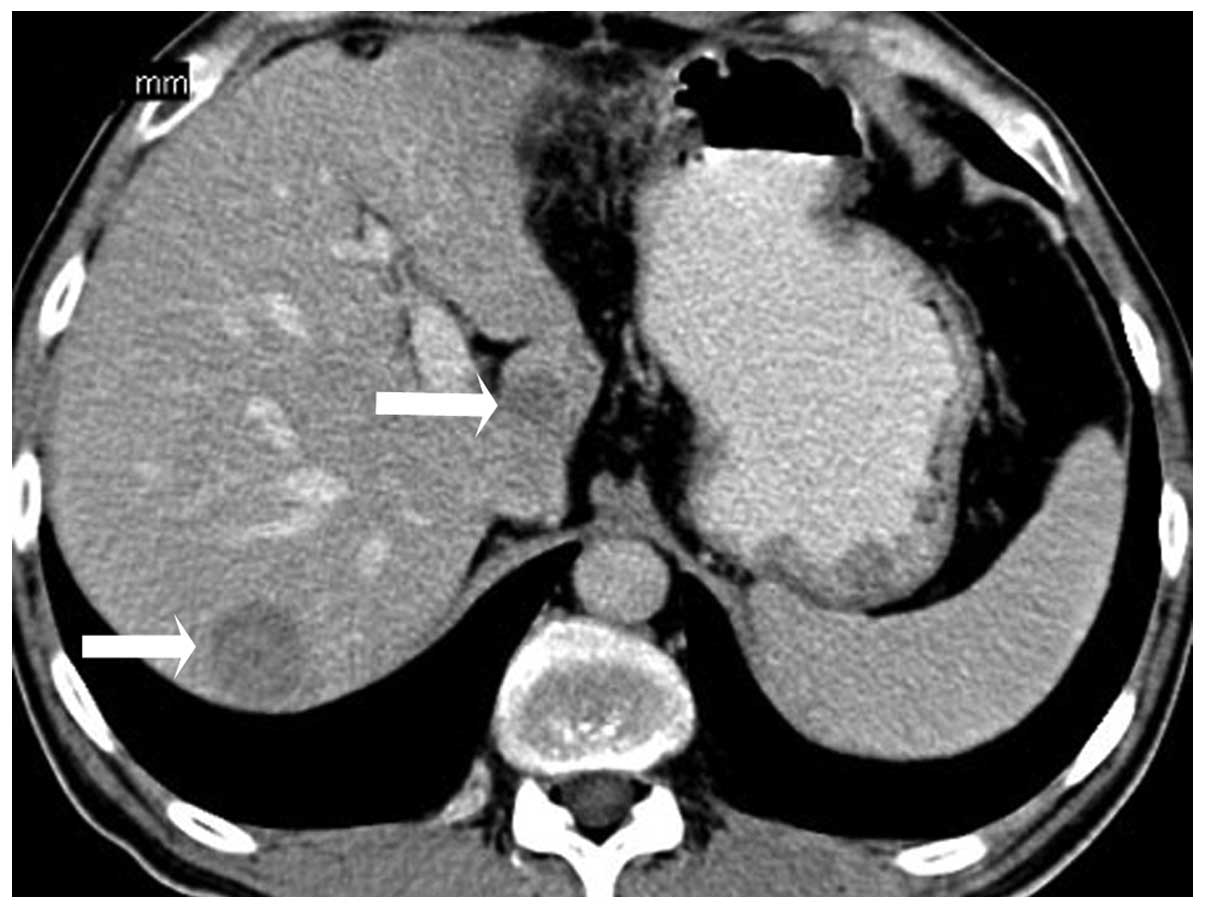
4). There were multiple masses scattered throughout the liver
that demonstrated hypodense lesions with minimal diffuse
heterogeneous enhancement (Fig.
5).
Surgical exploration revealed a small, encapsulated,
solid mass of 2.5 cm in size in the uncus of the pancreas.
Pancreaticoduodenectomy and wedge resection of a number of liver
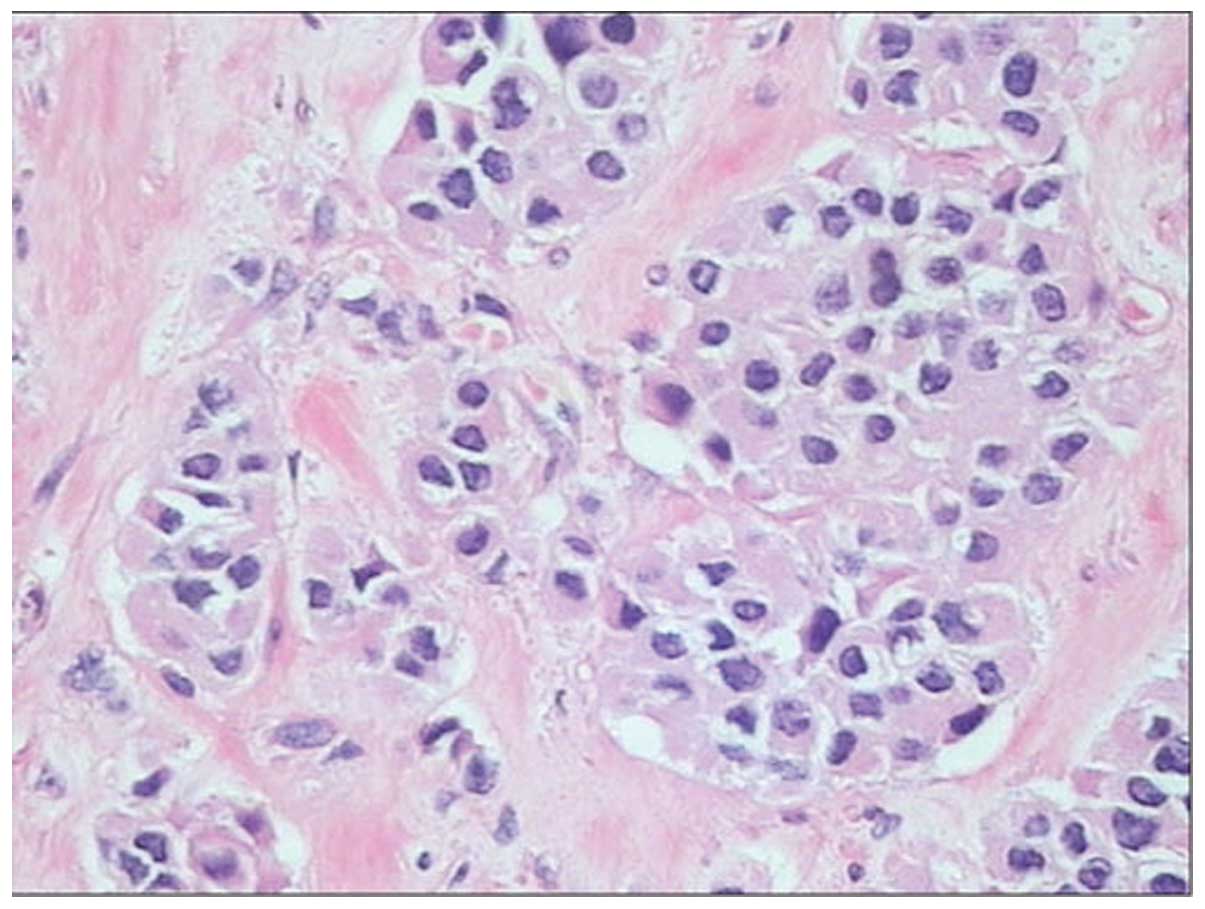
metastases was undertaken. Histopathology of the pancreatic mass
revealed the typical neuroendocrine features of nested cells,
coexisting with marked fibrotic stroma (Fig. 6). The immunohistochemical analysis of
the tumor for plasma VIP-immunoreactivity was markedly positive
(+++), and the tumor was also stained slightly positive for other
peptides, including NSE (++), AAT (+), AACT (++), CK-P (+), CgA
(++), Syn (+), and negative for ACTH and insulin. These findings
were consistent with the diagnosis criteria of VIPoma.
Postoperatively, the patient recovered immediately
from the symptoms and there has been no evidence of recurrence
during the past 27 months of follow-up since being discharged from
the hospital on May 12, 2013.
Discussion
VIPomas are rare, hormone-producing tumors; the
majority of cases (90%) originate from endocrine pancreatic cells
(6). The annual incidence of VIPoma
is low and reported to be ~1 per 10 million people in the USA.
VIPomas are more frequently diagnosed in women (65%) compared with
men (35%), with the age of onset ranging between 2–83 years (mean
age, 53.1 years). At the time of presentation, ≥70% of patients
already have metastases identified, and the great majority of these
tumors are malignant based on the presence of hepatic or lymph node
involvement and other distant metastasis (1). In humans, the majority of VIPomas occur
within the substance of the pancreas. Approximately 75% of VIPomas
are localized in the body and tail of the pancreas, while the
remaining 25% occur in the head of the pancreas (2). Clinically, production of large amounts
of VIP hormone results in watery diarrhea, hypokalemia, and
achlorhydria (7). This condition is
called WDHA syndrome and was first described by Verner and Morrison
in 1958 (7). The case described in
the present study occurred in the uncus of the pancreas, and
presented with WDHA syndrome, which was comparable with the
literature. The fasting plasma VIP levels were >200 pg/ml
(normal, 0–190 pg/ml) are required to establish the diagnosis
(2). The VIP-immunoreactivity of the
present case was markedly positive (+++), and combined with the
WDHA syndrome, the diagnosis of VIPoma was confirmed.
The classic and most common enhancement CT pattern
of syndromic pancreatic islet cell tumors illustrates a
hyperattenuating lesion in the arterial phase that becomes
inconspicuous in the venous phase (5,8). However,
to the best of our knowledge, only a few CT findings of pancreatic
VIPoma have previously been reported (3–5) and the
radiological manifestations of MPSCT have not previously been
described. The previous studies demonstrated that VIPoma appears as
a round, well-defined, homogeneous mass with central necrosis and
hypervascularized; heterogeneous on contrast enhanced CT with
internal septa, the CT attenuation values range between ~23.4–46 HU
in the non-enhancement phase, and ~76–116 HU following enhancement.
In addition, a previous study illustrated that half of VIPomas
present with calcification (5). In
the present case, the lesion was isodense compared with the
pancreatic parenchyma, and the process of contrast-enhanced
multiple-phase CT demonstrated a progressive-strengthening
modality, which was different from the previous comparable studies
and not consistent with the classic CT appearances of syndromic
pancreatic islet cell tumors.
VIPomas are hypervascularized and rich in tumor
cells and fibrosis. Since fibrous stroma is less vascularized, this
may result in contrast agent pooling within the tissue, and
therefore the fibrous area may demonstrate relative hypoattenuation
on early-phase images but hyperattenuation on late-phase images.
Conversely, as the viable tumor cells area requires an increased
blood supply, the development of a tumor is accompanied with
hypervascularity, it demonstrate relative hyperattenuation on
early-phase images (9). The present
authors hypothesized that the progressive-strengthening modality of
the VIPoma was mainly attributed to the large amount of fibrotic
stroma, and its hypervascularization.
Another notable observation in the current patient
was that, although the primary tumor measured only 2.2 cm in
diameter, multiple metastatic tumors were present. Semelka et
al (7) proposed that it may be a
feature of VIPoma to present as a small primary pancreatic tumor in
the setting of liver metastases. Comparably, other case studies
have reported large tumors without liver metastases (3,4).
Therefore, we propose that a small, primary, pancreatic VIPoma may
present with liver metastases while a large one may present without
liver metastases.
There are numerous differential diagnoses that
require consideration when a pancreatic mass is detected without
evidence of increased hormone release. VIPoma primarily needs to be
differentiated from pancreatic carcinoma, which is one of the most
common space occupying lesions of the pancreas. Previous studies
have demonstrated that contrast-enhancement images of pancreatic
carcinoma during the early phase on spiral CT usually present as
low attenuating lesions in comparison with the surrounding
pancreatic parenchyma; while on the late phase imaging, the
contrast on the pancreatic parenchyma generally reduces, resulting
in carcinomas presenting as a progressive-strengthening modality as
in the present case (9,10). However, Hiroyuki et al
(9) performed dynamic CT studies in
20 patients with pancreatic carcinoma and demonstrated that the CT
values (mean ± SD) of pancreatic carcinomas after contrast
injection were 28.3±12.8 HU in the hepatic artery phase, 36.7±14.5
HU in the hepatic portal venous phase, and 42.3±14.6 HU in the
hepatic parenchymal phase, respectively, which were notably lower
compared with what was observed in the present case (56 HU, 66 HU
and 74 HU in corresponding phase). In addition, pancreatic or bile
duct dilatation or local extension or distant metastases may
indicate pancreatic carcinoma. Although insulinoma, gastrioma,
glucagonoma, somatostatinoma and nonfunctioning pancreatic
endocrine tumors are all pancreatic endocrine tumors in addition to
VIPoma, the majority of these tumors are hypervascular in nature,
presenting as a hyperattenuating lesion in the arterial phase and
becoming inconspicuous in the venous phase (3,4), which is
different to the present case. In addition, other pancreatic
diseases, including mass-forming chronic pancreatitis, pancreatic
metastases, tuberculosis, solid pseudopapillary tumor and lymphoma,
should be considered in the further differential diagnosis. If
combined with the history and clinical manifestations, the
differentiation may be much easier.
At present, there is no consensus with regard to
standard guidelines for the treatment of VIPoma, due to its rare
occurrence, particularly in the uncus of the pancreas with liver
metastases. Surgery appears to be the most effective means of
treatment, and concurrent treatment with octreotide has advanced
the preoperative electrolyte management (11). In addition, the combination of
octreotide, chemotherapy, resection of tumor, radiofrequency tissue
ablation and liver transplantation may be selected for metastatic
VIPoma in the liver (11–13).
In summary, the present study reports a case of a
small VIPoma, only 2.2 cm in diameter, arising from the region of
the uncus of the pancreas with liver metastases. If a patient
presents to hospital with the following: WDHA syndrome (watery
diarrhea, hypokalemia, achlorhydria), particularly with watery
diarrhea; markedly elevated VIP serum levels; hypervascular lesion;
progressive-strengthening lesion; and with calcification in the
pancreas, the diagnosis of the VIPoma should be considered and a
small primary pancreatic tumor with liver metastases may also fit
this diagnosis.
References
|
1
|
Ghaferi AA, Chojnacki KA, Long WD, Cameron
JL and Yeo CJ: Pancreatic VIPomas: subject review and one
institutional experience. J Gastrointest Surg. 12:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delcore R and Friesen SR: Gastrointestinal
neuroendocrine tumors. J Am Coll Surg. 178:187–211. 1994.PubMed/NCBI
|
|
3
|
Tjon A, Tham RT, Jansen JB, et al: MR, CT,
and ultrasound findings of metastatic Vipoma in pancreas. J Comput
Assist Tomogr. 13:142–144. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Remme CA, de Groot GH and Schrijver G:
Diagnosis and treatment of VIPoma in a female patient. Eur J
Gastroenterol Hepatol. 18:93–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horton KM, Hruban RH, Yeo C and Fishman
EK: Multi-detector row CT of pancreatic islet cell tumors.
Radiographics. 26:453–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aspestrand F, Kolmannskog F and Jacobsen
M: CT, MR imaging and angiography in pancreatic apudomas. Acta
Radiol. 34:468–473. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Semelka RC, Custodio CM, Cem Balci N and
Woosley JT: Neuroendocrine tumors of the pancreas: spectrum of
appearances on MRI. J Magn Reson Imaging. 11:141–148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sheth S, Hruban RK and Fishman EK: Helical
CT of islet cell tumors of the pancreas: typical and atypical
manifestations. Am J Roentgenol. 179:725–730. 2002. View Article : Google Scholar
|
|
9
|
Hata H, Mori H, Matsumoto S, et al:
Fibrous stroma and vascularity of pancreatic carcinoma: correlation
with enhancement patterns on CT. Abdom Imaging. 35:172–180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu DS, Vedantham S, Krasny RM, Kadell B,
Berger WL and Reber HA: Two-phase helical CT for pancreatic tumors:
pancreatic versus hepatic phase enhancement of tumor, pancreas, and
vascular structures. Radiology. 199:697–701. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song S, Shi R, Li B and Liu Y: Diagnosis
and treatment of pancreatic vasoactive intestinal peptide endocrine
tumors. Pancreas. 38:811–814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiang G, Liu X, Tan C, Zhang H, Mai G and
Zheng Z: Diagnosis and treatment of VIPoma: a case report and
literature review in China. Pancreas. 41:806–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng SY, Li JT, Liu YB, et al: Diagnosis
and treatment of VIPoma in China: (case report and 31 cases review)
diagnosis and treatment of VI Poma. Pancreas. 28:93–97. 2004.
View Article : Google Scholar : PubMed/NCBI
|