Introduction
Prostate cancer is the second most frequently
diagnosed type of cancer and the sixth most common cause of
cancer-associated mortality in males, worldwide (1). The typical treatment strategies of
chemotherapy and radiotherapy have not provided significant
survival benefits for patients with advanced prostate cancer, and
the majority of available strategies are only palliative (2). Therefore, there is requirement for the
prompt identification of novel molecules to treat the increasing
number of prostate cancer cases, particularly cases that are
resistant to current chemotherapeutic agents (3,4).
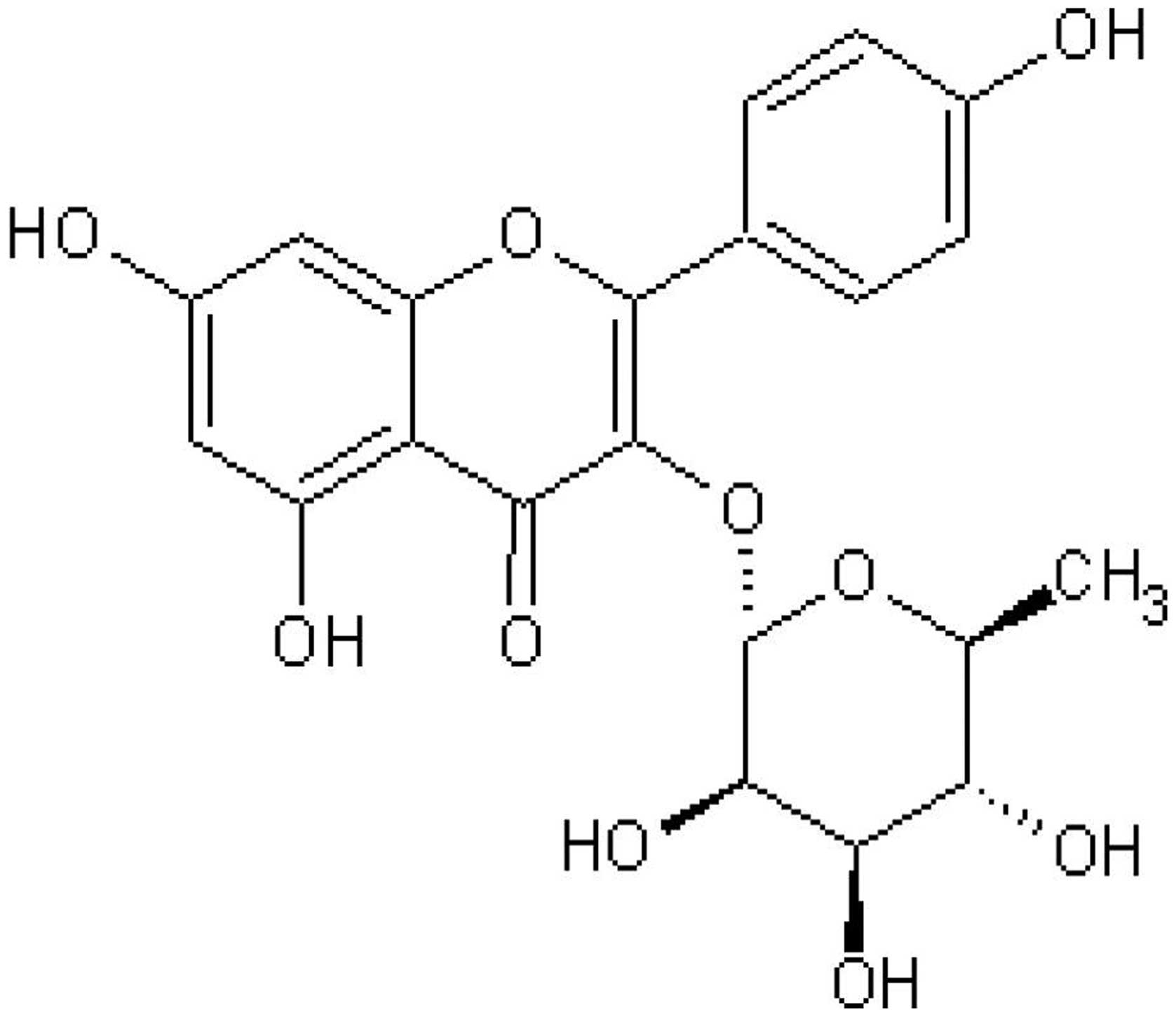
Afzelin is a flavonol glycoside found in Nymphaea
odorata, which has been identified to inhibit the growth of
breast cancer cells by stimulating apoptosis (5). In addition, afzelin has been
demonstrated to scavenge superoxide anion radicals in RAW264.7
cells (6). The structure of this
compound is shown in Fig. 1.
In eukaryotic cells, the actin cytoskeleton is
essential for mediating various biological functions, including
providing the structural framework of the cell, and driving
cellular motility and division. In particular, dynamic
reorganization of the actomyosin cytoskeleton and remodelling of
the extracellular matrix drive the multistep process of tumor cell
metastasis. Tumor cells are able to cross tissue boundaries into
the blood and lymphatic systems and migrate to distal regions of
the body. Therefore, cancer therapy has recently focused on gaining
a comprehensive understanding of the biological processes that
regulate actin organization (7).
Rho GTPase family proteins are key regulators of the
actin cytoskeleton and, with the aid of various target proteins,
maintain the tight regulation of normal cell growth and
differentiation (8–11). Eukaryotic cells exhibit a
predisposition to rapid and uncontrollable growth following genomic
alterations or carcinogenesis. For instance, increased expression
levels of LIM domain kinase 1 (LIMK1) are observed in prostate
cancer. The Rho GTPase myotonic dystrophy kinase-related
Cdc42-binding kinase α (MRCKα), Rho-associated coiled-coil
containing protein kinase (ROCK)1 and ROCK2 are responsible for the
activation of LIMK1 (9,12). Previous studies have demonstrated the
ability of ROCK inhibitors to reduce the invasive ability of tumor
cells in vitro and prevent the spread of tumor cells in
vivo, including melanoma and fibrosarcoma cells, as well as
liver, breast, lung and prostate cancer tumor cells (13–17).
Thus, inhibitors of LIMK1, MRCKα and ROCK1/2 are
considered to restore normal cell proliferation and provide a key
strategy for cancer treatment (18).
Therefore, the aim of the present study was to evaluate the in
vitro anti-prostate cancer activity of afzelin and its effect
on prostate cancer-associated kinases.
Materials and methods
Cell culture
Androgen-sensitive LNCaP (lymph node carcinoma of
the prostate) and androgen-independent PC-3 (prostate cancer-3)
cell lines were obtained from the American Type Culture Collection
(Manassas, VA, USA). The LNCaP cells were maintained in Eagle's
minimal essential medium (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum, glutamine, 2%
penicillin-streptomycin and 0.2% gentamicin (all obtained from
Sigma-Aldrich). According to the manufacturer's instructions, the
PC-3 cells were grown in Ham's F12K medium (Sigma-Aldrich)
supplemented with 2 mM L-glutamine adjusted to contain 1.5 g/l
sodium bicarbonate (90%) and 10% fetal bovine serum. The two cell
lines were incubated at 37°C in a humidified atmosphere of 95% air
and 5% CO2.
Cell proliferation assay
LNCaP and PC-3 cells were seeded in 96-well plates
at a density of 5×103 cells/well to a final volume of
100 µl. At 24 h after seeding, the medium was removed and replaced
with fresh medium, containing vehicle (dimethyl sulfoxide) or
increasing concentrations of afzelin (0.1, 1.0 and 10.0 µg/ml), in
a final volume of 100 µl. The cultures were prepared in
quadruplicate for each afzelin concentration and time point, and
maintained in a CO2 incubator for three days. After 24,
48 and 72 h, 10 µl water soluble tetrazolium salt (WST)-1 labeling
solution (WST-1 cell proliferation assay kit; Roche Diagnostics,
Indianapolis, IN, USA) was added to the cultures and the cells were
returned to the CO2 incubator for 2 h (19). The specific type of formazan product
formed was detected by measuring its absorbance in a 96-well
spectrophotometric plate reader (DiaSorin, Stillwater, MN, USA) at
a wavelength of 420 nm, as described by the manufacturer (20).
Cell cycle analysis
Cell cycle analysis was performed as described
previously (19). Briefly, cultured
LNCaP and PC-3 prostate cancer cells were exposed to various
concentrations of afzelin (0.1, 1.0 and 10.0 µg/ml) for 24 h.
Adherent cells were trypsinized (Sigma-Aldrich), pooled with the
cells in suspension and washed three times with ice-cold
phosphate-buffered saline (PBS). To determine cell viability, a
fraction of the cells were stained with trypan blue (Sigma-Aldrich)
and counted. The cultures were adjusted to a concentration of
1×106 cells/ml and fixed in a 2:1 ratio (v/v) of chilled
methanol overnight. The fixed cells were subsequently stained with
propidium iodide (Sigma-Aldrich) in the presence of RNase
(Sigma-Aldrich). A minimum of 1×104 cells from each
experimental group were analyzed using a flow cytometer (BD
Biosciences, San Jose, CA, USA) to determine the cell cycle
distribution and CellQuest cell cycle analysis software (version
5.1; BD Biosciences) to perform data analysis.
Western blot analysis
To determine the effects of afzelin on the protein
expression levels of MRCKα, LIMK1 and ROCK1, LNCaP and PC-3
prostate cancer cells were treated with varying concentrations of
afzelin (0.1, 1.0 and 10.0 µg/ml). The concentration of each
protein was measured spectrophotometrically using a modified Lowry
assay protocol (DC Protein assay; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Next, the lysates were electrophoresed through
a 7.5–12.0% denaturing polyacrylamide gel. The resolved proteins
were transferred to polyvinylidene fluoride membranes (EMD
Millipore, Bedford, MA, USA) and incubated with blocking solution
(PBS containing 0.05% Tween 20 and 5% skimmed milk) to block
non-specific binding. Next, the membranes were incubated with
monoclonal anti-rabbit MRCKα (1:1,000; cat. no. ab96659; Abcam,
Cambridge, USA), LIMK1 (1:1,000; cat. no. 3842; Cell Signaling
Technology, Inc., Beverly, MA, USA), p-LIMK1 (1:1,000; cat. no.
3841; Cell Signaling Technology, Inc.), ROCK1 (1:2,000; cat. no.
ab45171; Abcam) and p-ROCK1 (1:1,000; cat. no. ab203273; Abcam)
primary antibodies overnight at 4°C, followed by incubation with
horseradish peroxidase-conjugated secondary rabbit antibodies
(1:2,000; cat. no. G33-62G-1000; SignalChem, Richmond, BC, Canada)
for 1 h at room temperature. The positive protein bands were
visualized using enhanced chemiluminescence solution (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Results were analyzed by one way analysis of variance and all data
analysis was performed using SPSS version 21.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
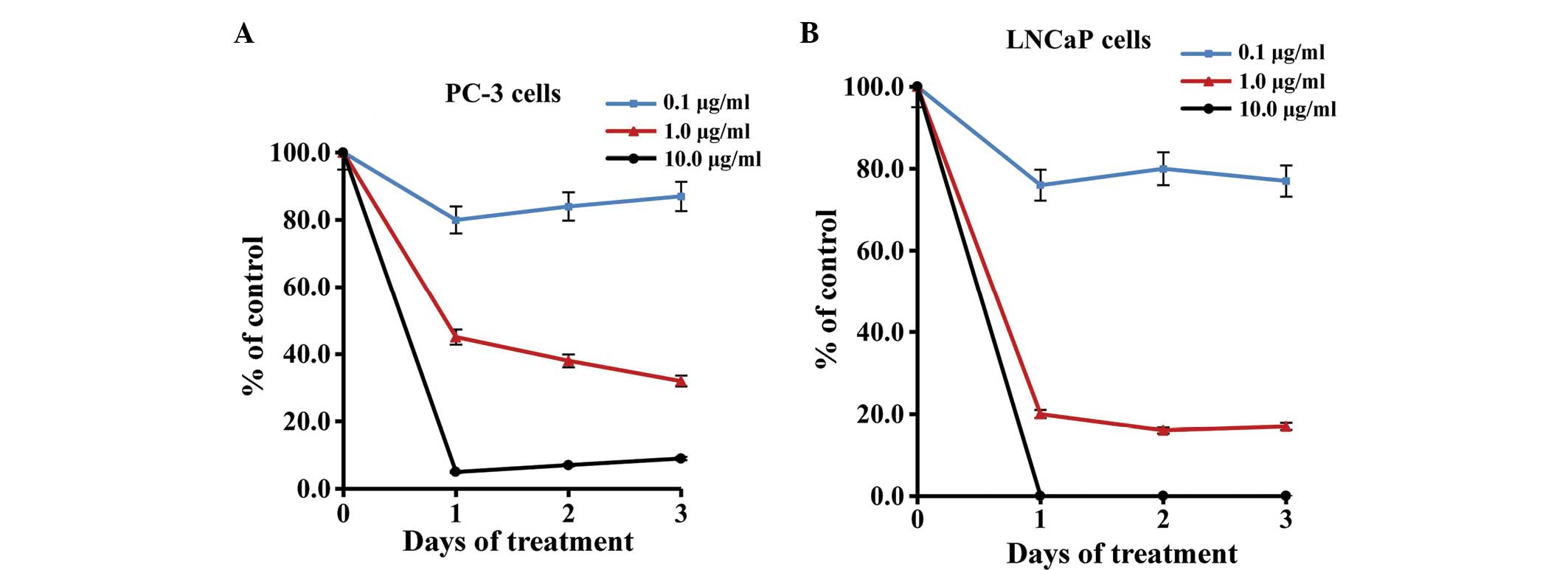
Afzelin decreases the growth of
prostate cancer cells in vitro
The present study analyzed the effect of afzelin on
the growth of two prostate cancer cell lines, PC-3 and LNCaP, by
exposing the cells to increasing concentrations of afzelin (0.1,
1.0 and 10.0 µg/ml) one day after seeding. As indicated in Fig. 2A and B, the viability of the PC-3 and
LNCaP cells, respectively, was monitored for 72 h using a WST-1
cell proliferation assay. The dose response curves are presented as
the percentage of cell growth in the control groups on the
corresponding day, and each value represents the mean of four
independent experiments. The results indicate that exposure to
afzelin at concentrations of 1.0 and 10.0 µg/ml markedly inhibited
the growth of PC-3 and LNCaP cells at 24, 48 and 72 h. Although
afzelin was a potent inhibitor of cell growth in the LNCaP and PC-3
cell lines (P<0.01), no growth inhibition was apparent in the
vehicle (0.1% DMSO)-treated control cells.
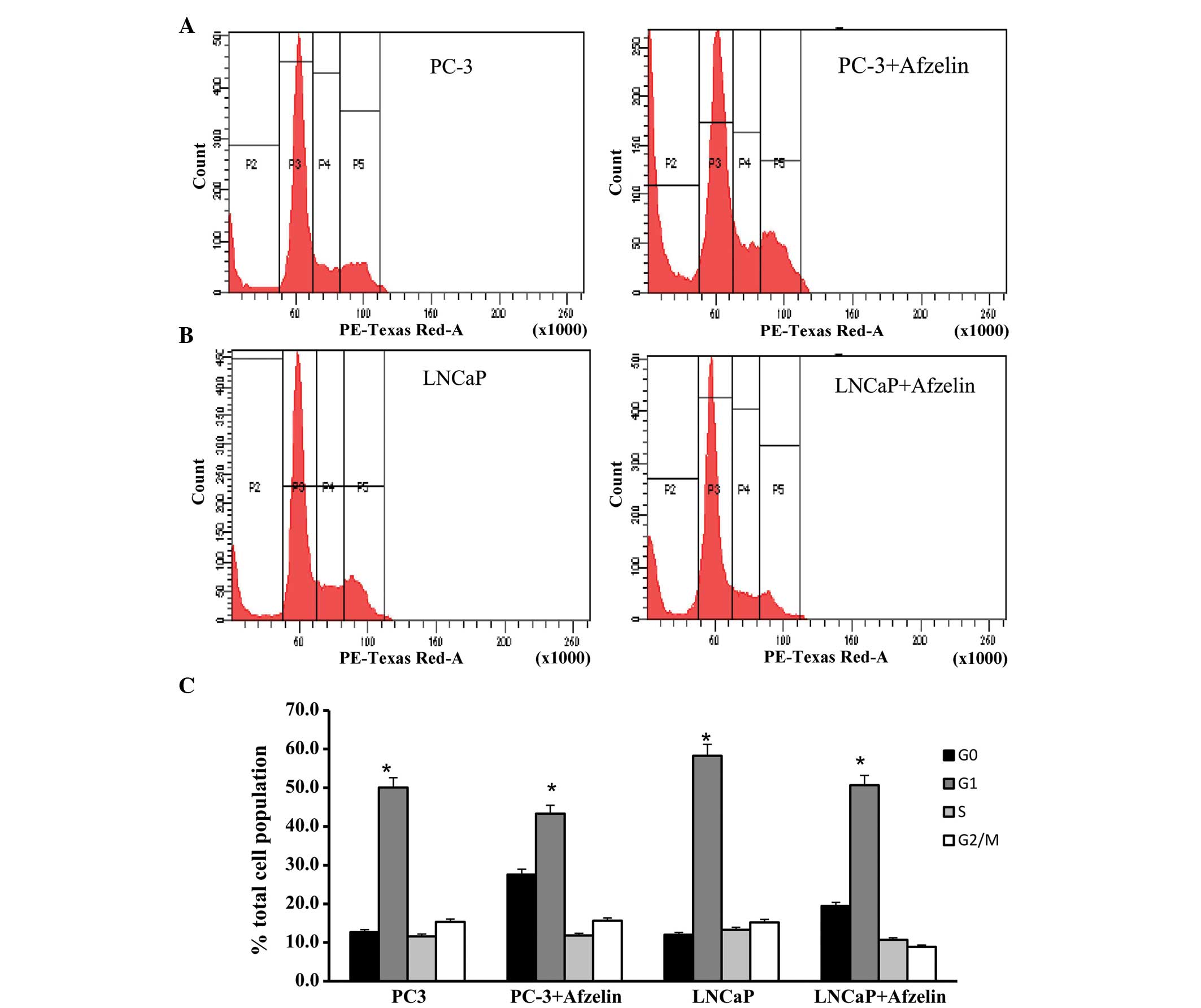
Afzelin induces apoptosis in LNCaP and
PC-3 cells
Flow cytometric analysis was used to determined the
cell cycle distribution of the LNCaP and PC-3 cells in the absence
and presence of afzelin for 24 h. As indicated in Fig. 3A, a broad peak of cells equivalent to
27.6% of the total cell population accumulated in the G0
population of the afzelin-treated (10.0 µg/ml) PC-3 cells, compared
with only 12.7% of the control cells. In addition, 43.3% of the
cells accumulated in the G1 phase of the cell cycle.
Thus, an increased proportion of PC-3 cells localized in the
G0 region following treatment with afzelin. Notably,
similar results were obtained in the LNCaP cells, where 12.0% of
the control cell population localized in the G0 phase
compared with 19.4% of the afzelin-treated (10.0 µg/ml) LNCaP cells
(Fig. 3B). Furthermore, 50.7% of
cells accumulated in G1 phase. The elevated population
of G0 cells observed subsequent to afzelin treatment
indicates the occurrence of extensive apoptosis or an ongoing
cytotoxic response. The results indicated that afzelin exhibits a
selective effect on the two cell types.
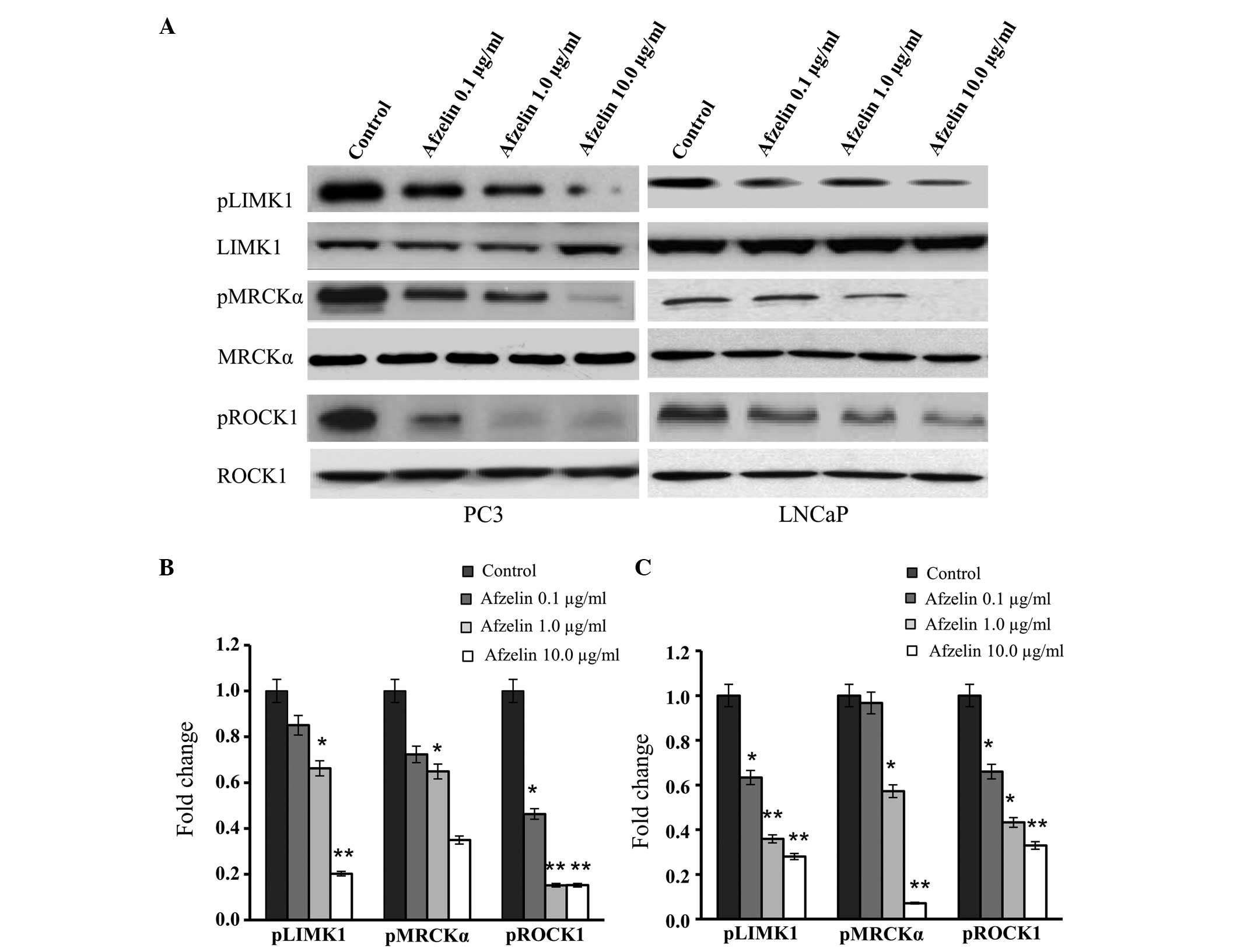
Afzelin decreases the expression of
Rho GTPase family proteins
To investigate the molecular effects of afzelin on
the LNCaP and PC-3 prostate cancer cell lines, an analysis was
conducted to determine the effect of afzelin treatment on the
expression levels of specific molecules that are critical in cell
cycle progression and apoptosis. Western blot analysis was
performed to determine the effect of increasing concentrations of
afzelin on the expression levels of members of the Rho GTPase
family proteins, including LIMK1, MRCKα and ROCK1. The results
identified that phosphorylation of these kinases progressively
diminished in the LNCaP and PC-3 cells as the dose of afzelin was
increased (Fig. 4A). However,
densitometry evaluation indicated that this suppression was more
significant in the PC-3 cells (Fig.
4B).
Discussion
Invasion and metastasis are critical events in
cancer progression, which often lead to patient mortality.
Metastatic cells undergo a sequence of changes that result in loss
of contact inhibition and enhanced motility, which subsequently
promotes cell migration from the primary tumor site, leading to
invasion and angiogenesis (21). At
present, the treatments available for patients with metastatic
tumors are limited. Thus, it is critical to identify and develop
compounds that target the molecular events involved in cell
invasion and metastasis in cancer therapy.
The contractility of the actin-myosin complex is
vital for cell motility and plays an important role in tumor cell
invasion and metastasis. Myosin II, a critical component of the
cytoskeletal contractile machinery, is regulated by the
phosphorylation of myosin II light chain proteins (MLC) at Thr18
and Ser19 (22). Furthermore, the
members of the Rho GTPase family are involved with the regulation
of actin cytoskeleton organization and dynamics, mediating the
development of focal adhesions and stress fibers (23–25).
Rho A and C proteins promote the actomyosin
contractile force generation via ROCK. ROCK1 and 2 cause the
phosphorylation of several downstream target proteins, including
MLC, the myosin binding subunit of MLC phosphatase, LIMK1 and LIMK2
(12,26). These target proteins catalyze various
processes involved in cancer progression, such as changes in
structural arrangement, alterations in cellular polarity,
proliferation, migration, invasion, transformation and cytokinesis
(26,27).
The phosphorylation of the LIMKs is also mediated by
MRCKα and p21-activated kinase, acting downstream of the
Rho/Rac/Cdc42 signaling pathway (28–31). In
addition, LIMK1 and 2 have been shown to exhibit a crucial role in
cancer progression, angiogenesis and metastasis (32). Previous studies have identified
increased LIMK1 expression in human breast cancer cell lines;
increased expression of LMK1 increased tumor invasion, whereas the
inhibition of LIMK1 expression, or blockade of LIMK1 activity
reduced the aggressive behavior of human MDA-MB-231 and MDA-MB-435
breast cancer cell lines (33,34). Thus
inhibitors of LIMK1, ROCK1 and MRCKα may inhibit tumor invasion and
metastasis effectively.
In the present study it was demonstrated that
afzelin, a flavonol glycoside found in Nymphaea odorata
(35), exhibits significant
anti-prostate cancer activities in androgen-sensitive LNCaP and
androgen-independent PC-3 prostate cancer cell lines. Afzelin has
been reported to exhibit antioxidant, DNA-protective, ultra-violet
radiation-absorbing and anti-inflammatory properties (36). In addition, a recent study revealed
that afzelin inhibits breast cancer cell proliferation by
stimulating apoptosis (5). Notbaly,
afzelin attenuated asthma in a murine model of asthma (37). In the present study, afzelin inhibited
cell proliferation in the two prostate cancer cell lines and
blocked the cell cycle in the G0 phase. Furthermore,
afzelin attenuated the expression of a number of kinases involved
in the maintenance of the actin cytoskeleton.
The effect of afzelin on cell growth in different
cell lines was evaluated in the current study, with afzelin
identified to markedly inhibit the growth of the prostate cancer
cell lines, LNCaP and PC-3. Furthermore, the effect of afzelin on
cell cycle progression was evaluated in the LNCaP and PC-3 cells.
The results revealed that afzelin (10.0 µg/ml) caused a markedly
increased population of PC-3 and LNCaP cells to accumulate in the
G0 phase when compared with the corresponding control
cells. To improve current understanding of the inhibitory effect of
afzelin on cell growth and cell cycle progression, the expression
levels of specific kinases were evaluated in the PC-3 and LNCaP
cell lines upon treatment with increasing concentrations of
afzelin. Afzelin was found to attenuate the protein expression
levels of LIMK1 in the LNCaP and PC-3 cells. Elevated expression of
LIMK1 has been previously identified in prostate cancer cell lines;
therefore, afzelin represents a novel strategy for prostate cancer
treatment. Furthermore, LIMK1 is activated by MRCKα and ROCK1
(9,12); thus, the effect of afzelin on the
expression of these kinases was also evaluated. The results of the
present study revealed that afzelin inhibited the protein
expression levels of MRCKα and ROCK1, clarifying that
downregulation of LIMK1 by afzelin is due to its inhibitory effect
on MRCKα and ROCK1, upstream in its signaling pathway. Previous
studies have demonstrated that ROCK inhibitors appear to reduce the
invasive ability of tumor cells in vitro and prevent the
dissemination of tumor cells in vivo in different types of
cancer, particularly in prostate cancer (10,13–17,38).
Furthermore, LIMK1, MRCKα and ROCK1 are proteins of interest in the
development of novel cancer therapies due to their involvement in
regulating actin organization (7)
and, in combination with other proteins, maintaining the tight
regulation of normal cell growth and differentiation (8,9).
Increased MRCKα expression has been reported in a
number of cancer types, including adenocarcinoma (39), cutaneous squamous cell carcinoma (SCC)
(40), in oral, hypopharyngeal, head
and neck SCC, oral cavity and tongue carcinoma (41–47). In
addition, increased MRCKα expression has also been identified in
in vitro studies using U937 histiocytic lymphoma, MDA-MB 231
breast cancer, A549 lung cancer and PLB-985 myelocytic leukemia
cell lines (46,47). Thus, the inhibition of MRCKα may
present an effective strategy to inhibit cancer. Furthermore,
Lourenço et al (48) revealed
that LIMK2 expression was decreased in the intestinal tumors of
cancer-prone genetically modified mice, as well as in human
colorectal cancer cell lines and tumors. In the present study,
afzelin was found to inhibit the expression of LIMK1, ROCK1 and
MRCKα, indicating that their inhibition may prevent tumor cell
invasion and metastasis. These results indicate that afzelin may
effectively reduce tumor progression, however, further studies are
required to investigate the association between azfelin and the
expression of LIMK1, ROCK1 and MRCKα as afzelin may also have
upstream targets, such as RhoA, Rac1a and Cdc42 that may effect the
activation and signal transduction downstream of LIMK1. ROCK1 and
MRCKα.
Conventional therapies, including radiotherapy and
chemotherapy, exhibit certain limitations in the treatment of
hormone-refractory prostate cancer; therefore, the development of
novel treatment strategies is required to enhance the number of
positive treatment outcomes. The results of the present study
indicate that afzelin may be an important candidate in prostate
cancer therapy. However, additional studies are required to
evaluate its therapeutic properties, particularly in animal
models.
Acknowledgements
This study was supported by the General Programs of
Shanghai Municipal Health Bureau (grant no. 20124345), the Second
Key Medical Specialties Construction Project of Shanghai (grant no.
201373) and the Science and Technology Commission of Shanghai
(grant no. 20111004).
References
|
1
|
Lowe HI, Watson CT, Badal S, et al:
Cycloartane-3,24,25-triol inhibits MRCKα kinase and demonstrates
promising anti prostate cancer activity in vitro. Cancer Cell Int.
12:462012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, et al: Cancer incidence and mortality patterns
in Europe: estimates for 40 countries in 2012. Eur J Cancer.
49:1374–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthold DR, Sternberg CN and Tannock IF:
Management of advanced prostate cancer after first-line
chemotherapy. J Clin Oncol. 23:8247–8252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diantini A, Subarnas A, Lestari K, et al:
Kaempferol-3-O-rhamnoside isolated from the leaves of Schima
wallichii Korth inhibits MCF-7 breast cancer cell proliferation
through activation of the caspase cascade pathway. Oncol Lett.
3:1069–1072. 2012.PubMed/NCBI
|
|
6
|
Mao YW, Tseng HW, Liang WL, et al:
Anti-inflammatory and free radial scavenging activities of the
constituents isolated from Machilus zuihoensis. Molecules.
16:9451–9466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heikkila T, Wheatley E, Crighton D, et al:
Co-crystal structures of inhibitors with MRCKβ, a key regulator of
tumor cell invasion. PloS One. 6:e248252011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaffe AB and Hall A: Rho GTPases:
biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilkinson S, Paterson HF and Marshall CJ:
Cdc42-MRCK and Rho-ROCK signaling cooperate in myosin
phosphorylation and cell invasion. Nat Cell Biol. 7:255–261. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakajima M, Hayashi K, Egi Y, et al:
Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16
melanoma. Cancer Chemother Pharmacol. 52:319–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakajima M, Hayashi K, Katayama K, et al:
Wf-536 prevents tumor metastasis by inhibiting both tumor motility
and angiogenic actions. Eur J Pharmacol. 459:113–120. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakajima M, Katayama K, Tamechika I, et
al: WF-536 inhibits metastatic invasion by enhancing the host cell
barrier and inhibiting tumour cell motiltiy. Clin Exp Pharmacol
Physiol. 30:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Genda T, Sakamoto M, Ichida T, et al: Cell
motility mediated by rho and Rho-associated protein kinase plays a
critical role in intrahepatic metastasis of human hepatocellular
carcinoma. Hepatology. 30:1027–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takamura M, Sakamoto M, Genda T, et al:
Inhibition of intrahepatic metastasis of human hepatocellular
carcinoma by Rho-associated protein kinase inhibitor Y-27632.
Hepatology. 33:577–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ying H, Biroc SL, Li WW, et al: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bemis DL, Capodice JL, Desai M, et al: A
concentrated aglycone isoflavone preparation (GCP) that
demonstrates potent anti-prostate cancer activity in vitro and in
vivo. Clin Cancer Res. 10:5282–5292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ngamwongsatit P, Banada PP, Panbangred W
and Bhunia AK: WST-1-based cell cytotoxicity assay as a substitute
for MTT-based assay for rapiddetection of toxigenic Bacillus
species using CHO cell line. J Microbiol Meth. 73:211–215. 2008.
View Article : Google Scholar
|
|
21
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banyard J, Anand-Apte B, Symons M and
Zetter BR: Motility and invasion are differentially modulated by
Rho family GTPases. Oncogene. 19:580–591. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hall A: The cytoskeleton and cancer.
Cancer Metastasis Rev. 28:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan Fisher, Wewer UM and Yoneda A:
Regulation of ROCK activity in cancer. J Histochem Cytochem.
61:185–198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gurkar AU, Chu K, Raj L, et al:
Identification of ROCK1 kinase as a critical regulator of
Beclin1-mediated autophagy during metabolic stress. Nat Commun.
4:21892013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edwards DC, Sanders LC, Bokoch GM and Gill
GN: Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase
signalling to actin cytoskeletal dynamics. Nat Cell Biol.
1:253–259. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohashi K, Nagata K, Maekawa M, Ishizaki T,
Narumiya S and Mizuno K: Rho associated kinase ROCK activates
LIM-kinase 1 by phosphorylation at threonine 508 within the
activation loop. J Biol Chem. 275:3577–3582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sumi T, Matsumoto K, Shibuya A and
Nakamura T: Activation of LIM kinases by myotonic dystrophy
kinase-related Cdc42-binding kinase alpha. J Biol Chem.
276:23092–23096. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dan C, Kelly A, Bernard O and Minden A:
Cytoskeletal changes regulated by the PAK4 serine/threonine kinase
are mediated by LIM kinase 1 and cofilin. J Biol Chem.
276:32115–32121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vlecken DH and Bagowski CP: LIMK1 and
LIMK2 are important for metastatic behavior and tumor cell-induced
angiogenesis of pancreatic cancer cells. Zebrafish. 6:433–439.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshioka K, Foletta V, Bernard O and Itoh
K: A role for LIM kinase in cancer invasion. Proc Natl Acad Sci
USA. 100:7247–7252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bagheri Yarmand, Mazumdar A, Sahin AA and
Kumar R: LIM kinase 1 increases tumor metastasis of human breast
cancer cells via regulation of the urokinase-type plasminogen
activator system. Int J Cancer. 118:2703–2710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, ElSohly HN, Li XC, et al:
Phenolic compounds from Nymphaea odorat. J Nat Prod.
66:548–550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shin SW, Jung E, Kim S, Kim JH, Kim EG,
Lee J and Park D: Antagonizing effects and mechanisms of afzelin
against UVB-induced cell damage. PLoS One. 8:e619712013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou W and Nie X: Afzelin attenuates
asthma phenotypes by downregulation of GATA3 in a murine model of
asthma. Mol Med Rep. 12:71–76. 2015.PubMed/NCBI
|
|
38
|
Benitaha SA, Valeróna PF, Aelstb L,
Marshallc CJ and Lacala JC: Rho GTPases in human cancer: an
unresolved link to upstream and downstream transcriptional
regulation. Biochimicaet Biophysica Acta. 1705:121–132. 2005.
|
|
39
|
Balasenthil S, Chen N, Lott ST, et al: A
migration signature and plasma biomarker panel for pancreatic
adenocarcinoma. Cancer Prev Res (Phila). 4:137–149. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lefort K, Mandinova A, Ostano P, et al:
Notch1 is a p53 target gene involved in human keratinocyte tumor
suppression through negative regulation of ROCK1/2 and MRCK-alpha
kinases. Genes Dev. 21:562–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pyeon D, Newton MA, Lambert PF, et al:
Fundamental differences in cell cycle deregulation in human
papillomavirus-positive and human papillomavirus-negative head/neck
and cervical cancers. Cancer Res. 67:4605–4619. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schlingemann J, Habtemichael N, Ittrich C,
et al: Patient-based cross-platform comparison of oligonucleotide
microarray expression profiles. Lab Invest. 85:1024–1039. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ginos MA, Page GP, Michalowicz BS, et al:
Identification of a gene expression signature associated with
recurrent disease in squamous cell carcinoma of the head and neck.
Cancer Res. 64:55–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim SM, Park YY, Park ES, et al:
Prognostic biomarkers for esophageal adenocarcinoma identified by
analysis of tumor transcriptome. PLoS One. 5:e150742010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang S, Zhan M, Yin J, et al:
Transcriptional profiling suggests that Barrett's metaplasia is an
early intermediate stage in esophageal adenocarcinogenesis.
Oncogene. 25:3346–3356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hao Y, Triadafilopoulos G, Sahbaie P, et
al: Gene expression profiling reveals stromal genes expressed in
common between Barrett's esophagus and adenocarcinoma.
Gastroenterology. 131:925–933. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kimchi ET, Posner MC, Park JO, et al:
Progression of Barrett's metaplasia to adenocarcinoma is associated
with the suppression of the transcriptional programs of epidermal
differentiation. Cancer Res. 65:3146–3154. 2005.PubMed/NCBI
|
|
48
|
Lourenço FC, Munro J, Brown J, et al:
Reduced LIMK2 expression in colorectal cancer reflects its role in
limiting stem cell proliferation. Gut. 63:480–493. 2014. View Article : Google Scholar : PubMed/NCBI
|