Introduction
Nasopharyngeal carcinoma (NPC) is a tumor of the
head and neck with a complex etiology. The incidence of NPC is low
in Western countries (0.5 per 100,000); however, in Southern China
and Asia NPC incidence peaks at 30 per 100,000 (1). Although radiotherapy (2) and chemoradiotherapy (3) have significantly improved the survival
rates of patients with NPC, the five-year survival probability
remains low due to disease recurrence and distant metastasis
(4). As a result of the high
incidence of recurrence and frequently fatal metastasis, the
identification of risk factors for recurrence and metastasis will
benefit patients with NPC.
Previous studies have demonstrated an association
between Epstein-Barr virus (EBV) infection and NPC. EBV-DNA may be
detected in NPC cells (5,6), and cell-free EBV-DNA may be detected in
the plasma of patients with NPC (7–13).
Furthermore, the levels of plasma EBV-DNA in recently diagnosed NPC
patients have been significantly correlated with tumor volume
(14,15), response to treatment (16), tumor clearance (17,18) and
tumor recurrence (19–21). Similarly, it has been observed that a
positive post-treatment detection of plasma EBV-DNA is predictive
of poor outcomes in terms of subsequent relapse rate, overall
survival (OS) and relapse-free survival (RFS) (11,13).
Therefore, the pre- and post-treatment levels of plasma EBV-DNA
have been used as reliable biomarkers for the screening, diagnosis,
monitoring and prognosis of NPC. However, previous studies have
included small cohorts of patients with NPC or short follow-up
times.
The identification of novel risk factors involved in
disease recurrence and poor survival is important for facilitating
early intervention. Furthermore, these risk factors will be of
significance for the prognosis and management of patients with NPC.
The objective of the present retrospective study was to determine
whether pre- and post-treatment levels of plasma EBV-DNA were
predictive of poor survival in a large sample of patients with
NPC.
Materials and methods
Patients and clinical specimens
In order to determine the potential value of pre-
and post-treatment plasma EBV-DNA levels for the prognosis of NPC,
637 patients with newly diagnosed NPC from the Department of
Otolaryngology - Head and Neck Surgery, Nanfang Hospital
(Guangzhou, China) and 245 healthy controls from the Physical
Examination Center, Nanfang Hospital were recruited between January
2006 and April 2013. NPC patients were diagnosed following the
pathological examination of biopsied tissues, and classified
according to the 2009 American Joint Committee on Cancer
tumor-node-metastasis (TNM) staging system (7th edition) (22). Patients with other types of tumor were
excluded from the study.
Venous blood samples were collected from patients
prior to treatment and at the first follow-up appointment. In
total, 8 ml whole blood was drawn into EDTA-containing tubes (BD
Biosciences, Franklin Lakes, NJ, USA) and separated into plasma and
cellular fractions by centrifugation at 1,500 × g for 5 min at 4°C.
The plasma was then stored at −80°C until further processing. The
present study was approved by the ethics committee of Nanfang
Hospital, Southern Medical University (Guangzhou, Guangdong, China)
and written informed consent was obtained from all patients prior
to treatment.
Clinical management
All patients received a uniform protocol of
conventional two-dimensional radiotherapy to the primary tumor and
neck region, with a total dose of 66–70 Gy over a six- to
eight-week period. In addition, 516 of the 637 (81.00%) patients
with advanced disease (T3-T4 or N2-N3) received induction
chemotherapy prior to radiation or adjuvant chemotherapy subsequent
to radiation.
Following the completion of treatment, all patients
were examined at 3, 6 and 12 months in the first year, every 6
months during the second and third years, and yearly thereafter.
Recurrence of NPC was evaluated by clinical physical examination,
chest radiography, magnetic resonance imaging and/or computed
tomography (CT; from the skull base to the whole neck), abdominal
sonography, whole-body bone scans, fiberoptic nasopharyngoscopy and
biopsied pathological verification if necessary and if the patient
agreed. Positron emission tomography-CT was used in order to
confirm the presence of distant metastasis.
Quantification of plasma EBV-DNA
The detection of plasma EBV-DNA was performed by the
Department of Laboratory Medicine, Nanfang Hospital. The total
plasma DNA was extracted using a QIAamp Blood Kit (Qiagen, Hilden,
Germany) and the content of plasma EBV-DNA was determined by
quantitative polymerase chain reaction (qPCR) using an EBV qPCR kit
(Liferiver, Shanghai, China), according to the manufacturer's
instructions. GAPDH (house keeping gene) was used as the internal
control. All reactions were performed in triplicate, including a
nontemplate control and a total of 5 µl DNA was used for each
reaction. Briefly, the following primers were used: Forward, 5′-GCT
GCG CTG CTG CTA TCT T-3′ and reverse, 5′-CAA GCC CAC TCC CCT GTC
T-3′ for the BamHI-W region of the EBV genome; and forward, 5′-GGC
GAC GCA AAA GAA GAT G-3′ and reverse, 5′-CCG TTG ACT CCG ACC TTC
AC-3′ for GAPDH (Life Technologies, Grand Island, NY, USA). PCR was
performed under the following conditions: Initial denaturation step
at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and
56°C for 1 min. EBV DNA copy number was calculated using the mean
values for the duplicate samples, according to the following
formula: C = Q × (VDNA/VPCR) × (1/Vext). C represents
the target concentration in plasma (copies/ml), Q represents the
target quantity (copies) determined by the sequence detector, VDNA
represents the total volume of DNA obtained after extraction, VPCR
represents the volume of DNA solution used for PCR amplification,
and Vext represents the volume of plasma extracted. In the present
study, 0 copies/ml was recorded if the plasma EBV-DNA was
undetectable by qPCR; a positive plasma EBV-DNA load was defined as
>0 copies/ml.
Statistical analyses
Previously reported cut-off values for pre- and
post-treatment plasma EBV-DNA levels were used in the present study
(1,500 copies/ml pre-treatment; 0 copies/ml post-treatment)
(11,13). Patients were stratified at 1,500
copies/ml pre-treatment and 0 copies/ml post-treatment. The relapse
and mortality rates of each group of patients were analyzed by the
χ2 test. The RFS (or OS) was calculated from the first
day of induction chemotherapy to the date of disease recurrence,
mortality or the final follow-up visit. The periods of RFS and OS
among the various groups of patients were evaluated by the
Kaplan-Meier method and analyzed by the log-rank test. The
potential risk of age, gender, tumor classification, lymph node
status, metastasis status, and status of pre-treatment and
post-treatment plasma EBV-DNA on the survival of NPC patients was
analyzed by the hazard ratio (HR), 95% confidence interval (CI) and
Wald test using the multivariate Cox proportional hazards model.
Levels of pre-treatment EBV-DNA between different tumor
classification, lymph node status, metastasis status and overall
stage were compared using Kruskal-Wallis and Wilcoxon tests. All
statistical analyses were performed using SPSS version 19.0 for
Windows (SPSS, Inc., Chicago, IL, USA). A two-tailed P-value of
<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The demographic and clinical characteristics of the
637 patients included in the present study are shown in Table I. During the median observation period
of 23 months (range, 5–75 months), there were 140 patients with NPC
recurrence and 101 patients who had succumbed to the disease.
 | Table I.Patients and disease characteristics
(n=637). |
Table I.
Patients and disease characteristics
(n=637).
| Characteristic | Value | n (%) |
|---|
| Age, years |
|
|
|
Median | 46 |
|
|
Range | 14–80 |
|
| Gender |
|
|
| Male | – | 477 (74.88) |
|
Female | – | 160 (25.12) |
| Overall stage |
|
|
| I | – | 21 (3.30) |
| II | – | 100 (15.70) |
| III | – | 244 (38.30) |
| IV | – | 272 (42.70) |
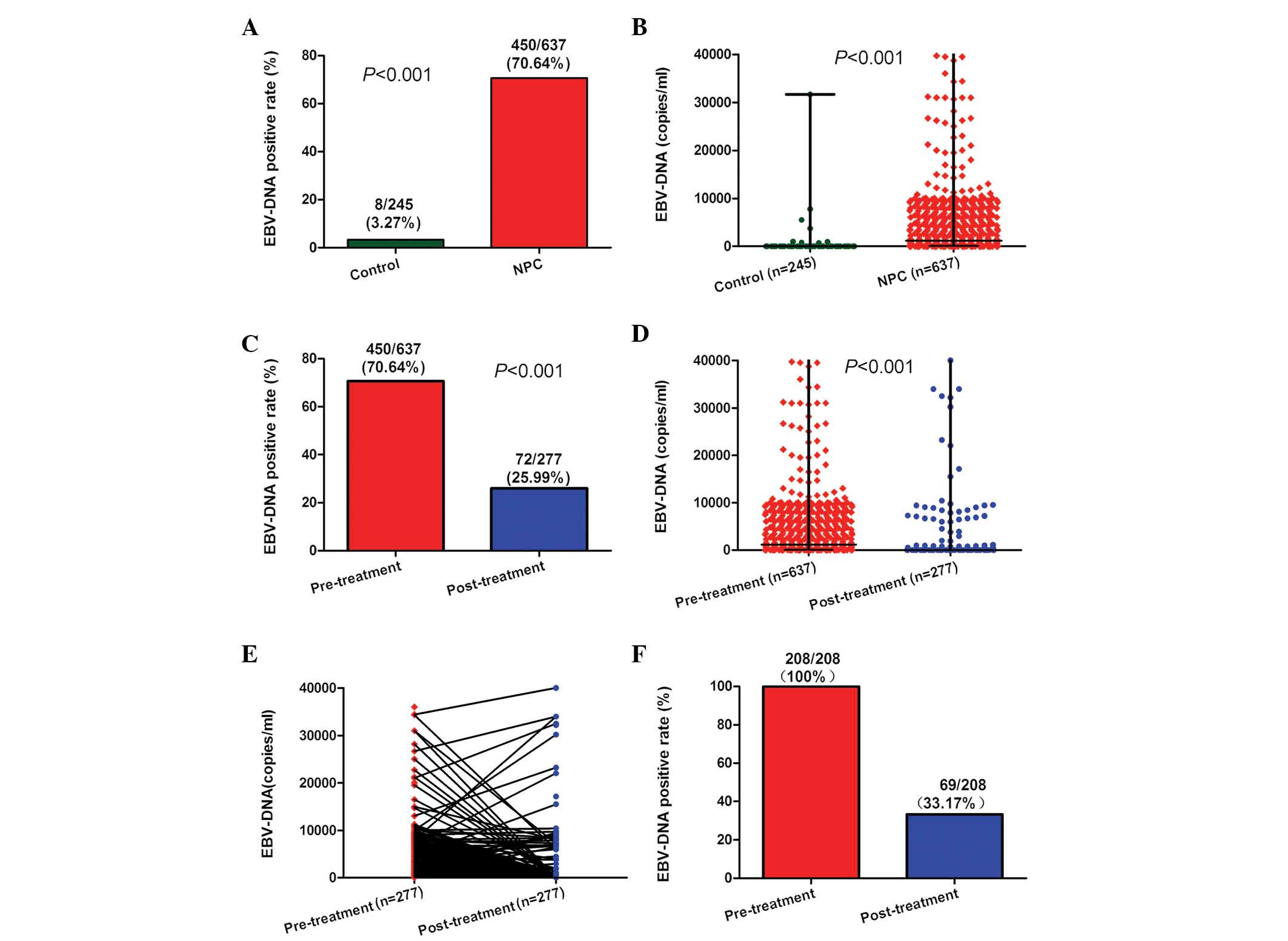
Alterations in pre- and post-treatment
plasma EBV-DNA
The analysis of plasma EBV-DNA was performed in 245
healthy controls, 637 NPC patients prior to treatment and 277 NPC
patients subsequent to treatment. In the present study, plasma
EBV-DNA was positive in 8 (3.27%) healthy controls and 450 (70.64%)
NPC patients prior to treatment (Fig.
1A; P<0.001). The pre-treatment plasma EBV-DNA loads were
significantly higher in NPC patients than those in healthy controls
(Fig. 1B; P<0.001). The percentage
of NPC patients positive for EBV-DNA was significantly higher prior
to treatment (70.64%) than following treatment (25.99%; Fig. 1C; P<0.001). Furthermore, the
pre-treatment plasma EBV-DNA loads were significantly higher
(median, 1150 copies/ml; range, 0–9.75×106 copies/ml)
than those post-treatment (median, 0 copies/ml; range,
0–3.83×106 copies/ml) (Fig.
1D; P<0.001). Of the 277 patients who were examined
following treatment (Fig. 1E), 208
had been positive for plasma EBV-DNA prior to treatment. Of these
208 patients, 139 (66.83%) were negative for plasma EBV-DNA
following treatment (Fig. 1F).
Next, the associations between pre-treatment plasma
EBV-DNA and patient clinical characteristics were evaluated. A
significant difference in the levels of pre-treatment plasma
EBV-DNA was identified with respect to tumor classification, lymph
node status, metastasis status and overall stage (Table II; P<0.001). It was revealed that
patients with a high plasma EBV-DNA load had a higher clinical
tumor classification, lymph node status, metastasis status and
overall cancer stage.
 | Table II.Pre-treatment plasma EBV-DNA levels
and clinical characteristics (n=637). |
Table II.
Pre-treatment plasma EBV-DNA levels
and clinical characteristics (n=637).
|
|
| EBV-DNA
(copies/ml) |
|
|---|
|
|
|
|
|
|---|
| Characteristics | n (%) | Median | Mean rank score | P-value |
|---|
| Tumor
classification |
|
|
| aP<0.001 |
| T1 | 108 (16.95%) | 630 | 251.88 |
|
| T2 | 151 (23.71%) | 942 | 284.63 |
|
| T3 | 191 (29.98%) | 2250 | 341.05 |
|
| T4 | 187 (29.36%) | 3500 | 363.00 |
|
| Lymph node
status |
|
|
| aP<0.001 |
| N0 | 74
(11.62%) | 0 | 205.24 |
|
| N1 | 191 (29.98%) | 832 | 269.27 |
|
| N2 | 270 (42.39%) | 2270 | 337.58 |
|
| N3 | 102 (16.01%) | 8900 | 445.48 |
|
| Metastasis
status |
|
|
| bP<0.001 |
| M0 | 606 (95.13%) | 1000 | 308.71 |
|
| M1 | 31 (4.87%) | 12200 | 520.06 |
|
| Overall stage |
|
|
| aP<0.001 |
| I | 21 (3.30%) | 0 | 148.69 |
|
| II | 100 (15.70%) | 420 | 212.58 |
|
|
III | 244 (38.30%) | 982 | 300.21 |
|
| IV | 272 (42.70%) | 4620 | 388.13 |
|
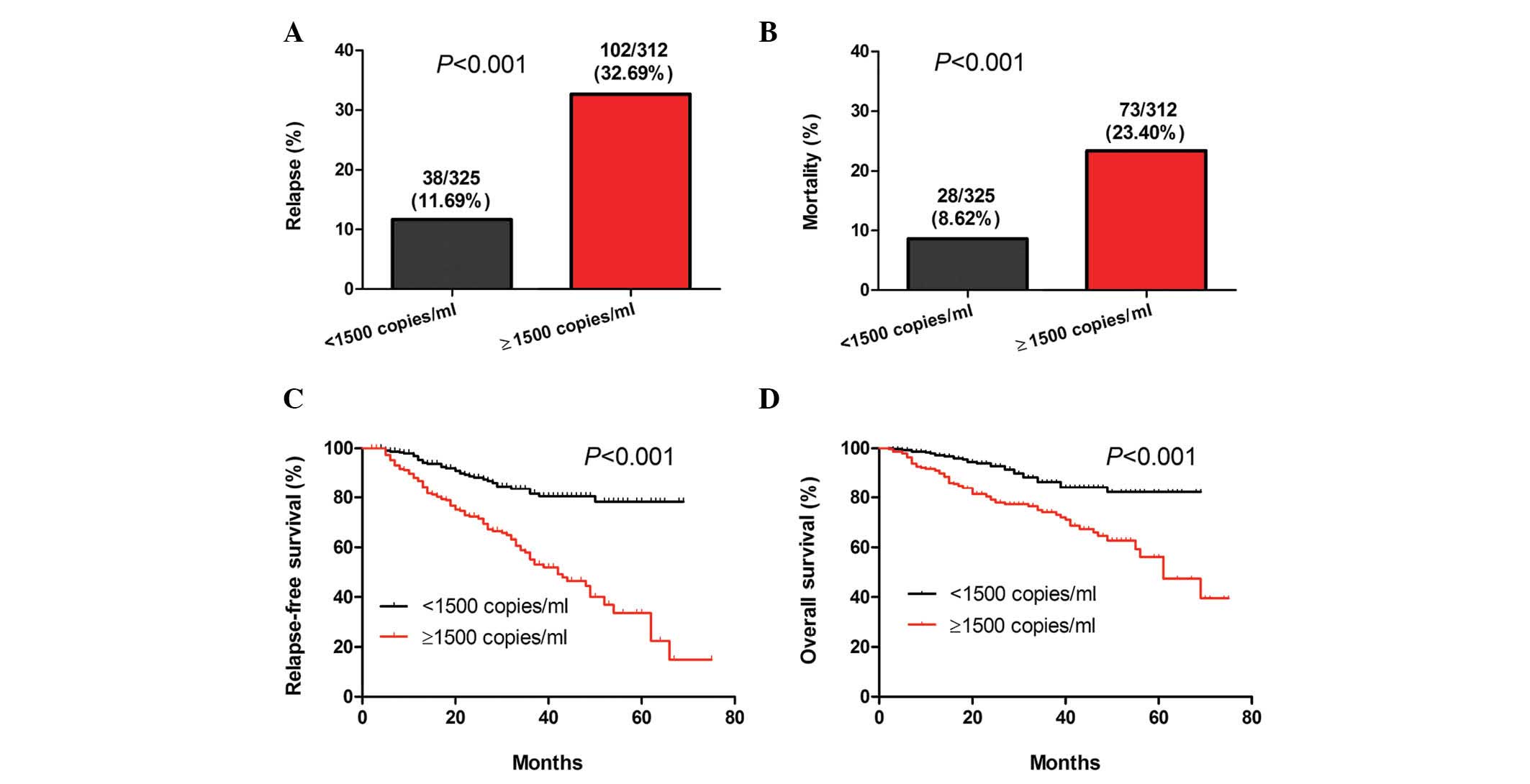
High pre-treatment plasma EBV-DNA
levels are associated with poor prognosis
The 637 patients were divided into two groups based
on a plasma EBV-DNA cut-off value of 1,500 copies/ml. Results of
the stratification analyses indicated that there was a significant
difference in the rates of NPC relapse and mortality between the
two groups of patients (P<0.001; Fig.
2A and B). In addition, a significant difference was identified
in the length of RFS and OS between the two groups (P<0.001;
Fig. 2C and D). Accordingly, the risk
of NPC relapse and mortality in patients with pre-treatment plasma
EBV-DNA levels of ≥1,500 copies/ml was higher than that of those
with pre-treatment plasma EBV-DNA levels of <1,500
copies/ml.
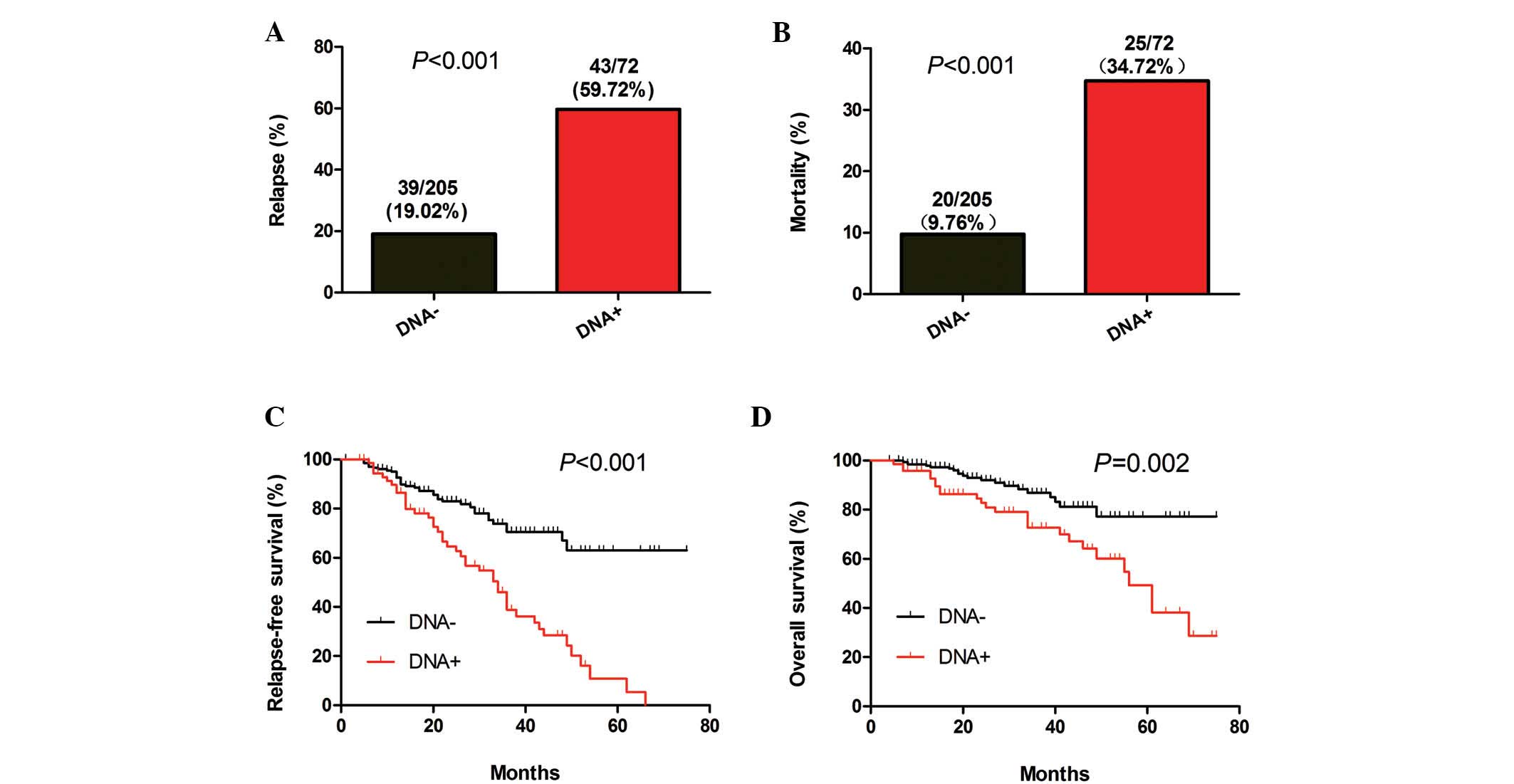
Positive post-treatment plasma EBV-DNA
is associated with poor prognosis
The 277 patients who were tested for the levels of
post-treatment plasma EBV-DNA were divided in two groups based on a
plasma EBV-DNA cut-off value of 0 copies/ml. The relapse and
mortality rates, as well as the periods of RFS and OS between the
two groups of patients were then analyzed. A significant difference
in the relapse and mortality rates was identified between the two
groups (P<0.001; Fig. 3A and B).
Similarly, there was a significant difference in the length of RFS
(P<0.001; Fig. 3C) and OS
(P=0.002; Fig. 3D) between the two
groups of patients. These data indicated that the risk for relapse
and mortality was higher amongst NPC patients with positive
post-treatment plasma EBV-DNA than that in patients with negative
post-treatment plasma EBV-DNA.
Positive post-treatment EBV-DNA and
the presence of distant metastasis are significant prognostic
factors for RFS and OS
Multivariate analysis, using the multivariate Cox
proportional hazard model, was applied to the data of the 277
patients who were tested for post-treatment plasma EBV-DNA. The Cox
analysis was based on age, gender, tumor classification, lymph node
status, metastasis, pre-treatment plasma EBV-DNA levels and
post-treatment detection of plasma EBV-DNA. Risk factors that
conferred a significantly shorter RFS period in patients with NPC
were high pre-treatment levels of plasma EBV-DNA (≥1,500 copies/ml;
HR, 1.954; 95% CI, 1.080–3.534; P=0.027) and a detectable
post-treatment level of plasma EBV-DNA (HR, 2.306; 95% CI,
1.427–3.727; P=0.001) (Table III).
Significant risk factors for a shorter OS period in patients with
NPC, included greater lymph node involvement (HR, 1.549; 95% CI,
1.054–2.277; P=0.026) and distant metastasis (HR, 4.216; 95% CI,
1.703–10.435; P=0.002). A detectable level of post-treatment plasma
EBV-DNA was the most important prognostic factor for RFS, whilst
the presence of distant metastasis was identified to be the most
significant prognostic factor for OS.
 | Table III.RFS and OS analyses using a
multivariate Cox proportional hazards model. |
Table III.
RFS and OS analyses using a
multivariate Cox proportional hazards model.
|
| RFS | OS |
|---|
|
|
|
|
|---|
| Parameter | HR (95% CI) | aP | HR (95% CI) | aP |
|---|
| Age, ≥45 years vs.
<45 years | 1.346 (0.695,
2.604) | 0.378 | 0.625 (0.283,
1.363) | 0.238 |
| Gender, male vs.
female | 1.124 (0.720,
1.753) | 0.607 | 1.328 (0.715,
2.468) | 0.369 |
| Tumor
classification, T1, T2, T3, T4 | 1.148 (0.913,
1.444) | 0.238 | 1.322 (0.939,
1.860) | 0.110 |
| Lymph node status,
N0, N1, N2, N3 | 1.261 (0.965,
1.648) | 0.090 | 1.549 (1.054,
2.277) | 0.026 |
| Metastasis status,
M1 vs. M0 | 0.139 (0.019,
1.027) | 0.053 | 4.216
(1.703, 10.435) | 0.002 |
| Pre-treatment
EBV-DNA, ≥1500 vs. <1500 copies/ml | 1.954 (1.080,
3.534) | 0.027 | 1.827 (0.787,
4.237) | 0.161 |
| Post-treatment
EBV-DNA, positive vs. negative | 2.306 (1.427,
3.727) | 0.001 | 1.432 (0.708,
2.894) | 0.318 |
Discussion
Elevated levels of circulating cell-free EBV-DNA are
able to be detected by qPCR in the plasma and serum of patients
with NPC (7–13). Although specificity is approximately
identical in the two sample types, it has been established that the
detection of EBV-DNA is more sensitive in plasma than in serum
(23). Plasma EBV-DNA has become a
well-known prognostic factor for the relapse and survival of NPC
patients (11–13,19).
Similarly, the presence of serum immunoglobulin (Ig)A antibodies
against the EBV capsid antigen (VCA-IgA) has also been identified
to be associated with an increased risk of NPC (24,25). It
has been demonstrated that NPC patients with higher levels of
VCA-IgA have poorer prognoses (26,27). Given
that plasma EBV-DNA is superior to VCA-IgA in sensitivity,
specificity and predicting the prognosis of patients with NPC
(28), plasma EBV-DNA may replace the
conventional VCA-IgA antibody test in the diagnosis and management
of NPC.
Previous studies have revealed that EBV-DNA loads in
NPC patients were increased as the cancer stage increased from
stage I to IV (29–31). In the present retrospective study, the
associations between pre-treatment levels of plasma EBV-DNA and the
clinical characteristics of 637 NPC patients were investigated. It
was identified that pre-treatment levels of plasma EBV-DNA in
patients with NPC correlated with clinical staging, tumor
classification and lymph node status, as well as with metastasis
status. The most significant difference in the pre-treatment levels
of plasma EBV-DNA was between varying metastasis statuses (1,000
vs. 12,200 copies/ml). The results also revealed that the plasma
EBV-DNA loads and the rates of positive detection in NPC patients
declined significantly following treatment. These data suggested
that the pre-treatment levels of plasma EBV-DNA reflect tumor
burden, which is consistent with the hypothesis that plasma EBV-DNA
is derived from tumor cells in patients with NPC (7).
Patients with NPC are routinely tested for EBV-DNA,
as it has been established that high levels of pre-treatment plasma
EBV-DNA or positive post-treatment plasma EBV-DNA are prognostic
factors for the relapse and survival of NPC patients. Lin et
al (11) and Wang et al
(13) reported that patients with
pre-treatment plasma EBV-DNA levels of >1,500 copies/ml or
persistently detectable post-treatment plasma EBV-DNA had a higher
probability of treatment failure. Lin et al (11) also identified that pre-treatment
plasma EBV-DNA levels were lower in patients with local recurrence
than in those with distant metastasis (1,311 vs. 4,253 copies/ml)
(11). A further study by Leung et
al (19) found that high
pre-treatment plasma EBV-DNA loads in early-stage NPC (≥4,000
copies/ml) was predictive of distant metastasis (19). In the present study, it was revealed
that patients with pre-treatment plasma EBV-DNA levels of ≥1,500
copies/ml exhibited poorer RFS and OS than patients with
pre-treatment plasma EBV-DNA levels of <1,500 copies/ml.
Furthermore, patients with positive post-treatment plasma EBV-DNA
had significantly shorter survival periods than those who tested
negative. The multivariate analyses established that pre-treatment
plasma EBV-DNA levels of ≥1,500 copies/ml and positive
post-treatment plasma EBV-DNA were risk factors associated with the
recurrence of NPC. With respect to predicting mortality, however,
lymph node metastasis and distant metastasis status were superior
to pre- or post-treatment levels of plasma EBV-DNA.
To the best of our knowledge, the sample size of the
present study was larger than that of any previous study
investigating the use of plasma EBV-DNA for predicting the
prognosis of patients with NPC. The findings of the present study
may therefore provide novel reference points for research and
clinical practice. However, certain limitations existed, including
the fact that it was a retrospective study with a limited sample
size and follow-up period. Therefore, the results of the present
study require validation from a prospective study with a larger
population and longer follow-up time.
In conclusion, the data indicated that the levels of
plasma EBV-DNA prior and subsequent to treatment were predictive of
the prognosis of patients with NPC. The analysis of pre- and
post-treatment plasma EBV-DNA may be valuable for elucidating the
prognosis of NPC in a clinical setting. Therefore, the findings of
the present study may provide a novel reference point for the
research and management of patients with NPC.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China - Guangdong Joint Fund (no. U1132003),
the Natural Science Foundation of Guangdong Province (no.
S2013010015685) and the Foundation for Distinguished Young Talents
in Higher Education of Guangdong, China (no. 2012LYM_0039).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee N, Xia P, Quivey JM, et al:
Intensity-modulated radiotherapy in the treatment of nasopharyngeal
carcinoma: An update of the UCSF experience. Int J Radiat Oncol
Biol Phys. 53:12–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS
and Wang WY: Phase III study of concurrent chemoradiotherapy versus
radiotherapy alone for advanced nasopharyngeal carcinoma: Positive
effect on overall and progression-free survival. J Clin Oncol.
21:631–637. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee AW, Sze WM, Au JS, et al: Treatment
results for nasopharyngeal carcinoma in the modern era: The Hong
Kong experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang YS, Tyan YS, Liu ST, Tsai MS and Pao
CC: Detection of Epstein-Barr virus DNA sequences in nasopharyngeal
carcinoma cells by enzymatic DNA amplification. J Clin Microbiol.
28:2398–2402. 1990.PubMed/NCBI
|
|
6
|
Pathmanathan R, Prasad U, Chandrika G,
Sadler R, Flynn K and Raab-Traub N: Undifferentiated,
nonkeratinizing and squamous cell carcinoma of the nasopharynx.
Variants of Epstein-Barr virus-infected neoplasia. Am J Pathol.
146:1355–1367. 1995.PubMed/NCBI
|
|
7
|
Lo YM, Chan LY, Lo KW, et al: Quantitative
analysis of cell-free Epstein-Barr virus DNA in plasma of patients
with nasopharyngeal carcinoma. Cancer Res. 59:1188–1191.
1999.PubMed/NCBI
|
|
8
|
Chan AT, Lo YM, Zee B, et al: Plasma
Epstein-Barr virus DNA and residual disease after radiotherapy for
undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst.
94:1614–1619. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adham M, Greijer AE, Verkuijlen SA, et al:
Epstein-Barr virus DNA load in nasopharyngeal brushings and whole
blood in nasopharyngeal carcinoma patients before and after
treatment. Clin Cancer Res. 19:2175–2186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan KC, Hung EC, Woo JK, et al: Early
detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus
DNA analysis in a surveillance program. Cancer. 119:1838–1844.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin JC, Wang WY, Chen KY, et al:
Quantification of plasma Epstein-Barr virus DNA in patients with
advanced nasopharyngeal carcinoma. N Engl J Med. 350:2461–2470.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WY, Twu CW, Chen HH, et al: Plasma
EBV DNA clearance rate as a novel prognostic marker for
metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res.
16:1016–1024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WY, Twu CW, Chen HH, et al: Long-term
survival analysis of nasopharyngeal carcinoma by plasma
Epstein-Barr virus DNA levels. Cancer. 119:963–970. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan H, Nicholls J, Chua D, et al:
Laboratory markers of tumor burden in nasopharyngeal carcinoma: A
comparison of viral load and serologic tests for Epstein-Barr
virus. Int J Cancer. 112:1036–1041. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan KC, Chan AT, Leung SF, et al:
Investigation into the origin and tumoral mass correlation of
plasma Epstein-Barr virus DNA in nasopharyngeal carcinoma. Clin
Chem. 51:2192–2195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu CL, Chang KP, Lin CY, et al: Plasma
Epstein-Barr virus DNA concentration and clearance rate as novel
prognostic factors for metastatic nasopharyngeal carcinoma. Head
Neck. 34:1064–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo YM, Leung SF, Chan LY, et al: Kinetics
of plasma Epstein-Barr virus DNA during radiation therapy for
nasopharyngeal carcinoma. Cancer Res. 60:2351–2355. 2000.PubMed/NCBI
|
|
18
|
To EW, Chan KC, Leung SF, et al: Rapid
clearance of plasma Epstein-Barr virus DNA after surgical treatment
of nasopharyngeal carcinoma. Clin Cancer Res. 9:3254–3259.
2003.PubMed/NCBI
|
|
19
|
Leung SF, Chan AT, Zee B, et al:
Pretherapy quantitative measurement of circulating Epstein-Barr
virus DNA is predictive of posttherapy distant failure in patients
with early-stage nasopharyngeal carcinoma of undifferentiated type.
Cancer. 98:288–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrari D, Codecà C, Bertuzzi C, et al:
Role of plasma EBV DNA levels in predicting recurrence of
nasopharyngeal carcinoma in a Western population. BMC Cancer.
12:2082012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan JY and Wong ST: The role of plasma
Epstein-Barr virus DNA in the management of recurrent
nasopharyngeal carcinoma. Laryngoscope. 124:126–130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB, Byrd DR, Compton CC, et al:
PharynxAJCC Cancer Staging Manual. 7th. Springer-Verlag; New York,
NY: pp. 422009
|
|
23
|
Yip TT, Ngan RK, Fong AH and Law SC:
Application of circulating plasma/serum EBV DNA in the clinical
management of nasopharyngeal carcinoma. Oral Oncol. 50:527–538.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chien YC, Chen JY, Liu MY, et al:
Serologic markers of Epstein-Barr virus infection and
nasopharyngeal carcinoma in Taiwanese men. N Engl J Med.
345:1877–1882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao SM, Liu Z, Jia WH, et al: Fluctuations
of Epstein-Barr virus serological antibodies and risk for
nasopharyngeal carcinoma: A prospective screening study with a
20-year follow-up. PLoS One. 6:e191002011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji MF, Wang DK, Yu YL, et al: Sustained
elevation of Epstein-Barr virus antibody levels preceding clinical
onset of nasopharyngeal carcinoma. Br J Cancer. 96:623–630. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai YL, Li J, Lu AY, et al: Prognostic
significance of serum anti-Epstein-Barr virus antibodies in
nasopharyngeal carcinoma. Zhonghua Shi Yan He Lin Chuang Bing Du
Xue Za Zhi. 27:119–122. 2013.(In Chinese). PubMed/NCBI
|
|
28
|
Twu CW, Wang WY, Liang WM, et al:
Comparison of the prognostic impact of serum anti-EBV antibody and
plasma EBV DNA assays in nasopharyngeal carcinoma. Int J Radiat
Oncol Biol Phys. 67:130–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lo YM, Leung SF, Chan LY, et al: Plasma
cell-free Epstein-Barr virus DNA quantitation in patients with
nasopharyngeal carcinoma. Correlation with clinical staging. Ann NY
Acad Sci. 906:99–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leung SF, Lo YM, Chan AT, et al: Disparity
of sensitivities in detection of radiation-naïve and
postirradiation recurrent nasopharyngeal carcinoma of the
undifferentiated type by quantitative analysis of circulating
Epstein-Barr virus DNA1,2. Clin Cancer Res. 9:3431–3434.
2003.PubMed/NCBI
|
|
31
|
Hou X, Zhao C, Guo Y, et al: Different
clinical significance of pre- and post-treatment plasma
Epstein-Barr virus DNA load in nasopharyngeal carcinoma treated
with radiotherapy. Clin Oncol (R Coll Radiol). 23:128–133. 2011.
View Article : Google Scholar : PubMed/NCBI
|