Introduction
Hepatic cancer is the most common malignancy and is
responsible for the highest number of cancer-associated mortalities
worldwide (1). Platinum-based agents
are an important class of chemotherapy drugs for hepatic cancer,
among which cisplatin is typical. This class of drugs are DNA
alkylating agents that cross-link the DNA molecules to affect their
replication, transcription and other normal functions, leading to
cell growth arrest, apoptosis and death (2). Cisplatin is indicated for patients with
advanced inoperable diseases, and also as part of comprehensive
therapeutic regimens, including surgical operation (3). However, tumor cells tend to develop drug
resistance to cisplatin with continuous drug treatment, resulting
in treatment failure (4). At this
point, the only option for these patients is to select other
chemotherapeutic drugs, despite their limited clinical benefits.
One of the reasons for the limited benefits of other drugs is that
tumor cells are likely to develop resistance to the other
chemotherapeutic drugs as they develop resistance to cisplatin;
this is called multidrug resistance (5). Several key regulatory proteins have been
identified in the past decade as a result of the considerable
progress in the biological studies of tumorigenesis and tumor
progression; and a number of successes have been achieved regarding
the therapies targeting these proteins in clinical practice
(6). These key proteins, including
WEE1 G2 checkpoint kinase (WEE1), regulate tumor cell growth and
metastasis, and serve a role in the development of drug resistance
in tumor cells. WEE1 is a cell cycle-associated kinase (7) and has been demonstrated to be expressed
in certain cisplatin-resistant cells from human epidermal carcinoma
(8). To investigate the role of WEE1
in the cisplatin-resistant hepatic cancer cell line HepG2/DDP, the
current study examined the reversal of cisplatin resistance in
HepG2/DDP by silencing WEE1, and explored the mechanisms of
triptolide-mediated reversal of drug resistance by probing the
aspects associated with the mechanisms of tumor drug
resistance.
Materials and methods
Cells and cell culture
The multidrug resistant human hepatic cancer cell
line HepG2/DPP was obtained from The Third Affiliated Hospital of
Xinxiang Medical University (Xinxiang, China) and cultured with
RPMI 1640 (containing 10% calf serum; Corning Life Sciences,
Manassas, VA, USA) in an incubator under 37°C, 5% CO2
and saturated humidity conditions. Trypsin-EDTA [0.25% in
phosphate-buffered saline (PBS); Corning Life Sciences] was used
for digestion and passaging. Cells in the logarithmic growth phase
were used in all experiments. The present study was approved by the
ethics committee of Luoyang Central Hospital Affiliated to
Zhengzhou University (Luoyang, China).
Silencing of WEE1 expression with
lentivector-wee1 short hairpin (sh)RNA
The cells were cultured in 24-well plates, at a
density of 3×104 cells/well. The culture was continued
until ~70% cells reached confluence. The wee1 shRNA (h) viral
vectors (Lentiviral Particles; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) were prepared in accordance with the
procedures described in the manufacturer's instructions. The cells
were transfected with different multiplicity of infection (MOI)
values (10 and 20). The culture medium was switched to virus-free
medium (PT3414-1; Corning Life Sciences) 24 h following
transfection. The culture was continued for 48 h for passaging. The
silencing status of WEE1 was evaluated by western blotting and
quantitative polymerase chain reaction (qPCR). The HepG2/DDP cells
were the parental control group, the HepG2/DDP cells transfected
with blank vector were the negative control group and the HepG2/DDP
cells transfected with WEE1 short hairpin (sh)RNA were the shRNA#1
(MOI, 10) and shRNA#2 (MOI, 20) silencing groups.
Determination of cell sensitivity to
cisplatin with MTS assay
Cells (5×104 cells/ml) were seeded in
96-well microplates, at 100 µl/well and cultured overnight to allow
cell adherence. Next, different concentrations of cisplatin (0, 30,
60, 90, 120, 150, 180, 210, 240, 270 and 300 µM; Sigma-Aldrich, St.
Louis, MO, USA) were added and the culture was continued for 72 h.
Following aspiration of the culture medium, 50 µl MTS (Promega
Corporation, Madison, WI, USA) was added in accordance with the
reagent instructions and cultured for 4 h. The optical density (OD)
was measured at 490 nm wavelength with a Bio-Rad 3550 microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the
inhibition rate of the drug on the cells was calculated as follows:
Inhibition rate = (1 - OD of experimental group/OD of control
group) × 100. Using the cisplatin concentration as abscissa and the
inhibition rate as ordinate, the inhibition curve was plotted and
fitted to obtain the half maximal inhibitory concentration
(IC50). Reversal fold (RF) was defined as RF =
IC50 (silence group)/IC50 (parental
group).
Determination of intracellular
rhodamine-123 (Rh-123) content, cell surface P-glycoprotein (P-gp)
expression, apoptosis and caspase −3/8 activity with flow
cytometry
Following addition of 100 µl of 10 µM Rh-123
staining solution (Beyotime Institute of Biotechnology, Shanghai,
China), the cells in logarithmic growth phase were cultured for 1 h
and harvested. The fluorescence intensity of intracellular Rh-123
was detected with a flow cytometer (FACSAria™ I; BD Biosciences,
Franklin Lakes, NJ, USA) to indicate intracellular Rh-123 content.
P-gp expression was determined with flow cytometry using specific
procedures as follows: 106 cells were added to 0.1 ml
culture medium for each sample and stained in accordance with the
kit instructions [Anti-P-gp/fluorescein isothiocyanate (FITC)
fluorescent antibody kit; Abcam, Cambridge, MA, USA]; cell
fluorescence intensity was detected with a flow cytometer to
indicate the P-gp expression level. Apoptosis was detected using
the annexin V-FITC/propidium iodide (PI; BD Biosciences, Franklin
Lakes, NJ, USA) double-staining technique; the cells were treated
with 20 µM cisplatin for 24 h and harvested to determine apoptosis
in accordance with the manufacturer's instructions. Following
similar cisplatin treatment, intracellular caspase-3 and −8
activities were determined with phycoerythrin-labeled anti-active
rabbit IgG caspase-3 (cat no. sc-7147) and −8 antibodies (cat no.
sc-7890; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.) using
the flow cytometer in accordance with the manufacturer's
instructions.
Determination of protein levels of
WEE1 G2 checkpoint kinase (WEE1), multidrug resistance protein 1
(MDR1), multidrug resistance associated protein 1 (MRP1),
lipoprotein receptor-related protein (LRP), B-cell lymphoma 2
(BCL-2), survivin and glutathione S-transferase (GST); and
phosphorylation levels of mitogen-activated protein kinase kinase
(MEK) and extracellular-signal-regulated kinase (ERK) in HepG2/DPP
cells using western blotting
Cells in the logarithmic growth phase were lysed
using a cell lysis kit (Beyotime Institute of Biotechnology) to
extract the total protein content. The proteins were separated in
12% SDS-PAGE (Beyotime Institute of Biotechnology) at 220 V and
transferred onto a polyvinylidene difluoride (PVDF) membrane
(Beyotime Institute of Biotechnology). Antibodies for WEE1 (cat no.
sc-9037; rabbit anti-human IgG; 1:2,000 dilution), MDR1 (cat no.
sc-55510; mouse anti-human IgG; 1:2,000 dilution), MRP1 (cat no.
sc-13960; rabbit anti-human IgG; 1:2,000 dilution), LRP (cat no.
sc-390134; mouse anti-human IgG; 1:2,000 dilution), BCL-2 (cat no.
sc-492; rabbit anti-human IgG; 1:2,000 dilution), survivin (cat no.
sc-10811; rabbit anti-human IgG; 1:2,000 dilution), GST (cat no.
sc-459; rabbit anti-human IgG; 1:2,000 dilution), p-MEK (cat no.
sc-130203; rabbit anti-human IgG; 1:1,500 dilution) and p-ERK (cat
no. sc-13073; rabbit anti-human IgG; 1:1,500 dilution) were used to
detect the target proteins, and β-actin (cat no. sc-1616; goat
anti-human IgG; 1:5,000 dilution) and ERK (cat no. sc-94; rabbit
anti-human IgG; 1:2,000 dilution) were used as the internal
reference genes. All antibodies were purchased from Santa Cruz
Biotechnology, Inc. Cells were incubated with the antibodies at 4°C
overnight. Subsequent to washing off the primary antibody with 10%
PBS, horseradish peroxidase (HRP)-conjugated secondary antibody
(cat no. sc-2005; goat anti-mouse IgG-HRP; 1:2,000 dilution; and
cat no. sc-2004; goat anti-rabbit IgG-HRP; 1:2000 dilution) was
added to the cells and incubated for 1 h. Cells were washed with
10% PBS, then an enhanced chemiluminescence (ECL) kit (Millipore,
Boston, MA, USA) was used to identify the immunoreactive bands
using methods according to the manufacturer's instructions.
Determination of the mRNA levels of
WEE1, MDR1, MRP1, LRP, BCL-2, survivin and GST in HepG2/DPP tumor
cells using reverse transcription (RT)-qPCR
Following the extraction of total RNA from each
group of cells in the logarithmic growth phase using TRIzol
(Invitrogen Life Technologies, Carslbad, CA, USA), RT was conducted
with Real-Time PCR kit (Ambion, Austin, TX, USA) to obtain the
cDNA. The primer sequences were as follows: WEE1, F
5′-GATGAGCAGAACGCTTTGAGAG-3′ and R 5′-CAGAGGCAGCATTTGGGATT-3′;
MDR1, F 5′-AAAAAGATCAACTCGTACCACTC-3′ and R
5′-GCACAAAATACACCAACAA-3′; MRP1, F 5′-ACTTCCACATCTGCTTCGTCAGTG-3′
and R 5′-ATTCAGCCACAGGAGGTAGAGAGC-3′; LRP, F
5′-AGTCAGAAGCCGAGAAAG-3′ and R 5′-CCCAGCCACAGCAAGGT-3′; BCL-2, F
5′-ACGGGGTGAACTGGGGGAGGA-3′ and R 5′-TGTTTGGGGCAGGCATGTTGACTT-3′;
survivin, F 5′-GCATGGGTGCCCCGACGTTG-3′ and R
5′-GCTCCGGCCAGAGGCCTCAA-3′; GST, F 5′-ACGTGGCAGGAGGGCTCACTC-3′ and
R 5′-TACTCAGGGGAGGCCAGCAA-3′; GAPDH, F 5′-CTTAGATTTGGTCGTATTGG-3′
and R 5′-GAAGATGGTGATGGGATT-3′. Following denaturation at 94°C for
3 min, 40 cycles of amplification were conducted under the
following conditions: 95°C for 5 sec, 65°C for 35 sec, 72°C for 60
sec, and extension at 72°C for 5 min.
Statistical analysis
The experimental data are presented as the mean ±
standard deviation and analyzed with SPSS software, version 13.0
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
used for comparison; P<0.05 was considered to indicate a
statistically significant difference.
Results
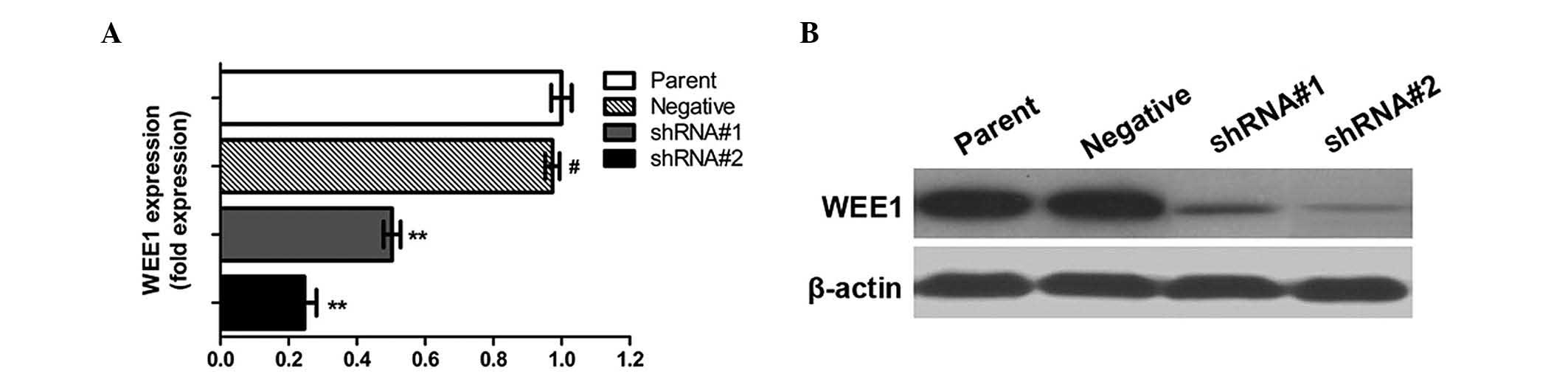
Lentivector-wee1 shRNA significantly
downregulated WEE1 expression in HepG2/DDP cells
Transfection of HepG2/DDP cells with different MOI
values resulted in varying degrees of WEE1 silencing. Subsequent to
screening, cells transfected with the MOI values of 10 and 20 were
selected for subsequent studies. As presented in Fig. 1, WEE1 expression levels in the shRNA#1
(MOI, 10) and shRNA#2 (MOI, 20) groups were 53 and 22% of the
parental group cells, respectively (P<0.05). Blank vector shRNA
cells (negative group) were observed to have no effect on WEE1
expression levels (P>0.05).
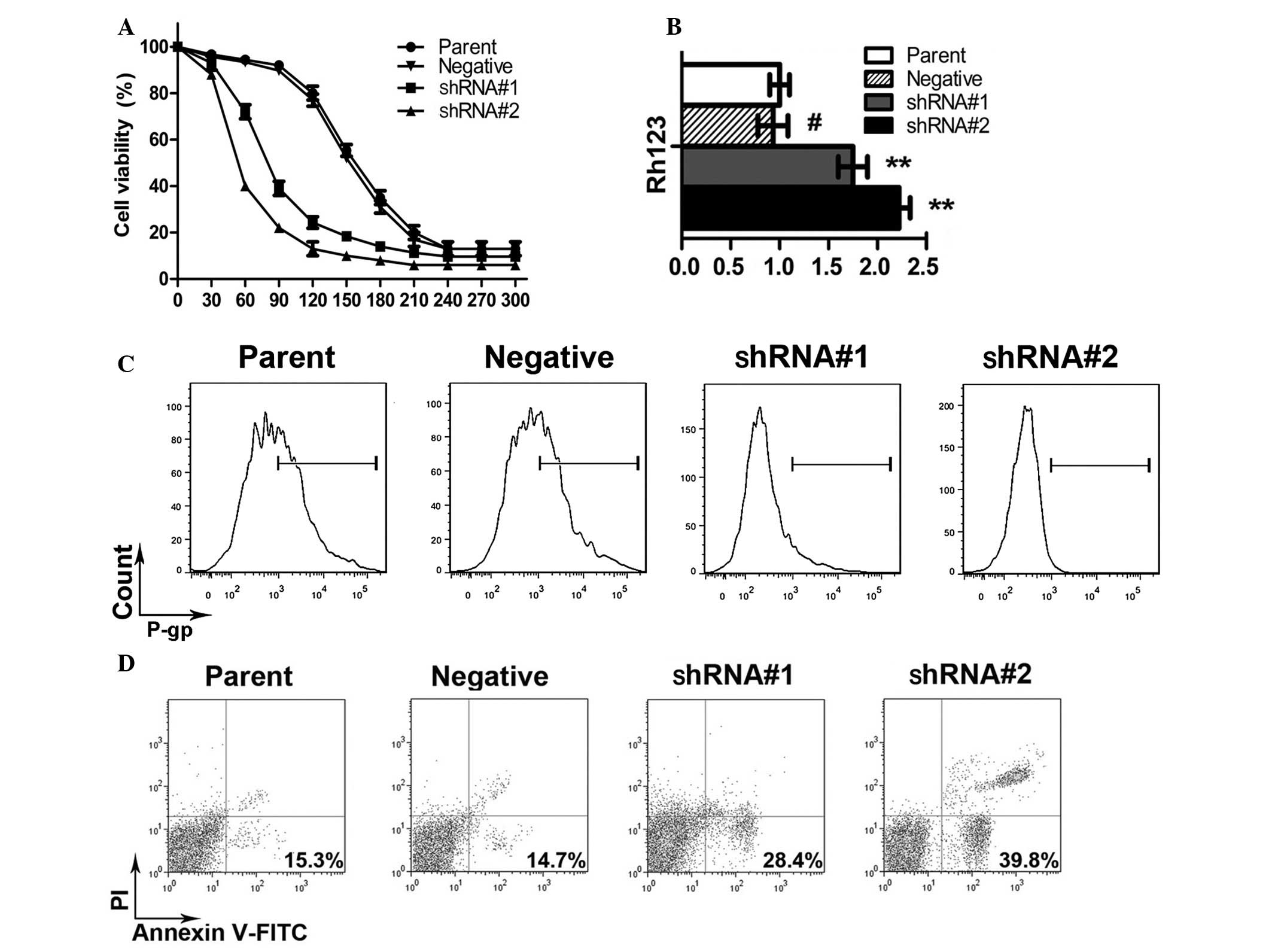
WEE1 silencing increased the
sensitivity of HepG2/DPP cells to cisplatin
MTS assay results (Fig.
2A) demonstrated that the IC50 of cisplatin-mediated
inhibition on HepG2/DPP cell proliferation was 156 µM, while the
IC50s for the shRNA#1 and shRNA#2 groups were 70.2 and
43.5 µM, respectively, suggesting that silencing WEE1 enabled an
increase in the sensitivity of HepG2/DPP cells to cisplatin. The
RFs calculated based on the IC50s for shRNA#1 and
shRNA#2 groups were 2.2 and 3.6 fold, respectively.
WEE1 silencing increased Rh-123
content, downregulated P-gp expression, and increased the rate of
apoptosis and caspase-3/8 activity in HepG2/DPP cells
The fluorescence intensity of intracellular Rh-123
in the shRNA#1 and shRNA#2 groups increased by 1.75- and 2.23-fold
compared with the parental control group (Fig. 2B). By contrast, P-gp expression was
downregulated to 73.2 and 34.5% of the control group, respectively
(Fig. 2C), suggesting that elevation
of intracellular Rh-123 was associated with the downregulation of
P-gp, which may increase intracellular cisplatin. In addition,
following treatment with 20 µM cisplatin for 24 h, the apoptosis
rate of the HepG2/DDP parental group was 15.3%, while those of the
shRNA#1 and shRNA#2 groups were 28.4 and 39.8% (Fig. 2D; P<0.05).
WEE1 silencing downregulated the
expression of drug resistance-associated genes and the
phosphorylation of MEK and ERK, and upregulated the activity of
caspase-3/8 in HepG2/DPP cells
RT-qPCR analysis demonstrated that the gene
transcription levels of MDR1, MRP1, LRP and GST decreased
significantly following WEE1 silencing (Fig. 3A). Western blot results demonstrated
that, compared with the control group, the protein levels were also
reduced (Fig. 3B). These results
indicate that the downregulation of protein expression was
regulated at the transcriptional level.
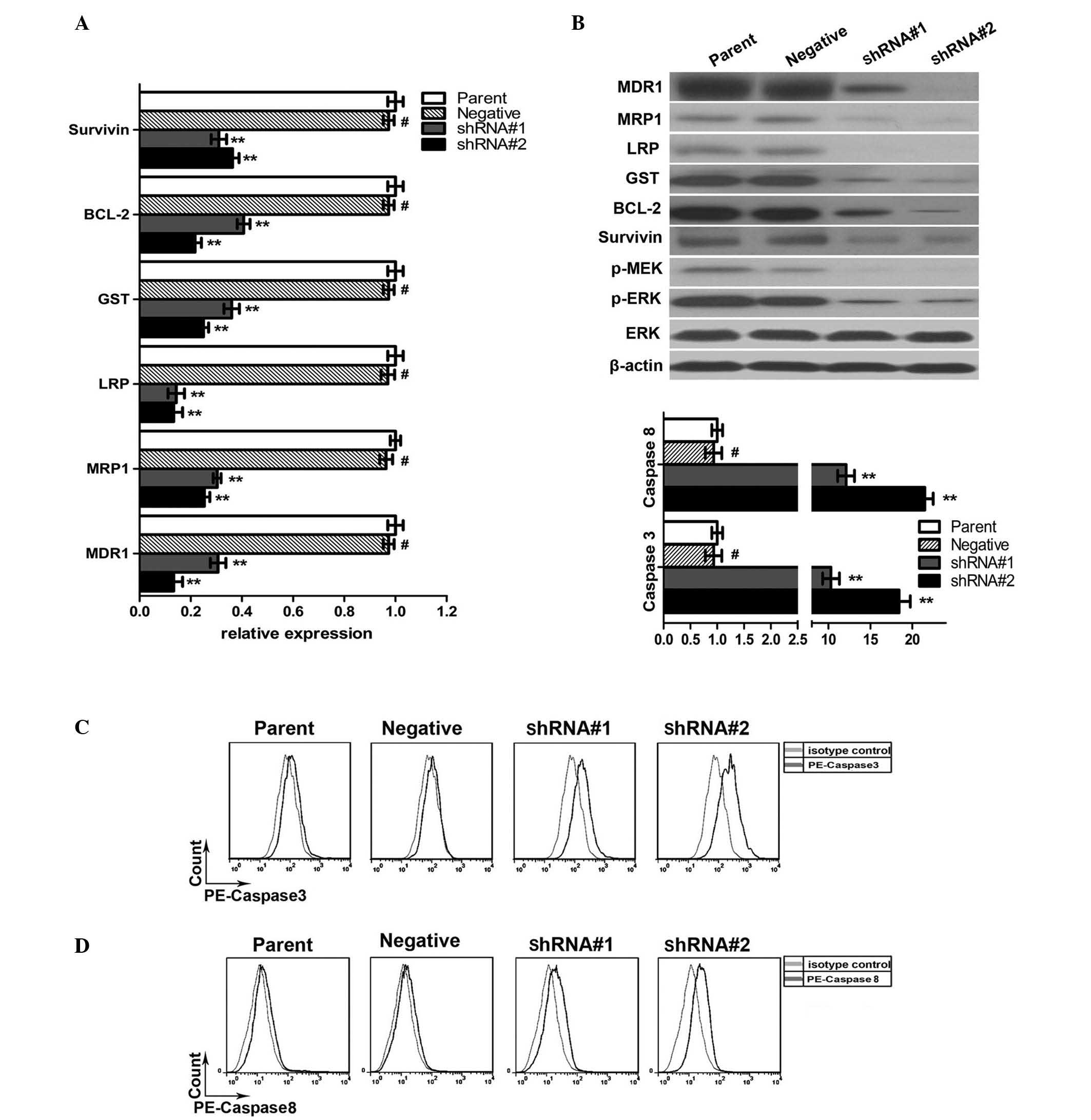 | Figure 3.WEE1 silencing downregulated the
expression of drug resistance-associated genes in HepG2/DPP cells.
(A) Reverse transcription-quantitative polymerase chain reaction
demonstrated decreased mRNA expression levels of survivin, BCL-2,
GST, LRP, MRP1 and MDR1 in the shRNA#1 and shRNA#2 groups compared
with parental and negative groups. Data are presented as the mean ±
standard deviation. #P>0.05 and **P<0.05 vs. the
parental group, n=5. (B) Western blot assay demonstrated reduced
expression levels of survivin, BCL-2, GST, LRP, MRP1, MDR1 p-MEK,
p-ERK in the shRNA#1 and shRNA#2 groups compared with the parental
and negative groups. β-actin and ERK were used as internal
controls. (C and D) Flow cytometry demonstrated an increased
activity of caspase-3/8 in the shRNA#1 and shRNA#2 groups compared
with the parental and negative groups. Data are presented as the
mean ± standard deviation. #P>0.05 and **P<0.05
vs. the parental group, n=3. shRNA, short hairpin RNA; shRNA#1,
WEE1-silenced group 1; shRNA#2, WEE1-silenced group 2; BCL-2,
B-cell lymphoma 2; GST, glutathione S-transferase; LRP, lipoprotein
receptor-related protein; MRP1, multidrug resistance associated
protein 1; MDR1, multidrug resistance protein 1; p-,
phosphorylated; MEK, mitogen-activated protein kinase kinase; ERK,
extracellular-signal-regulated kinase; PE, phycoerythrin. |
Since WEE1 silencing enhanced apoptosis, the
expression of 2 anti-apoptotic proteins, BCL-2 and survivin, were
assessed. Western blotting indicated that WEE1 silencing was
accompanied by downregulation of BCL-2 and survivin expression.
RT-qPCR analysis demonstrated that the downregulation of expression
was achieved at transcriptional level (P<0.05).
In order to explain these results, the MEK/ERK
pathway was considered as the possible intracellular signaling
pathway regulated by WEE1. Western blotting demonstrated that
levels of phosphorylated MEK and ERK decreased following WEE1
silencing, indicating that the activity of this pathway was
significantly suppressed.
Furthermore, the shRNA#1 and shRNA#2 groups were
treated with 20 µM cisplatin for 24 h, and the activated caspase-3
content was increased by 10.3 and 18.7-fold, respectively, compared
with the control group; the activated caspase-8 content increased
12.1 and 21.5-fold, compared with the control group (Fig. 3).
Discussion
In the present study, WEE1-silenced HepG2/DDP cell
lines were constructed using the lentiviral vector transfection
technique and then 2 representative cell lines with different
degrees of WEE1 silencing were selected for subsequent studies.
Previous studies have demonstrated the correlation between WEE1 and
the resistance to certain tumor drugs (9,10). The MTS
assay results of the present study confirmed that WEE1 silencing
increased the sensitivity of HepG2/DDP to cisplatin. Further
experiments demonstrated that WEE1 silencing resulted in the
elevation of intracellular Rh-123 concentration and apoptosis. The
2 main mechanisms for tumor cells in the development of resistance
to drugs include the overexpression of a variety of multidrug
resistance genes to enhance drug efflux and lower intracellular
drug concentration, and the resistance to drug-induced apoptosis
(11). Thus, the current study
hypothesized that WEE1 also reversed the drug resistance of
HepG2/DDP through the regulation of these 2 drug resistance
mechanisms.
To assess this hypothesis, the effects of WEE1
silencing on the expression of various multidrug resistant proteins
were detected. The expression of cell surface P-gp was examined
with flow cytometry; the results demonstrated that its expression
was significantly downregulated. In addition, MDR1 and MRP1
expression levels were detected with western blotting; the results
demonstrated that these proteins were also significantly
downregulated. These 2 proteins belong to the ABC super-family and
are able to pump chemotherapeutic drugs out of the cells (12). Furthermore, the present study also
demonstrated that LRP protein expression was downregulated.
Although LRP is not a member of the ABC family, it is also able to
pump drugs out of the cells (13). We
hypothesized that downregulation of the expression of these
proteins may increase the concentration of intracellular
chemotherapeutic drugs. This is consistent with the elevation of
intracellular drug concentration of Rh-123 observed in the present
study. RT-qPCR analysis demonstrated that the regulation of these
expression levels was achieved at the transcriptional level.
The effects of WEE1 suppression on
apoptosis-associated proteins was also analyzed. BCL-2 and survivin
are two common anti-apoptotic proteins (14). It has previously been demonstrated
that the BCL-2 protein expression level is significantly higher in
drug-resistant HepG2/DDP cells compared with non-drug-resistant
mother cells (15); this was
confirmed in the current study. Western blotting results indicated
that BCL-2 and survivin levels declined significantly following
WEE1 silencing; RT-qPCR analysis demonstrated that the regulation
of these expression levels was achieved at the transcriptional
level. Flow cytometry analysis demonstrated that WEE1 silencing
enhanced apoptosis and also significantly increased caspase-3/8
activity, clarifying the pathway of apoptosis.
Tumor cells can also develop resistance to
chemotherapeutic drugs through another mechanism by which
chemotherapeutic drugs are inactivated in metabolism. For example,
GST can acylate and inactivate cisplatin (16,17). The
current study demonstrated that GST levels significantly increased
in HepG2/DDP cells and significantly decreased subsequent to WEE1
silencing, suggesting that GST was also involved in WEE1-mediated
drug resistance to cisplatin.
Since WEE1 is able to interact with multiple cell
signaling pathways (7), the present
study assessed the key ERK/MEK signaling pathway, which is closely
associated with cell growth (18) and
drug resistance to cisplatin (19).
Western blotting demonstrated that ERK and MEK phosphorylation
levels significantly decreased with the silencing of WEE1,
suggesting that the activity of this signaling pathway declined and
it was involved in WEE1-mediated cisplatin drug resistance.
In summary, the current study demonstrated that
silencing WEE1 is able to reverse the multidrug resistance of
HepG2/DDP cells. The mechanisms of action may be associated with
the downregulation of multidrug resistance genes to inhibit drug
efflux, the enhancement of apoptosis, and the inhibition of MEK/ERK
pathway activity.
References
|
1
|
Zhu Y, Zhu L, Lu L, Zhang L, Zhang G, Wang
Q and Yang P: Role and mechanism of the alkylglycerone phosphate
synthase in suppressing the invasion potential of human glioma and
hepatic carcinoma cells in vitro. Oncol Rep. 1:431–436. 2014.
|
|
2
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brock MV, Hooker CM, Syphard JE, Westra W,
Xu L, Alberg AJ, Mason D, Baylin SB, Herman JG, Yung RC, et al:
Surgical resection of limited disease small cell lung cancer in the
new era of platinum chemotherapy: Its time has come. J Thorac
Cardiovasc Surg. 129:64–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao L, Li W, Zhou Y, Zhang Y, Huang S, Xu
X, Li Z and Guo Q: The overexpression and nuclear translocation of
Trx-1 during hypoxia confers on HepG2 cells resistance to DDP, and
GL-V9 reverses the resistance by suppressing the Trx-1/Ref-1 axis.
Free Radic Biol Med. 82:29–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsudomi T: Advances in target therapy
for lung cancer. Jpn J Clin Oncol. 40:101–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCarthy N: Cell cycle: A WEE pointer. Nat
Rev Cancer. 12:378–379. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pouliot LM, Chen YC, Bai J, Guha R, Martin
SE, Gottesman MM and Hall MD: Cisplatin sensitivity mediated by
WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Cancer
Res. 72:5945–5955. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kogiso T, Nagahara H, Hashimoto E,
Ariizumi S, Yamamoto M and Shiratori K: Efficient induction of
apoptosis by wee1 kinase inhibition in hepatocellular carcinoma
cells. PLoS One. 6:e1004952014. View Article : Google Scholar
|
|
10
|
Tsai SC, Yang JS, Peng SF, Lu CC, Chiang
JH, Chung JG, Lin MW, Lin JK, Amagaya S, Chung C Wai-Shan, et al:
Bufalin increases sensitivity to AKT/mTOR-induced autophagic cell
death in SK-HEP-1 human hepatocellular carcinoma cells. Int J
Oncol. 4:1431–1442. 2012.
|
|
11
|
de Figueiredo-Pontes LL, Pintão MC,
Oliveira LC, Dalmazzo LF, Jácomo RH, Garcia AB, Falcão RP and Rego
EM: Determination of P-glycoprotein, MDR-related protein 1, breast
cancer resistance protein, and lung-resistance protein expression
in leukemic stem cells of acute myeloid leukemia. Cytometry B Clin
Cytom. 74:163–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fridman E, Skarda J, Pinthus JH, Ramon J
and Mor Y: Expression of multidrug resistance-related protein
(MRP-1), lung resistance-related protein (LRP) and topoisomerase-II
(TOPO-II) in Wilms' tumor: Immunohistochemical study using TMA
methodology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
152:47–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kerr EH, Frederick PJ, Egger ME, Stockard
CR, Sellers J, DellaManna D, Oelschlager DK, Amm HM, Eltoum IE,
Straughn JM, et al: Lung resistance-related protein (LRP)
expression in malignant ascitic cells as a prognostic marker for
advanced ovarian serous carcinoma. Ann Surg Oncol. 20:3059–3065.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li K, Chen B, Xu L, Feng J, Xia G, Cheng
J, Wang J, Gao F and Wang X: Reversal of multidrug resistance by
cisplatin-loaded magnetic Fe3O4 nanoparticles in A549/DDP lung
cancer cells in vitro and in vivo. Int J Nanomedicine. 8:1867–1877.
2013.PubMed/NCBI
|
|
16
|
Surowiak P, Materna V, Kaplenko I,
Spaczyński M, Dietel M, Lage H and Zabel M: Augmented expression of
metallothionein and glutathione S-transferase pi as unfavourable
prognostic factors in cisplatin-treated ovarian cancer patients.
Virchows Arch. 447:626–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Liu XJ, Yang P, Zhao M, Lv LX,
Zhang GD, Wang Q and Zhang L: Alkylglyceronephosphate synthase
(AGPS) alters lipid signaling pathways and supports chemotherapy
resistance of glioma and hepatic carcinoma cell lines. Asian Pac J
Cancer Prev. 7:3219–3226. 2014. View Article : Google Scholar
|
|
18
|
Montagut C and Settleman J: Targeting the
RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 283:125–134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brozovic A and Osmak M: Activation of
mitogen-activated protein kinases by cisplatin and their role in
cisplatin-resistance. Cancer Lett. 251:1–16. 2007. View Article : Google Scholar : PubMed/NCBI
|