Introduction
Radiotherapy following breast-conserving surgery is
an integral part of breast-conserving treatment in early-stage
breast cancer (1). Traditionally, the
entire breast is irradiated, with an additional boost dose to the
tumor bed (TB) in patients with a higher risk of local recurrence
(2). Although the geometric precision
of modern radiation dose delivery is high, target delineation
uncertainties are often large (3). In
particular, delineation of the TB area, which is performed on the
postoperative radiotherapy planning computed tomography (CT) scan,
is highly variable among radiation oncologists (4–8). This may
potentially lead to treatment inaccuracies, which are of particular
concern due to the increasing use of Accelerated Partial Breast
Irradiation (APBI), in which only the breast tissue surrounding the
TB is irradiated. Furthermore, postoperative seroma formation may
lead to large TB volumes, which are associated with an increased
risk of subcutaneous fibrosis and poorer cosmetic results (9–12).
There are a number of potential options to improve
TB visualization for standard CT-guided TB delineation. Magnetic
resonance imaging (MRI) may have additional value due to its
excellent soft-tissue contrast compared with CT. The use of
different MRI sequences makes it possible to differentiate between
fibroglandular tissue, fluid and fat, while it can also detect the
heterogeneity and irregularity of seromas (13). Another potential strategy for
improving CT-guided TB delineation is to increase the observer's
knowledge of the original tumor location. Currently, the
preoperative diagnostic mammogram or MRI scan are used by the
radiation oncologist to reconstruct the original tumor location on
the planning CT scan. This diagnostic imaging is not acquired in
the supine radiotherapy treatment position, which presents
challenges in interpreting the original tumor position on the
supine planning CT scan. Preoperative imaging acquired in the
treatment position may improve the observer's knowledge regarding
the original tumor location and, thereby, potentially reduce
interobserver variability (IOV). The addition of a preoperative
contrast-enhanced (CE)-CT in the supine radiotherapy treatment
position to the standard postoperative planning CT was investigated
by Boersma et al (14), who
reported a minor reduction of the IOV. However, in our recent
delineation study on preoperative breast tumor delineation, CE-MRI
in the radiotherapy supine position was demonstrated to be superior
to CE-CT for tumor detection and the visualization of tumor
irregularities and spiculations (15). Therefore, in the current study, CE-CT,
which is most commonly available in radiotherapy institutes, and
CE-MRI were investigated as additional imaging modalities with
which to improve post-lumpectomy CT-guided TB definition.
The aim of the present study was to investigate
whether the IOV of standard CT-guided post-lumpectomy TB
delineation is reduced through the use of additional postoperative
MRI, preoperative CE-CT or preoperative CE-MRI in the supine
radiotherapy treatment position.
Materials and methods
Patients and selection
The study population included NTR3198 study patients
(15), who had received CT and MRI
scans prior to and following lumpectomy as part of the study to
quantify pre- and postoperative treatment volumes. This study was
approved by the institutional review committee of the University
Medical Center Utrecht (Utrecht, The Netherlands) and registered in
the International Clinical Trials Registry. Written informed
consent was obtained from all patients. Patients eligible for
inclusion had a clinical TNM T1-T2, N0-staged adenocarcinoma of the
breast (16), and were scheduled for
breast-conserving therapy. Patients with lobular carcinoma, a
history of ipsilateral breast surgery, contraindications for 1.5
Tesla MRI or iodine allergy, and patients who received neoadjuvant
treatment, were not eligible. Patients were enrolled between
November 2011 and November 2012.
Image acquisition
The eligible patients underwent preoperative CE-CT
and CE-MRI scans prior to lumpectomy, and a standard planning CT
scan directly followed by an MRI scan at a median of 21 days
(range, 14–50 days) after lumpectomy. All scans were performed in
the supine treatment position.
For CT and MRI, the patients were placed with the
arms in abduction and hands above the head at 10° inclination and
with the use of a knee support (C-Qual™ and Thorawedge
for CT and MRI, respectively; CIVCO Medical Solutions, Reeuwijk,
The Netherlands). The tumor or surgical scar was marked on the skin
with a CT/MRI compatible wire.
For MRI, a wide bore (70 cm) MRI scanner (Ingenia
1.5T; Philips Medical Systems, Best, The Netherlands) and anterior
receive coil were used. To prevent breast deformation by the
anterior receive coil, a polymethyl methacrylate support was
designed, which was adjustable to patient habitus and breast size.
The following three-dimensional high resolution MRI images were
used in this study: T1 weighted (T1w) fast field echo (FFE) with
and without fat suppression (Dixon), T2 weighted (T2w) turbo spin
echo (TSE) with fat suppression [spectral adiabatic inversion
recovery (SPAIR)] and preoperative dynamic series of CE T1w Dixon
images (17). The MRI sequence
parameters are provided in Table I.
The total acquisition times of the pre- and postoperative MRI
protocols were 21 and 14 min, respectively.
 | Table I.Magnetic resonance imaging sequence
parameters. |
Table I.
Magnetic resonance imaging sequence
parameters.
| Parameter | Postoperative T2 TSE
SPAIR | Postoperative T1
Dixon FFE | Preoperative dynamic
T1 Dixon FFE |
|---|
| Orientation | Transverse | Transverse | Transverse |
| Acquisition mode | 3D | 3D | 3D |
| FOV, mm | 250×450×200 | 250×448×200 | 250×448×200 |
| Matrix size | 200×357×167 | 252×447×182 | 208×388×167 |
| Acquired voxel size,
mm | 1.25×1.25×2.40 | 0.99×1.00×2.20 | 1.20×1.21×2.40 |
| Reconstructed voxel
size, mm | 0.78×0.78×1.2 | 0.95×0.93×1.10 | 1.16×1.18×1.20 |
| TR/TE, ms/ms | 2000/172 | 7.1/1.71 | 6.1/1.87 |
| Flip angle | 90 | 12 | 10 |
| Refocusing angle | 120 | NA | NA |
| Turbo factor | 74 | 117 | 84 |
| NSA | 1 | 2 | 1 |
| Fat suppression | SPAIR | Dixon | Dixon |
| Acquisition time,
min | 5:40 | 7:51 | 4:13 |
Target volume delineation
All the images were transferred to the in-house
developed delineation software (18).
The postoperative MRI was registered to the postoperative planning
CT by rigid mutual information registration on a box around the
tumor, using the T1w images with fat-suppression. The preoperative
CE-CT and CE-MRI were registered to the planning CT by automatic
registration on the chest wall. Subsequently, four experienced
breast radiation oncologists independently delineated the TB, using
written delineation instructions. These instructions were
formulated in a consensus meeting with all observers, supervised by
an experienced breast radiologist. The consensus meeting was
repeated once, to answer questions regarding MRI and to discuss
ambiguities in the delineation instructions. Delineation took place
as follows: Firstly, the observer delineated the TB on standard
postoperative planning CT and assigned a cavity visualization score
(CVS) of 1 (no cavity visible), 2 (heterogeneous cavity with
indistinct margins), 3 (heterogeneous cavity with some distinct
margins), 4 (mildly heterogeneous cavity with mostly distinct
margins), or 5 (homogeneous cavity with clearly identified margins)
(19). Next, the CT-based delineation
was duplicated and adjusted according to findings of the
co-registered postoperative MRI, preoperative CT and preoperative
MRI. The original CT-guided delineation was duplicated to prevent
influencing the data by intraobserver variations and to solely
study the influence of additional imaging on this standard
delineation method.
Data analysis
The conformity index (CI) and distance between the
centers of mass (dCOM) of the TB contours were calculated for all
the possible observer pairs. The CI per observer pair was
calculated using the following formula: CI = volume of agreement /
total encompassing volume. Consequently, a CI of 1 implies a
perfect agreement among observers, while a CI of 0 indicates no
overlap in delineations. For dCOM, a value of 0 indicates that two
delineations are centered at the same position.
Median values and accompanying ranges were used to
describe the data, as not all variables were normally distributed.
A Wilcoxon signed-rank test was performed to compare paired
variables using SPSS version 20 (IBM SPSS, Armonk, NY, USA) with a
significance level of 0.05. To visualize the effect of additional
imaging on the CI, the change in CI per observer pair was plotted
against the original CI of that observer pair on CT using GraphPad
Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Furthermore,
these outcomes were categorized as CVS≤3 and CVS≥4.
Results
Patients
A total of 14 patients were prospectively included
in the present study (Table II). The
majority of patients underwent full thickness closure of the
excision cavity, which consisted of suturing the deep and
superficial layers of the cavity's breast tissue. A representative
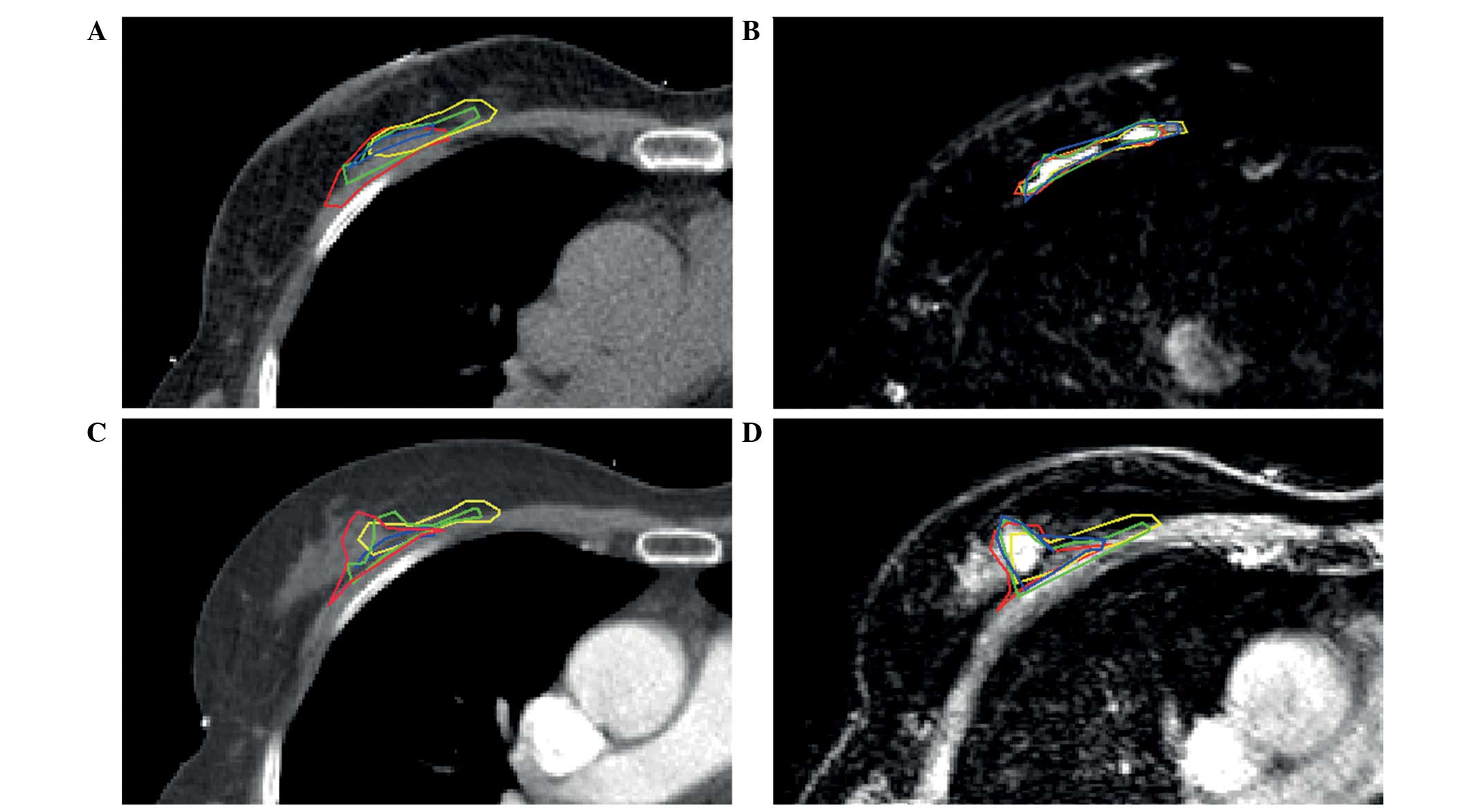
example of standard postoperative CT, registered to postoperative
MRI, preoperative CE-CT and preoperative CE-MRI in one of the
patients is shown in Fig. 1. The
different features of the TB as visualized by different MRI
sequences are shown in Fig. 2. Fat
suppressed T1w (Fig. 2B) and T2w
(Fig. 2D) images enable distinction
between fibroglandular tissue and seroma. Surgical clips can be
visualized by the T1w images without fat-suppression (Fig. 2C).
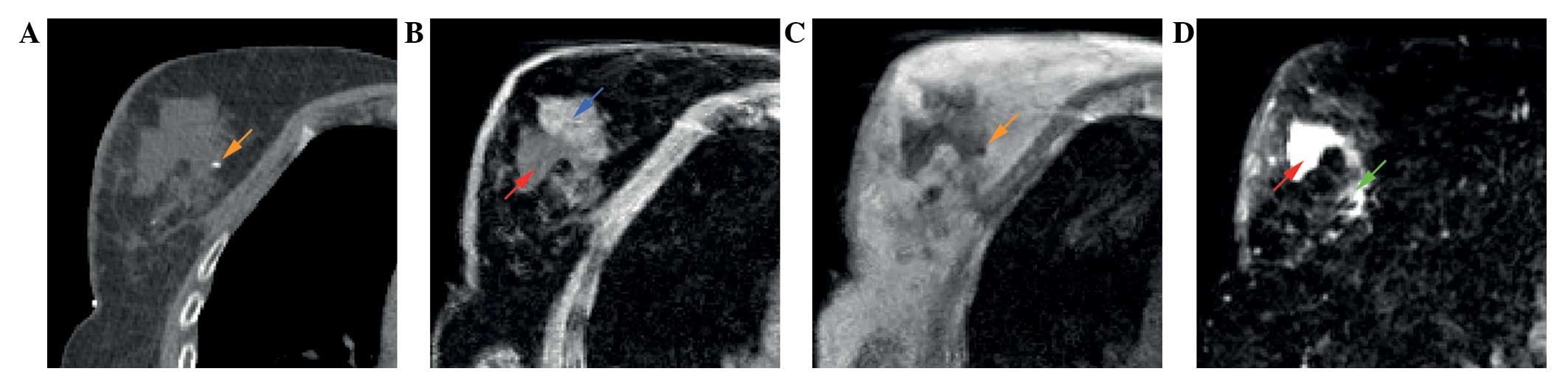 | Figure 2.A 62-year-old patient with pT1cN0(sn)
ductal carcinoma of the right breast. Different features of the
tumor bed as shown on (A) postoperative planning CT, (B)
postoperative T1w MRI with fat suppression, (C) postoperative T1w
MRI and (D) postoperative T2w MRI with fat suppression. Arrows:
Blue, fibroglandular tissue; red, seroma; orange, surgical clip;
green, area with intermediate signal intensity. CT, computed
tomography; MRI, magnetic resonance imaging; T1w, T1 weighted; T2w,
T2 weighted. |
 | Table II.Patient characteristics (n=14). |
Table II.
Patient characteristics (n=14).
| Characteristic | Value |
|---|
| Age, years |
|
|
Median | 61 |
|
Range | 48–70 |
| Microscopic tumor
diameter, mm |
|
|
Median | 12 |
|
Range | 6–29 |
| Histology, n |
|
| Ductal
carcinoma | 10 |
|
Ductal-lobular carcinoma | 3 |
| Tubular
carcinoma | 1 |
| Side, n |
|
|
Left | 7 |
|
Right | 7 |
| Time between
surgery and postoperative imaging, days |
|
|
Median | 21 |
|
Range | 14–50 |
| Surgical technique,
n |
|
| Open
cavity | 2 |
| Full
thickness closure | 12 |
| Number of clips
placed |
|
|
Median | 5 |
|
Range | 4–6 |
| Cavity
visualization scores, n |
|
| 1 - no
cavity visible | 1 |
| 2 -
heterogeneous cavity, indistinct margins | 5 |
| 3 -
heterogeneous cavity, some distinct margins | 2 |
| 4 -
mildly heterogenous cavity, mostly distinct margins | 5 |
| 5 -
homogenous cavity, clearly identified margins | 1 |
| Mean
score | 3 |
IOV in volume and dCOM
TB delineation determined by the four independent
observers resulted in wide ranges in volume, CI and dCOM on
standard postoperative planning CT (Table III), which did not improve with any
of the additional imaging methods. The lowest limit of the CI range
(CI, 0.00) was observed in cases with an absolute disagreement
among observers, with no overlap of TB delineations. This
disagreement occurred in one patient with a centrally located TB
and a CVS of 1 (no cavity visible), assigned unanimously by all
observers. One observer contoured a region different from the other
three observers. Excluding this outlier from the analysis did not
influence the outcomes. This observer did not cause outliers in any
of the other patients. Data analysis revealed that none of the
observers deviated from the other observers with regard to volume,
CI and dCOM.
 | Table III.Volume, conformity index and dCOM of
the tumor bed delineations. |
Table III.
Volume, conformity index and dCOM of
the tumor bed delineations.
| Parameter | Median | Range |
P-valuea |
|---|
| Volume,
cm3 |
|
|
|
| CT | 22 | 4–934 |
|
| CT +
postoperative MRI | 28 | 3–964 | <0.001 |
| CT +
preoperative CT | 26 | 6–933 | <0.001 |
| CT +
preoperative MRI | 25 | 7–933 | <0.001 |
| Conformity
index |
|
|
|
| CT | 0.57 | 0.00–0.90 |
|
| CT +
postoperative MRI | 0.61 | 0.00–0.89 |
0.176 |
| CT +
preoperative CT | 0.58 | 0.00–0.90 | <0.001 |
| CT +
preoperative MRI | 0.59 | 0.00–0.89 | <0.001 |
| dCOM, mm |
|
|
|
| CT | 5.11 | 0–53 |
|
| CT +
postoperative MRI | 3.72 | 0–52 |
0.110 |
| CT +
preoperative CT | 4.69 | 1–42 |
0.004 |
| CT +
preoperative MRI | 4.56 | 0–48 |
0.001 |
Addition of a postoperative MRI to the standard
postoperative planning CT did not influence the CI (P=0.176) or
dCOM (P=0.110; Table III). However,
the TB volumes increased significantly (P<0.001) with a median
increase of 6 cm3.
Addition of a preoperative CT or MRI significantly
increased the CI (P<0.001 for both) and dCOM (P=0.004 and
P=0.001, respectively; Table III).
A statistically significant absolute volume increase of 4
cm3 and 3 cm3, was observed following the
addition of preoperative CE-CT and CE-MRI, respectively (both
P<0.001).
The change in CI per observer pair following the
addition of an imaging method was plotted against the original CI
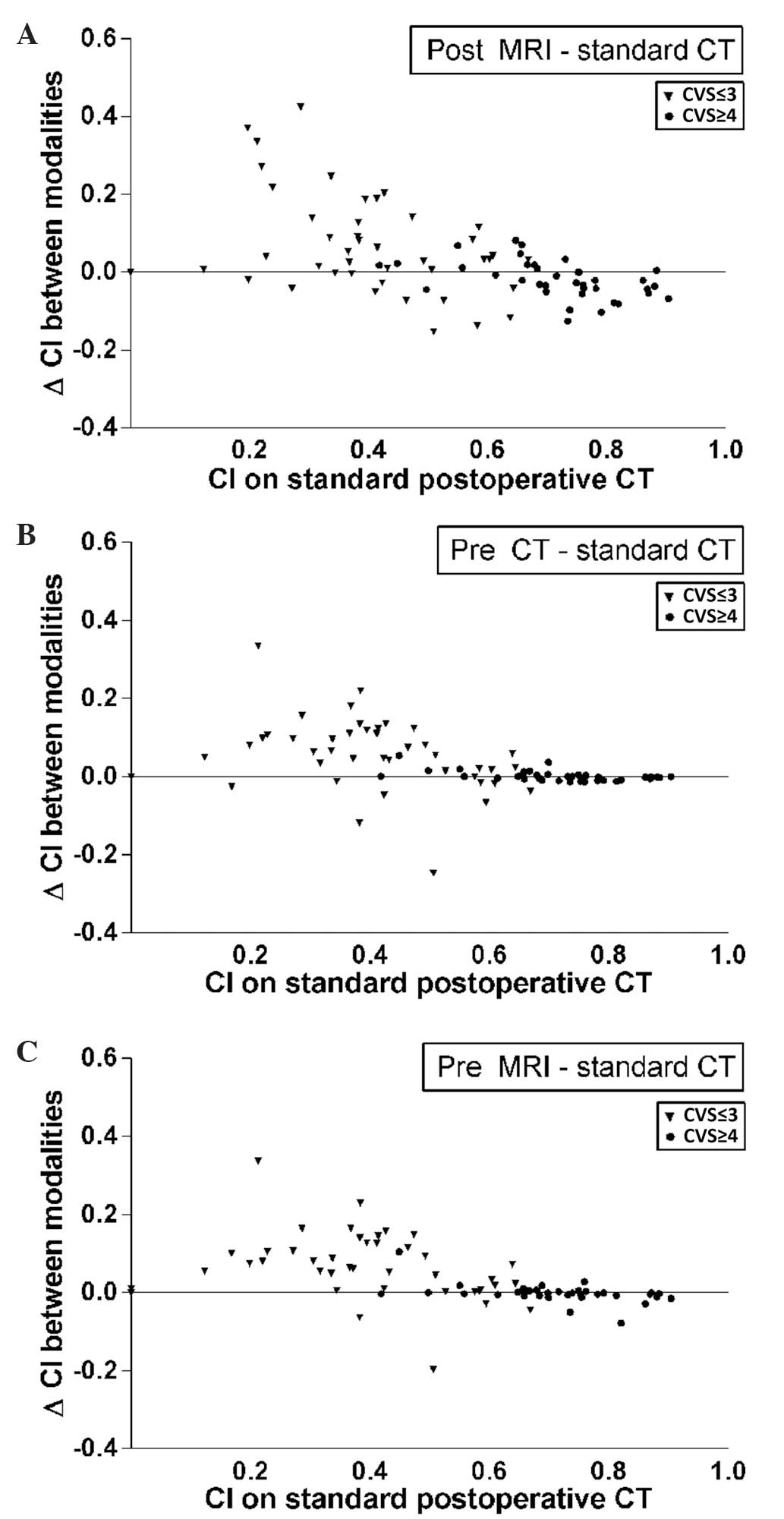
of that observer pair on standard postoperative CT (Fig. 3). These outcomes were categorized as
CVS≤3 and CVS≥4. In general, the CI was higher in patients with a
high CVS score (Fig. 3, circles). In
these patients, conformity did not increase with the use of
additional imaging modalities and significantly decreased following
the addition of postoperative MRI (P=0.016). By contrast, in
patients with a low CI and a more heterogeneous TB (CVS≤3), an
increase in CI was observed, from a median of 0.40 on CT to 0.52 on
CT with additional preoperative MRI. In this same subgroup, volumes
also increased, from 17 cm3 on CT to 23 cm3
on CT with additional postoperative MRI.
Discussion
To the best of our knowledge, the present study was
the first to investigate the value of additional pre- and
postoperative CT and MRI, with all imaging acquired in the
radiotherapy supine position using wide bore CT and MRI scanners,
and its effect on the IOV of the TB delineation.
The results indicated that the addition of
postoperative MRI did not improve the IOV of standard CT-guided TB
delineation. This was unexpected, as the use of different MRI
sequences enables differentiation between various soft tissues
(Fig. 2). In the fat-suppressed
images, MRI is unique in its clear contrast between seroma and
fibroglandular tissue. However, the interpretation of the different
MRI sequences in combination with the available patient information
appears to be observer-dependent, despite the training and written
delineation instructions. The observers were found to expand their
target volume rather than reduce their original CT-guided
delineation based on the information provided by additional
imaging. For instance, in Fig. 1B,
the area of seroma and architectural distortion on T2w MRI was
included. Furthermore, when the observers were provided with
preoperative imaging, they expanded the original CT-based
delineation in the direction of the original tumor, and did not
adjust, for instance, the medial borders. It appears that observers
will expand their contour based on additional information, but are
unlikely to reduce it when an area may not be part of the TB. In
that case, they seem to favor their interpretation of the standard
planning CT, which they are most familiar with. This finding may
also explain the volume increase that is observed subsequent to
providing additional imaging.
The heterogeneity in CI, as shown in Fig. 3, indicates that the subgroup of
patients with CVS≤3 potentially benefits from the use of MRI, since
the CI mostly increases in this subgroup. However, the clinical
relevance of this finding is debatable as the median CI following
the addition of preoperative MRI for this patient subgroup was only
0.52. Furthermore, these findings must be interpreted with caution,
as delineated volumes also increased by a median value of up to 6
cm3. It may be of interest to focus on the subgroup of
patients with a CVS of ≤3 in future studies in a larger patient
cohort, particularly as a higher incidence of these lower CVS
scores may be expected with the increasing use of full-thickness
closure following lumpectomy.
In line with the results of the present study, Kirby
et al (20) reported increased
TB volumes delineated on CT-MRI datasets. The current study
investigated whether additional MRI, registered to the standard
postoperative planning CT, was able to reduce the IOV of TB
delineation. In a previous study investigating TB delineation using
MRI-only, the conformity among observers was even lower (21), which is also consistent with the
results reported by Giezen et al (22). However, in a study by Jolicoeur et
al (23), the IOV improved and
the volumes were smaller on MRI-only, compared with that of
CT-based delineation. This contradiction may be due to the MRI
quality and the definition of the TB. In the study by Jolicoeur
et al (23), the TB was
defined as an architectural change on primarily T2w MRI. By
contrast, in other studies, the TB was reconstructed according to
architectural changes, original tumor location on preoperative
diagnostic imaging, physical examination and the placement of
surgical clips (20,22). Furthermore, Jolicoeur et al
(23) primarily used T2w sequences,
while the present study used multiple sequences. The use of
different surgical techniques may also have influenced the
differences in outcomes between these studies: The majority of
patients in the present study underwent full thickness closure,
while Jolicoeur et al (23)
excluded patients who underwent oncoplastic techniques, which is
likely to have included full thickness closure. Subsequent to
suturing the cavity walls, seroma may follow the suturing lines,
the shapes of which may be more subject to interpretational
differences compared with clearly defined cavity walls in
superficially closed cavities (Fig.
2, red and green arrows).
Giezen et al (22) and Jolicoeur et al (23) investigated TB delineation using MRI
separately and compared it with CT-guided delineation. In the
present study, the additional value of MRI registered to standard
postoperative CT was assessed, as this would be the application in
clinical practice. In this setting, no added value of postoperative
MRI was observed for the general postoperative patient
population.
Despite statistically significant differences, no
clinically relevant effects of either additional preoperative MRI
or CT imaging on the CI of postoperative CT-guided TB delineation
were observed in the current study. These findings are consistent
with those of Boersma et al (14), who found no increase in CI following
the addition of a preoperative CE-CT.
The study results may be influenced by structural
observer outliers, observer training and observer knowledge of MRI
interpretation. As there is no gold standard with which to validate
the ‘correct’ imaging modality for TB delineation, consensus among
observers is used as an alternative method. Upon the inspection of
the data, no observer deviated with regard to volume, CI or dCOM.
Even in the presence of guidelines, training and an adequate number
of clips, considerable variation exists (24). A possible limitation of the current
study is the small number of patients used. However, with increases
and decreases in IOV and volumes, a larger cohort is unlikely to
alter the average difference considerably.
For the overall patient population, additional
imaging will not improve consistency in TB delineation. However, it
may be possible to further improve the IOV of postoperative
CT-guided TB delineation. A number of studies have proposed
irradiation of the high-risk breast tissue surrounding the tumor
prior to lumpectomy, while the tumor remains in situ
(15,25–27). This
preoperative approach would result in a markedly lower IOV compared
with the current standard postoperative treatment (15,27).
Furthermore, preoperative image-guided target volume definition may
be validated using pathological studies as a gold standard, which
may improve confidence in an accurate treatment of the high-risk
area (28).
In conclusion, the addition of postoperative MRI,
preoperative CE-CT or preoperative CE-MRI did not result in a
considerable reduction of the IOV of postoperative CT-guided TB
delineation, while target volumes increased marginally. The
influence of additional imaging may be dependent on CVS.
Acknowledgements
This article is part of the PhD thesis entitled
‘Towards MRI-guided radiotherapy in early-stage breast cancer
patients’ (29).
References
|
1
|
Darby S, McGale P, Correa C, et al: Early
Breast Cancer Trialists' Collaborative Group (EBCTCG): Effect of
radiotherapy after breast-conserving surgery on 10-year recurrence
and 15-year breast cancer death: Meta-analysis of individual
patient data for 10,801 women in 17 randomised trials. Lancet.
378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartelink H, Horiot JC, Poortmans PM, et
al: Impact of a higher radiation dose on local control and survival
in breast-conserving therapy of early breast cancer: 10-year
results of the randomized boost versus no boost EORTC 22881-10882
trial. J Clin Oncol. 25:3259–3265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malinen E and Muren LP: Image guided
therapy - do we get the picture? Acta Oncol. 53:3–5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Struikmans H, Wárlám-Rodenhuis C, Stam T,
Stapper G, Tersteeg RJ, Bol GH and Raaijmakers CP: Interobserver
variability of clinical target volume delineation of glandular
breast tissue and of boost volume in tangential breast irradiation.
Radiother Oncol. 76:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coles CE, Wilson CB, Cumming J, Benson JR,
Forouhi P, Wilkinson JS, Jena R and Wishart GC: Titanium clip
placement to allow accurate tumour bed localisation following
breast conserving surgery: Audit on behalf of the IMPORT Trial
Management Group. Eur J Surg Oncol. 35:578–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurkmans C, Admiraal M, van der Sangen M
and Dijkmans I: Significance of breast boost volume changes during
radiotherapy in relation to current clinical interobserver
variations. Radiother Oncol. 90:60–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Mourik AM, Elkhuizen PH, Minkema D,
Duppen JC and van Vliet-Vroegindeweij C: Dutch Young Boost Study
Group: Multiinstitutional study on target volume delineation
variation in breast radiotherapy in the presence of guidelines.
Radiother Oncol. 94:286–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landis DM, Luo W, Song J, Bellon JR,
Punglia RS, Wong JS, Killoran JH, Gelman R and Harris JR:
Variability among breast radiation oncologists in delineation of
the postsurgical lumpectomy cavity. Int J Radiat Oncol Biol Phys.
67:1299–1308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
den Hartogh MD, van Asselen B, Monninkhof
EM, et al: Excised and irradiated volumes in relation to the tumor
size in breast-conserving therapy. Breast Cancer Res Treat.
129:857–1865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collette S, Collette L, Budiharto T, et
al: EORTC Radiation Oncology Group: Predictors of the risk of
fibrosis at 10 years after breast conserving therapy for early
breast cancer: A study based on the EORTC Trial 22881-10882 ‘boost
versus no boost’. Eur J Cancer. 44:2587–2599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vrieling C, Collette L, Fourquet A, et al:
EORTC Radiotherapy and Breast Cancer Cooperative Groups: The
influence of patient, tumor and treatment factors on the cosmetic
results after breast-conserving therapy in the EORTC ‘boost vs. no
boost’ trial. Radiother Oncol. 55:219–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukesh MB, Barnett G, Cumming J, Wilkinson
JS, Moody AM, Wilson C, Wishart GC and Coles CE: Association of
breast tumour bed seroma with post-operative complications and late
normal tissue toxicity: Results from the Cambridge Breast IMRT
trial. Eur J Surg Oncol. 38:918–924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whipp EC and Halliwell M: Magnetic
resonance imaging appearances in the postoperative breast: The
clinical target volume-tumor and its relationship to the chest
wall. Int J Radiat Oncol Biol Phys. 72:49–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boersma LJ, Janssen T, Elkhuizen PH,
Poortmans P, van der Sangen M, Scholten AN, Hanbeukers B, Duppen
JC, Hurkmans C and van Vliet C: Reducing interobserver variation of
boost-CTV delineation in breast conserving radiation therapy using
a pre-operative CT and delineation guidelines. Radiother Oncol.
103:178–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
den Hartogh MD, Philippens ME, van Dam IE,
et al: MRI and CT imaging for preoperative target volume
delineation in breast-conserving therapy. Radiat Oncol. 9:632014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (UICC): TNM Classification of
Malignant Tumors (7th). Chichester, UK: Wiley-Blackwell. 2009.
|
|
17
|
Dixon WT: Simple proton spectroscopic
imaging. Radiology. 153:189–194. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bol GH, Kotte AN, van der Heide UA and
Lagendijk JJ: Simultaneous multi-modality ROI delineation in
clinical practice. Comput Methods Programs Biomed. 96:133–140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smitt MC, Birdwell RL and Goffinet DR:
Breast electron boost planning: Comparison of CT and US. Radiology.
219:203–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirby AM, Yarnold JR, Evans PM, Morgan VA,
Schmidt MA, Scurr ED and desouza NM: Tumor bed delineation for
partial breast and breast boost radiotherapy planned in the prone
position: What does MRI add to X-ray CT localization of titanium
clips placed in the excision cavity wall? Int J Radiat Oncol Biol
Phys. 74:1276–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
den Hartogh MD, van den Bongard HJ,
Davidson MT, Kotte AN, Verkooijen HM, Philippens ME, van Vulpen M,
van Asselen B and Pignol JP: Full-thickness closure in
breast-conserving surgery: The impact on radiotherapy target
definition for boost and partial breast irradiation. A
multimodality image evaluation. Ann Surg Oncol. 21:3774–3779. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giezen M, Kouwenhoven E, Scholten AN,
Coerkamp EG, Heijenbrok M, Jansen WP, Mast ME, Petoukhova AL and
Struikmans H: MRI-versus CT-based volume delineation of lumpectomy
cavity in supine position in breast-conserving therapy: An
exploratory study. Int J Radiat Oncol Biol Phys. 82:1332–1340.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jolicoeur M, Racine ML, Trop I, Hathout L,
Nguyen D, Derashodian T and David S: Localization of the surgical
bed using supine magnetic resonance and computed tomography scan
fusion for planification of breast interstitial brachytherapy.
Radiother Oncol. 100:480–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirby AN, Jena R, Harris EJ, Evans PM,
Crowley C, Gregory DL and Coles CE: Tumour bed delineation for
partial breast/breast boost radiotherapy: What is the optimal
number of implanted markers? Radiother Oncol. 106:231–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Palta M, Yoo S, Adamson JD, Prosnitz LR
and Horton JK: Preoperative single fraction partial breast
radiotherapy for early-stage breast cancer. Int J Radiat Oncol Biol
Phys. 82:37–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nichols EM, Feigenberg SJ, Marter K,
Cheston SB, Lasio G, Tkaczuk K, Kesmodel S, Buras R and Regine WF:
Preoperative radiation therapy significantly increases patient
eligibility for accelerated partial breast irradiation using
3D-conformal radiotherapy. Am J Clin Oncol. 36:232–238. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van der Leij F, Elkhuizen PH, Janssen TM,
Poortmans P, van der Sangen M, Scholten AN, van Vliet-Vroegindeweij
C and Boersma LJ: Target volume delineation in external beam
partial breast irradiation: Less inter-observer variation with
preoperative-compared to postoperative delineation. Radiother
Oncol. 110:467–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schmitz AC, van den Bosch MA, Loo CE, Mali
WP, Bartelink H, Gertenbach M, Holland R, Peterse JL, Rutgers EJ
and Gilhuijs KG: Precise correlation between MRI and histopathology
- exploring treatment margins for MRI-guided localized breast
cancer therapy. Radiother Oncol. 97:225–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
den Hartogh MD: Towards MRI-guided
radiotherapy in early-stage breast cancer patients. https://www.umcutrecht.nl/getmedia/b6fd022c-ffc9-49d0-a26f-602916d5d225/Proefschrift_MdenHartogh.pdf.aspxPhD
dissertation. University Medical Center Utrecht (Utrecht, The
Netherlands). 2014.
|