Introduction
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer worldwide (1).
It is the third leading cause of cancer-related mortality globally
and the second leading cause in China (2,3).
Worldwide, there are 560,000 new cases of HCC reported per year
(4). The highest incidence rates are
in areas of Asia and Africa, where individuals are at a high risk
of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection,
and it is generally accepted that hepatitis viruses have a major
role in HCC development (5). A
previous study (6) reported that the
five-year survival rate of HCC patients following surgical
resection for small and large HCC was 63.4 and 39.6%, respectively,
whereas the five-year survival rate for patients with unresectable
HCC treated with cytoreduction therapy was 64.7%. The poor
prognosis of HCC may be attributed to the recurrent and metastatic
nature of the disease (6). Therefore,
the identification of oncogenes and progression markers may
contribute greatly to the prevention and treatment of HCC.
c-Src, the human homolog of the Rous sarcoma
virus-transforming gene, is a non-receptor tyrosine kinase. It is a
critical modulator of multiple signaling pathways mediated by
integrins, G protein-coupled receptors, cell adhesion proteins and
hormone receptors (7). Src remains
inactive in the cytoplasm when it is phosphorylated at Y530.
However, once activated, Src translocates to the membrane and
becomes fully activated by autophosphorylation at Y416 (8,9). Activated
Src [phosphorylated (p-) Y416Src] at the cell membrane initiates
signaling pathways that induce cell proliferation, adhesion and
migration/invasion (10). Src is
characterized as an oncogene, and the overexpression and/or
elevated activity of Src appears to be involved in the progression
of various tumor types, including HCC (11,12).
Notably, although higher Src activity has been detected by an in
vitro kinase assay in HCC (13)
and its activation is reported to be involved in the cancerous
behaviors of HCC cells (14,15), it has yet to be clarified whether Src
is involved in the pathogenesis and progression of HCC, and whether
it influences specific HCC clinicopathological factors.
Therefore, the present study aimed to characterize
the expression and distribution of total Src (t-Src) and p-Y416Src
in HCC tissues, and in two HCC cell lines with different metastatic
potentials derived from a single Chinese HCC patient. Furthermore,
the associations between the expression of t-Src and p-Y416Src and
various clinicopathological characteristics were analyzed.
Materials and methods
Cell lines and culture
MHCC97-L and HCCLM3 cell lines, derived from MHCC97
parental HCC cells and exhibiting different metastatic potentials,
were provided by the Liver Cancer Institute, Shanghai Medical
College of Fudan University (Shanghai, China). MHCC97 cells are
derived from a single Chinese patient with HCC (16,17). All
cell lines were cultured in Dulbecco's modified Eagle's medium
(DMEM; GE Healthcare Life Sciences, Logan, UT, USA), containing 10%
fetal bovine serum (Gibco Life Technologies, Carlsbad, CA, USA) and
1% antibiotic (100 IU/ml penicillin and 100 µg/ml streptomycin;
Mediatech, Inc., Manassas, VA, USA). Cells were maintained at 37°C
in an atmosphere of 5% CO2.
Tissue samples
A total of 52 paraffin-embedded HCC tissue samples
and 52 control tissues samples from the adjacent noraml liver were
obtained from Chinese patients at The First Affiliated Hospital of
Harbin Medical University (Harbin, China) between 2010 and 2012.
The patients were well-characterized for clinical, pathological and
phenotypic markers. Sample collection was approved by the Harbin
Medical University Institutional Ethics Committee and written
informed consent was obtained from all patients. Long-term
follow-up data was not available as all HCC cases were recent;
therefore, survival curves could not be calculated. The diagnoses
of HCC were established by clinical features, according to the
National Comprehensive Cancer Network (18) and British Society of Gastroenterology
guidelines (19), and confirmed by
histological analysis. Liver specimens were obtained by needle
biopsy or surgical resection and transferred to 10% neutral
formalin within 15 min to minimize loss of phospho-antigens. All
patient features, including age, tumor size, tumor node metastasis
(TNM) stage (20), HBV status and
α-fetoprotein (AFP) levels, were obtained from the pathological
case reports.
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded liver sections (5
µm thick) were prepared. t-Src and activated p-Y416Src expression
were assessed by IHC using rabbit anti-human antibodies against
t-Src (monoclonal IgG; 36D10; #2109; Cell Signaling Technology,
Inc., Danvers, MA, USA) and p-Y416Src (polyclonal; #2101; Cell
Signaling Technology, Inc.). The standard indirect IHC method was
performed, as previously described in our laboratory (21). Briefly, the sections were placed in
citrate buffer (pH 6.0) and heated in a microwave oven for antigen
retrieval (95°C for 3 min). Endogenous peroxidase activity was
inhibited by incubation in 3% hydrogen peroxide
(H2O2) for 15 min. After blocking with 10%
goat serum for 1 h, slides were incubated overnight at 4°C with
antibodies to t-Src (1:400 dilution) and p-Y416Src (1:50 dilution).
The secondary antibody staining kit (horseradish
peroxidase-conjugated goat anti-rabbit IgG polymer; PV-6001;
ZSGB-Bio, Beijing, China) was then applied for 45 min at 37°C, and
a 3,3′-diaminobenzidine substrate kit (ZLI-9019; ZSGB-Bio) was
added to the tissue for 2 min. Sections were counterstained with
hematoxylin (Sigma-Aldrich, St. Louis, MO, USA), dehydrated and
mounted. The specificity of immunostaining was evaluated by
replacing the primary t-Src and p-Y416Src antibodies with
non-specific, isotype-matched rabbit IgG (24E10; #3195; 1:200
dilution; Cell Signaling Technology, Inc.) in phosphate-buffered
saline.
Scoring
Src expression in tumor samples was assessed using
the histoscore method developed by Allred et al (22). Cellular location (cytoplasm and
membranes) of Src staining was scored separately for each sample.
In each specimen, an intensity score and a proportion score were
determined. The staining intensity was scored based on visual
assessment of brown color within the cytoplasm or cell membrane on
a scale of 0–3, as follows: 0, no staining; 1, weak staining; 2,
moderate staining; or 3, strong staining). The proportion score
represented the percentage of positively stained cells in the
entire tissue section under microscopic observation (Eclipse E800;
Nikon Corporation, Tokyo, Japan) observation 0, none; 1, <5%; 2,
5–25%; 3, 26–50%; 4, 51–75%; 5, >75%). Overall Src expression in
each tumor sample was then calculated as a sum of the intensity
score (0–3) and the proportion score (0–5) to give a range of 0–8
(22). Scores of 0 were categorized
as negative staining and scores of 1–8 were categorized as positive
staining (weak, 1–2; moderate, 3–6; positive, 7–8). Three
investigators, blinded to the patient characteristics, scored the
slides independently and an agreement was reached for all
samples.
Immunocytochemistry
MHCC97-L and HCCLM-3 cells were cultured and fixed
in 95% ethanol (Tianjin Ke Mi Ou Chemical Reagent Co., Tianjin,
China) for 5 min at room temperature. Cell slides were then washed
and permeabilized by incubation with 0.2% Triton X-100
(Sigma-Aldrich) for 15 min at 4°C. Endogenous peroxidase activity
was inhibited by incubation of the cells in 3%
H2O2 for 20 min. Staining of the cells using
the standard indirect horseradish peroxidase method was performed
as described for IHC.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 10.0; SPSS, Inc., Chicago, IL, USA). The
χ2 test was used to analyze the frequency of expression,
activation and subcellular localization of Src between cancer and
normal tissue samples, and between different groups of clinical
data. The histoscore values were reported as the mean ± standard
error of the mean. The Mann-Whitney U test was used to analyze
differences in the expression of t-Src and p-Y416Src in the
cytoplasm and membrane between cancer tissue and normal tissue, and
between lymph nodes with different metastatic statuses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The clinical features and pathological findings of
the patients investigated are indicated in Table I. Age, gender distribution, percentage
of HBV- and HCV-positive patients, pathological characteristics and
blood marker expression were similar to previous results in Chinese
patients with HCC (23).
 | Table I.Demographics, pathological features
and clinical markers in the patients with hepatocellular
carcinoma. |
Table I.
Demographics, pathological features
and clinical markers in the patients with hepatocellular
carcinoma.
| Characteristic | Value |
|---|
| Patients, n | 52 |
| Gender, n |
|
| Male | 42 |
|
Female | 10 |
| Age, years |
|
| Mean | 54 |
|
Range | 22–72 |
| HBV-positive, % | 65.38 |
| HCV-positive, % | 9.62 |
| Cirrhosis-positive,
% | 51.92 |
| TNM stage, n |
|
| I | 0 |
| II | 20 |
|
III | 25 |
| IV | 7 |
| Differentiation,
n |
|
|
Well | 13 |
|
Moderate | 28 |
|
Poor | 11 |
| Lymph node
metastasis-positive, % | 42.31 |
| Marker
expression |
|
| AFP
>400 ng/ml, % | 73.08 |
| CEA
>5 µg/l, % | 30.77 |
| SF
>13 µg/l, % | 75.00 |
| CA19-9
>35 U/ml, % | 32.69 |
Expression pattern of Src in HCC and
adjacent normal liver tissues
To identify the expression pattern of Src in HCC and
adjacent normal liver tissues, the present study initially detected
and compared the expression rate of Src in two groups of tissues.
The t-Src positive expression rate in HCC tissues was 65.38%, which
was significantly higher than that in adjacent normal liver tissue
(30.76%; P<0.001; Table II).
Similarly, the active Src (p-Y416Src) positive frequency was
significantly higher in HCC tissue when compared with adjacent
normal liver tissue (P=0.010; Table
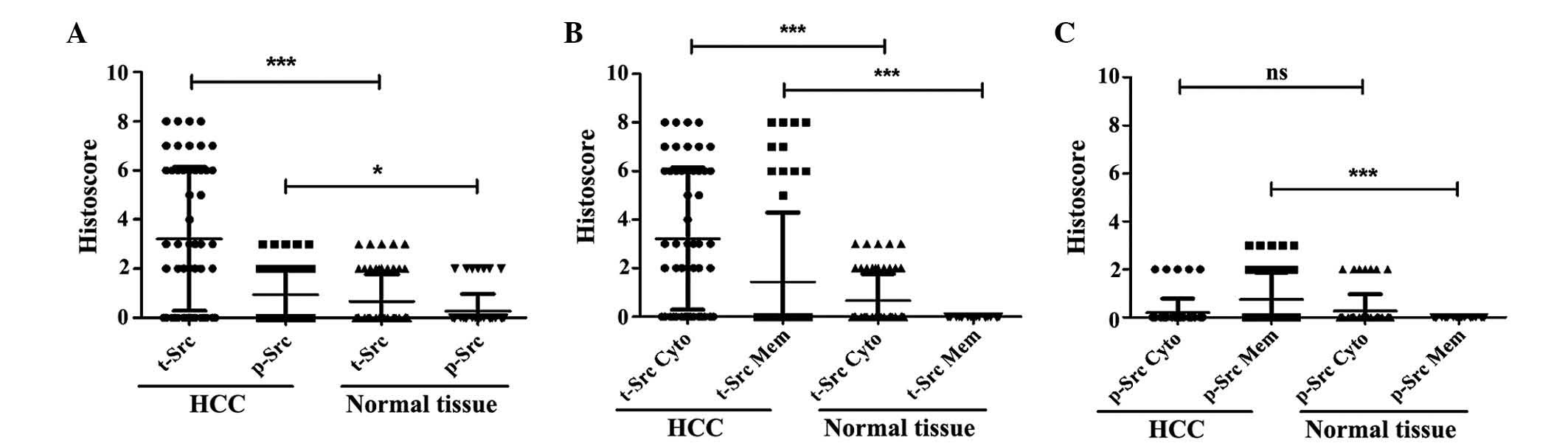
II). In addition, the histoscore of t-Src and p-Y416Src
staining were analyzed using a Mann-Whitney U test. The statistical
analysis revealed that the staining scores of t-Src (mean
histoscore, 3.21) and p-Y416Src (mean histoscore, 0.94) were both
significantly higher in HCC tissues compared with in adjacent
normal liver tissues (P<0.001 and P=0.023, respectively;
Fig. 1A).
 | Table II.Expression of t-Src and p-Y416Src in
HCC tissue and adjacent normal liver tissue (n=52). |
Table II.
Expression of t-Src and p-Y416Src in
HCC tissue and adjacent normal liver tissue (n=52).
| Variable | HCC, % (n) | Normal % (n) | χ2 | P-value |
|---|
| t-Src positive | 65.38 (34/52) | 30.76 (16/52) | 12.48 | <0.001 |
| p-Y416Src
positive | 42.30 (22/52) | 13.46 (7/52) | 10.76 |
0.010 |
Subsequently, the subcellular distribution of Src
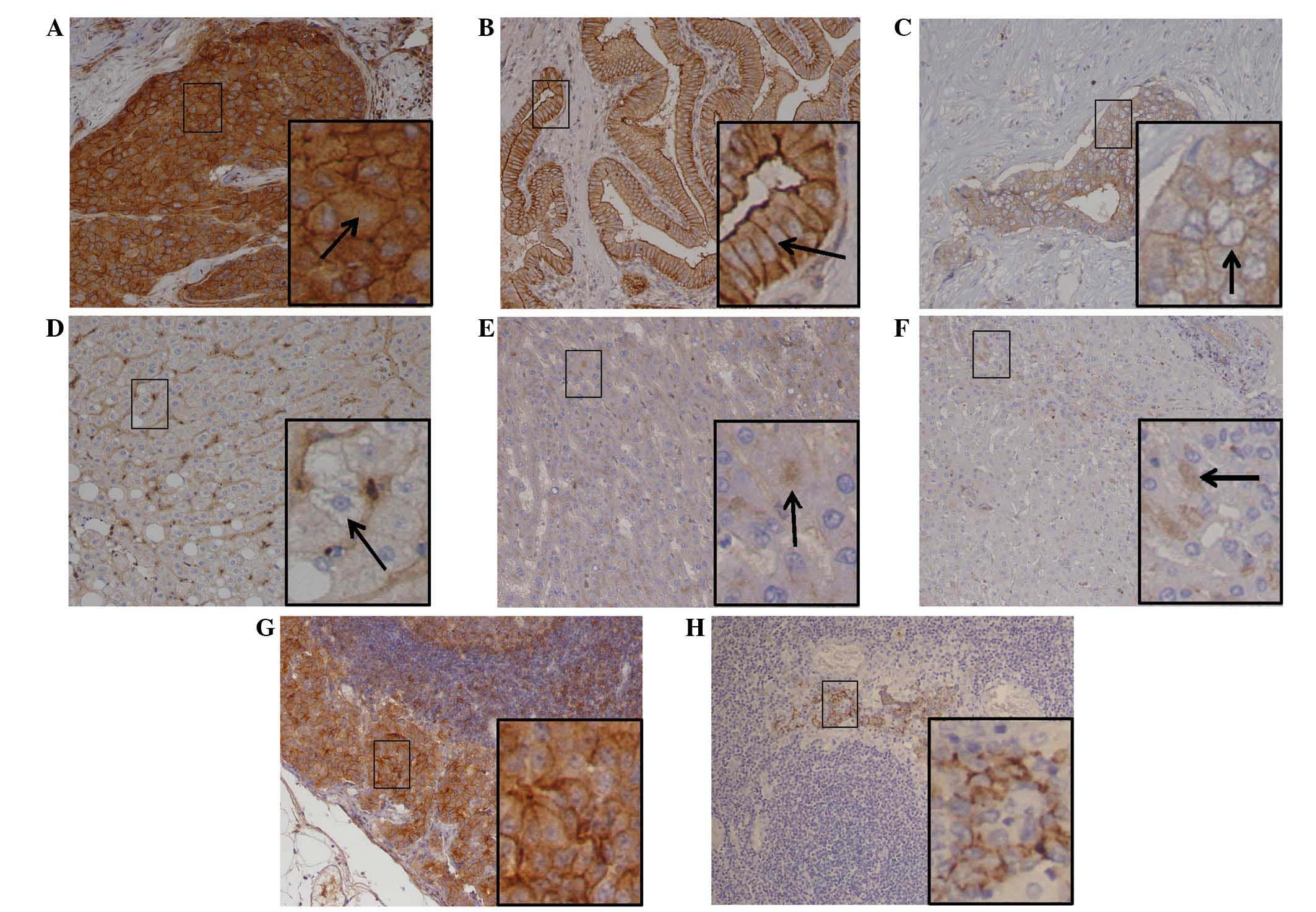
was detected in the two groups of tissues. Of the HCC tissue
samples, 34 exhibited positive t-Src staining in the cytoplasm,
with different histoscores. Positive t-Src staining in the membrane
was observed in 11 of the t-Src cytoplasm positive cases (Table III and Fig. 2A). Furthermore, positive t-Src
staining in the membrane only appeared in cases of HCC with strong
cytoplasmic staining; no cases exhibited only t-Src membrane
staining. In comparison, positive t-Src staining exhibited only
weak cytoplasmic distribution in hepatocytes of the adjacent normal
tissue samples (Fig. 2E), whilst
membrane t-Src was not detected in the normal hepatocytes (Table III). The positive staining frequency
and histoscores of t-Src subcellular location in the cytoplasm
(mean histoscore, 3.21; P<0.001 and P<0.001, respectively)
and membrane (mean histoscore, 1.44; P<0.001 and P<0.001,
respectively) were significantly higher in HCC tissues when
compared with adjacent normal tissues (mean cytoplasm and membrane
histoscores, 0.67 and 0.00, respectively; Table III and Fig. 1B).
 | Table III.Subcellular localization of t-Src and
p-Y416Src in HCC tissue and adjacent normal liver tissue
(n=52). |
Table III.
Subcellular localization of t-Src and
p-Y416Src in HCC tissue and adjacent normal liver tissue
(n=52).
| Variable | HCC, % (n) | Normal, % (n) | χ2 | P-value |
|---|
| t-Src |
|
|
|
|
|
Cytoplasm | 65.38 (34/52) | 30.76 (16/52) | 12.48 | <0.001 |
|
Membrane | 21.15 (11/52) | 0.00 (0/0) | 12.30 | <0.001 |
| p-Y416Src |
|
|
|
|
|
Cytoplasm | 9.62 (5/52) | 13.46 (7/52) |
0.38 |
0.539 |
|
Membrane | 32.69 (17/52) | 0.00 (0/0) | 20.32 | <0.001 |
With regard to p-Y416Src, positive cytoplasmic
staining was identified in 9.62% (5/52) of HCC cases and positive
staining for membrane p-Y416Src was observed in 32.69% (17/52) of
HCC cases (Table III and Fig. 2C). By contrast, all p-Y416Src-positive
adjacent normal tissue cases (n=7) exhibited only weak cytoplasmic
staining (Fig. 2F) and no positive
staining of the membrane was detected. Statistical analysis
revealed that membrane p-Y416Src expression was detected
significantly more frequently (P<0.001) and more strongly
(P<0.001) in HCC tissue (mean histoscore, 0.75) than in normal
liver tissue (mean histoscore, 0.00; Table III and Fig. 1C). However, there was no significant
difference in cytoplasmic p-Y416Src staining between HCC and normal
tissues (Table III and Fig. 1C).
Clinical and pathological
correlations
As t-Src expression occurred at a high frequency in
HCC tissues compared with adjacent normal tissues, the correlation
between t-Src expression and the patients' clinicopathological
characteristics was analyzed. t-Src expression was not associated
with patient age, gender, HBV/HCV infection status, cirrhosis,
tumor size, or AFP, carcinoembryonic antigen or serum ferritin
levels (P>0.05). However, high expression of t-Src in HCC was
significantly associated with more advanced TNM cancer stage
(P=0.002), poor cellular differentiation (P=0.007), the presence of
lymph node metastasis (P=0.030) and high carbohydrate antigen 19-9
(CA19-9) level (P=0.016; Table IV).
However, positive p-Y416Src expression was only significantly
associated with more advanced TNM stage (P=0.010), and no other
factors. Furthermore, strong staining of t-Src and p-Y416Src was
observed in lymph nodes with HCC metastasis (Fig. 2G and H), and high Src expression
scores in HCC tissues were associated with metastasis-positive
lymph nodes (P=0.007, P=0.008; Table
V). These data indicated that elevated t-Src expression was
significantly associated with HCC metastasis, as well as tumor
stage, cellular differentiation and CA19-9 level.
 | Table IV.Correlation of Src expression with
clinicopathological characteristics in patients with HCC. |
Table IV.
Correlation of Src expression with
clinicopathological characteristics in patients with HCC.
|
|
| t-Src, n (%) |
|
| p-Y416Src, n
(%) |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | n | Positive | Negative | χ2 | P-value | Positive | Negative | χ2 | P-value |
|---|
| Gender |
|
|
| 0.001 | 0.977 |
|
| 0.271 | 0.603 |
|
Male | 42 | 28 (66.67) | 14 (33.33) |
|
| 19 (45.24) | 23 (54.76) |
|
|
|
Female | 10 | 6
(60.00) | 4
(40.00) |
|
| 3
(30.00) | 7
(70.00) |
|
|
| Age, years |
|
|
| 0.051 | 0.820 |
|
| 0.552 | 0.458 |
|
≤50 | 22 | 14 (63.64) | 8
(36.36) |
|
| 8
(36.36) | 14 (63.64) |
|
|
|
>50 | 30 | 20 (66.67) | 10 (33.33) |
|
| 14 (46.67) | 16 (53.33) |
|
|
| HBV |
|
|
| 1.868 | 0.172 |
|
| 1.868 | 0.172 |
|
Positive | 34 | 20 (58.82) | 14 (41.18) |
|
| 14 (41.18) | 20 (58.82) |
|
|
|
Negative | 18 | 14 (77.78) | 4
(22.22) |
|
| 8
(44.44) | 10 (55.56) |
|
|
| HCV |
|
|
| 0.052 | 0.820 |
|
| 0.134 | 0.714 |
|
Positive | 5 | 4
(80.00) | 1
(20.00) |
|
| 3
(40.00) | 2
(60.00) |
|
|
|
Negative | 47 | 30 (63.83) | 17 (36.17) |
|
| 19 (40.43) | 28 (59.57) |
|
|
| Cirrhosis |
|
|
| 0.041 | 0.84 |
|
| 0.785 | 0.376 |
|
Present | 27 | 18 (66.67) | 9
(33.33) |
|
| 13 (48.15) | 14 (51.85) |
|
|
|
Absent | 25 | 16 (64.00) | 9
(36.00) |
|
| 9
(36.00) | 16 (64.00) |
|
|
| TNM stage |
|
|
| 9.253 | 0.002a |
|
| 6.626 | 0.010b |
|
I–II | 20 | 8
(40.00) | 12 (60.00) |
|
| 4
(20.00) | 16 (80.00) |
|
|
|
III–IV | 32 | 26 (81.25) | 6
(18.75) |
|
| 18 (56.25) | 14 (43.75) |
|
|
| Cellular
differentiation |
|
|
| 7.251 | 0.007a |
|
| 2.626 | 0.105 |
|
Well | 13 | 4
(30.77) | 9
(69.23) |
|
| 3
(23.08) | 10 (76.92) |
|
|
|
Moderate/poor | 39 | 30 (76.92) | 9
(23.08) |
|
| 19 (48.72) | 20 (51.28) |
|
|
| Tumor diameter,
cm |
|
|
|
0.569 | 0.451 |
|
| 0.908 | 0.341 |
| ≤5 | 18 | 13 (72.22) | 5
(27.78) |
|
| 6
(33.33) | 12 (66.67) |
|
|
|
>5 | 34 | 21 (61.76) | 13 (38.24) |
|
| 16 (47.06) | 18 (52.94) |
|
|
| Lymph node
metastasis |
|
|
| 4.550 | 0.030b |
|
| 2.340 | 0.126 |
|
Present | 22 | 18 (81.82) | 4
(18.18) |
|
| 12 (54.55) | 10 (45.45) |
|
|
|
Absent | 30 | 16 (53.33) | 14 (46.67) |
|
| 10 (33.33) | 20 (66.67) |
|
|
| AFP, ng/ml |
|
|
| 0.052 | 0.820 |
|
| 0.341 | 0.559 |
|
≤400 | 14 | 10 (71.43) | 4
(28.57) |
|
| 5
(35.71) | 9
(64.29) |
|
|
|
>400 | 38 | 24 (63.16) | 14 (36.84) |
|
| 17 (44.74) | 21 (55.26) |
|
|
| CEA, µg/l |
|
|
| 0.944 | 0.331 |
|
| 0.560 | 0.454 |
| ≤5 | 36 | 22 (61.11) | 14 (38.89) |
|
| 14 (38.89) | 22 (61.11) |
|
|
|
>5 | 16 | 12 (75.00) | 4
(25.00) |
|
| 8
(50.00) | 8
(50.00) |
|
|
| SF, µg/l |
|
|
| 0.453 | 0.501 |
|
| 0.945 | 0.331 |
|
≤13 | 13 | 7
(53.85) | 6
(46.15) |
|
| 4
(30.77) | 9
(69.23) |
|
|
|
>13 | 39 | 27 (69.23) | 12 (30.77) |
|
| 18 (46.15) | 21 (53.85) |
|
|
| CA19-9, U/ml |
|
|
| 5.827 | 0.016b |
|
| 2.823 | 0.093 |
|
≤35 | 35 | 19 (54.29) | 16 (45.71) |
|
| 12 (34.29) | 23 (65.71) |
|
|
|
>35 | 17 | 15 (88.24) | 2
(11.76) |
|
| 10 (58.82) | 7 (41.18) |
|
|
 | Table V.Association between lymph node status
and Src expression in HCC tissues. |
Table V.
Association between lymph node status
and Src expression in HCC tissues.
| Variable | Lymph node
metastasis positive, % (n=22) | Lymph node
metastasis negative, % (n=30) | P-value |
|---|
| t-Src | 4.45±2.91 | 2.30±2.62 | 0.008 |
| p-Y416Src | 1.36±1.09 | 0.53±1.00 | 0.007 |
Src expression in HCC cell lines
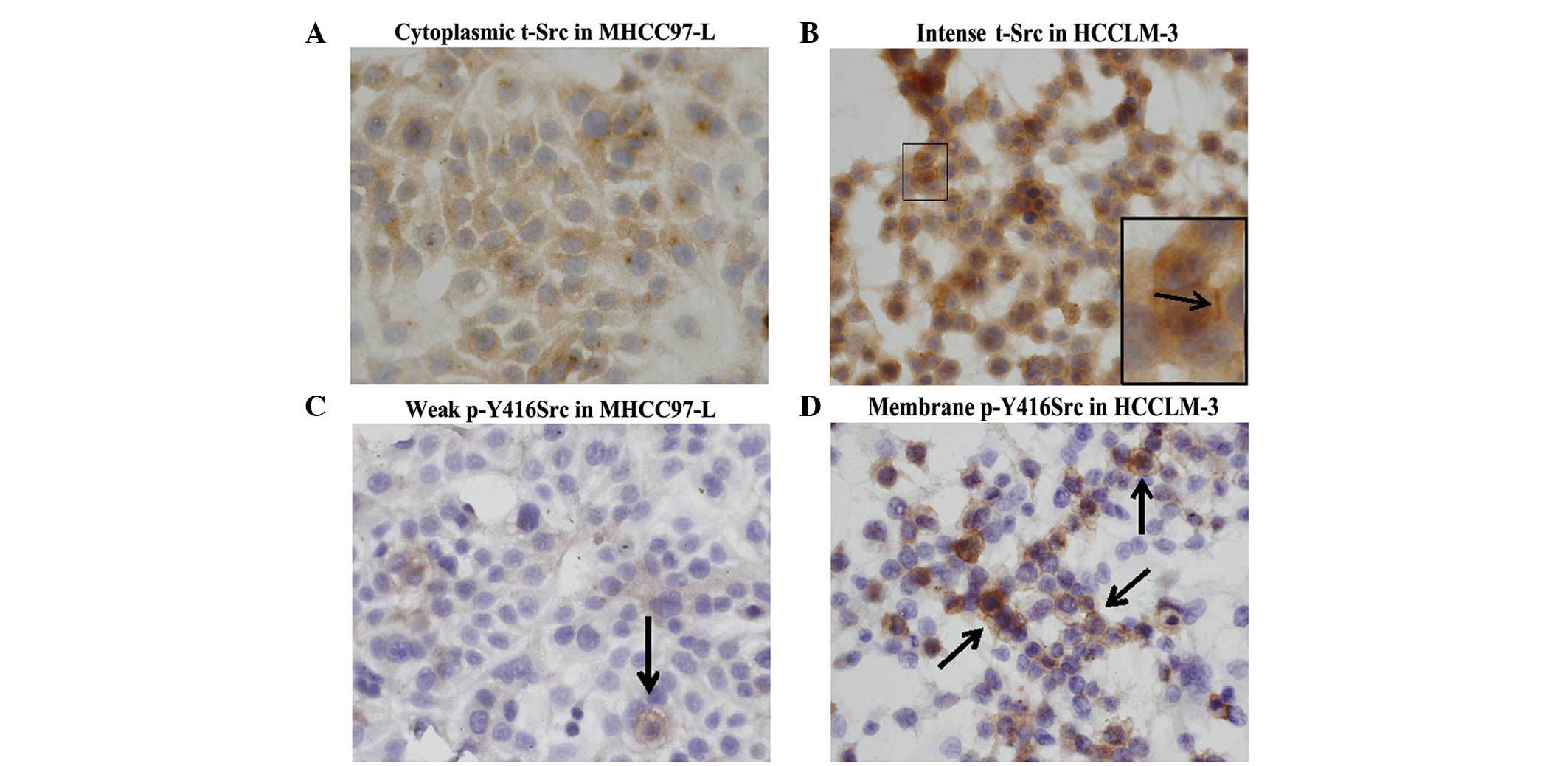
Additionally, the expression of t-Src and p-Y416Src
was detected by immunocytochemistry in two HCC cell lines with
different metastatic potentials derived from a single Chinese HCC
patient. Stonger expression of both t-Src and p-Y416Src was
detected in the higher metastatic potential cell line, HCCLM-3
(Fig. 3B and D). However, only weak
expression of these two forms of Src was detected in the lower
metastatic potential cell line, MHCC97-L (Fig. 3A and C). These results were consistent
with those obtained from HCC tissues, which indicated that elevated
Src expression is associated with HCC metastasis.
Discussion
Src and focal adhesion kinase (FAK) are two
components of non-receptor intracellular tyrosine kinases that are
linked by integrin-extracellular matrix interactions (9). The Src and FAK proteins function as a
complex in cellular signaling networks and control numerous
important biological processes within the cell (9). Although the aberrant expression and
activity of Src is well documented in colon and breast cancer
(12,24,25),
immunohistological data regarding Src expression in HCC and the
association between Src expression and HCC metastasis is still
lacking.
To the best of our knowledge, the current study is
the first to demonstrate increased Src expression in HCC tissue
from Chinese patients compared with adjacent normal liver tissue.
It was identified that 65.38% (34/52) of HCC samples from Chinese
patients had positive t-Src expression, as detected by IHC.
Furthermore, the localization of t-Src was cytoplasmic, consistent
with previous reports (23,26). Furthermore, considering that the
patients in the aforementioned Japanese study of HCC (26) were predominantly positive for HCV
infection, while the Chinese patients in the current study were
predominantly positive for HBV infection, we propose that positive
t-Src expression is not induced by a specific viral infection. Ito
et al (26) identified
p-Y416Src expression using IHC on liver sections from 87 Japanese
patients with HCC and detected active Src in 46% of the cohort.
Similarly, the present study observed that 42.30% of Chinese HCC
cases exhibited positive p-Y416Src expression. By contrast,
p-Y416Src was not detected in normal liver samples in a previous
report (23) but presented weak
cytoplasmic localization in the present study. Considering that Src
functions in normal and tumor cells, and its activity is dependent
on phosphorylation sites, we propose that Src, including p-Y416Src,
is expressed in normal hepatocytes at low levels of activation. In
the current study, 16 of 34 cases with t-Src and 7 of 22 cases with
p-Y416Src positive expression in HCC tissues exhibited weak
cytoplasmic localization in normal hepatocytes.
The clinical and pathological implications of Src
expression and HCC remain to be clarified. Masaki et al
(13) identified higher Src kinase
activity in a small cohort of poorly differentiated HCC cases
compared with normal liver tissue. Higher expression of Src was
more common in well or moderately differentiated carcinomas than in
poorly differentiated ones (13),
contradicting data that it is typically associated with more
advanced cancer. It has also been reported that Src expression is
correlated with Ki-67 expression, intrahepatic metastasis, TNM
stage, tumor grade and AFP expression (23,26,27). The
current data demonstrates that increased t-Src expression is
significantly associated with more advanced TNM cancer stage, poor
cellular differentiation, the presence of lymph node metastasis and
high CA19-9 expression levels. These data are partly consistent
with previous studies (23,26,27). It is
well-known that the phosphorylation of Y416 in the activation of
the kinase domain upregulates the enzyme activity of Src (28,29), which
is involved in the induction of pathways related to cell
proliferation, adhesion and migration/invasion (10). Thus, increased p-Y416Src expression
may facilitate further HCC progression and is consequently
associated with poor prognosis. Although it has been reported that
increased p-Y416Src expression is associated with poor patient
survival (27), the current data
demonstrated that elevated p-Y416Src expression is independent of
the majority of clinical and pathological parameters.
The MHCC97 cell line, a human HCC cell line with
high metastatic potential, was established by Tian et al
(16) using a subcutaneous xenograft
of a metastatic model of human HCC from a Chinese patient in nude
mice. Based on MHCC97 as the parental cells, three cell lines
(MHCC97-L, MHCC97-H and HCCLM3) were subsequently established with
increasing metastatic potential (17). In the present study, to support the
findings of increased t-Src expression in HCC tissue, the
expression of Src was detected in MHCC97-L and HCCLM-3 cell lines
using immunocytochemistry. Notably, Src was more highly expressed
in the cell line with the highest metastatic potential, HCCLM-3,
compared with that in MHCC97-L cells (with lower metastatic
potential). These data suggest that Src may be involved in HCC
progression. Src overexpression or overactivation has also been
identified in a variety of human biopsies from primary tumors and
their metastases (11). It has been
established that active Src is associated with integrin adhesion
dynamics and E-cadherin dysregulation during the Src-induced
epithelial-mesenchymal transition. However, the mechanisms of Src
induced metastasis in HCC require further investigation.
In conclusion, the present study demonstrated that
the expression of t-Src and p-Y416Src were markedly elevated in HCC
tissue. In addition, it was observed that t-Src expression was
associated with cancer stage, cellular differentiation, lymph node
metastasis and CA19-9 level. Similarly to previous studies, the
present results demonstrated the potential value of Src as
predictor of HCC outcome. In addition, to the best of our
knowledge, the present study is the first to identify an
association between elevated t-Src and CA19-9 expression. Notably,
elevated t-Src expression was observed in HCC tissues with lymph
node metastasis and in an HCC cell line with a high metastatic
potential. Collectively, the current data suggests that Src may be
important in HCC metastasis. However, the underlying mechanisms
require further investigation.
Acknowledgements
The present study was supported by the Academic
Backbone Support Plan of Heilongjiang Province Department of
Education (grant no. 1254G040). The authors would also like to
thank the Heilongjiang Provincial Science and Technology Innovation
Team in Higher Education Institutes for Infection and Immunity, and
Heilongjiang Provincial Key Laboratory for Infection and Immunity,
Harbin Medical University (Harbin, China) for their assistance.
References
|
1
|
Farazi PA and Depinho RA: The genetic and
environmental basis of hepatocellular carcinoma. Discov Med.
6:182–186. 2006.PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistic, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hussain SA, Ferry DR, El-Gazzaz G, Mirza
DF, James ND, McMaster P and Kerr DJ: Hepatocellular carcinoma. Ann
Oncol. 12:161–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimizu I, Kohno N, Tamaki K, Shono M,
Huang HW, He JH and Yao DF: Female hepatology: Favorable role of
estrogen in chronic liver disease with hepatitis B virus infection.
World J Gastroenterol. 13:4295–4305. 2007.PubMed/NCBI
|
|
6
|
Tang Z, Zhou X, Lin Z, Yang B, Ma Z, Ye S,
Wu Z, Fan J, Liu Y, Liu K, et al: Surgical treatment of
hepatocellular carcinoma and related basic research with special
reference to recurrence and metastasis. Chin Med J (Engl).
112:887–891. 1999.PubMed/NCBI
|
|
7
|
Collett MS and Erikson RL: Protein kinase
activity associated with the avian sarcoma virus src gene product.
Proc Natl Acad Sci USA. 75:2021–2024. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ingley E: Src family kinases: Regulation
of their activities, levels and identification of new pathways.
Biochim Biophys Acta. 56(65): 17842008.
|
|
9
|
Bolos V, Gasent JM, Lopez-Tarruella S and
Grande E: The dual kinase complex FAK-Src as a promising
therapeutic target in cancer. Onco Targets Ther. 3:83–97. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frame MC: Src in cancer: Deregulation and
consequences for cell behaviour. Biochim Biophys Acta.
1602:114–130. 2002.PubMed/NCBI
|
|
11
|
Yeatman TJ: A renaissance for SRC. Nat Rev
Cancer. 4:470–480. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J: Is Src the key to understanding
metastasis and developing new treatments for colon cancer? Nat Clin
Pract Gastroenterol Hepatol. 5:306–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masaki T, Okada M, Shiratori Y, Rengifo W,
Matsumoto K, Maeda S, Kato N, Kanai F, Komatsu Y, Nishioka M and
Omata M: pp60c-src activation in hepatocellular carcinoma of human
and LEC rats. Hepatology. 27:1257–1264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun CK, Man K, Ng KT, Ho JW, Lim ZX, Cheng
O, Lo CM, Poon RT and Fan ST: Proline-rich tyrosine kinase 2 (Pyk2)
promotes proliferation and invasiveness of hepatocellular carcinoma
cells through c-Src/ERK activation. Carcinogenesis. 29:2096–2105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Toni EN, Thieme SE, Herbst A, Behrens
A, Stieber A, Jung A, Blum H, Göke H and Kolligs FT: OPG is
regulated by beta-catenin and mediates resistance to TRAIL-induced
apoptosis in colon cancer. Clin Cancer Res. 14:4713–4718. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY,
Chen J and Xue Q: New human hepatocellular carcinoma (HCC) cell
line with highly metastatic potential (MHCC97) and its expressions
of the factors associated with metastasis. Br J Cancer. 81:814–821.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL,
Liu YK and Tang ZY: Stepwise metastatic human hepatocellular
carcinoma cell line model system with multiple metastatic
potentials establish through consecutive in vivo selection
and studies on metastatic characteristics. J Cancer Res Clin Oncol.
130:460–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Comprehensive Cancer Network.
NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary
cancers. Version 2. 2012.http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdfAccessed.
August 27–2015
|
|
19
|
Ryder SD: British Society of
Gastroenterology: Guidelines for the diagnosis and treatment of
hepatocellular carcinoma (HCC) in adults. Gut. 52(Suppl 3):
iii1–iii8. 2003.PubMed/NCBI
|
|
20
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM and Haller DG: Liver Staging Form. AJCC Cancer Staging
Manual (6th). (New York). Springer-Verlag. 1312002.
|
|
21
|
Wu Y, Wang T, Ye S, Zhao R, Bai X, Wu Y,
Abe K and Jin X: Detection of hepatitis B virus DNA in
paraffin-embedded intrahepatic and extrahepatic cholangiocarcinoma
tissue in the northern Chinese population. Hum Pathol. 43:56–61.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Allred DC, Clark GM, Elledge R, Fuqua SA,
Brown RW, Chamness GC, Osborne CK and McGuire WL: Association of
p53 protein expression with tumor cell proliferation rate and
clinical outcome in node-negative breast cancer. J Natl Cancer
Inst. 85:200–206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lau GM, Lau GM, Yu GL, et al: Expression
of Src and FAK in hepatocellular carcinoma and the effect of Src
inhibitors on hepatocellular carcinoma in vitro. Dig Dis
Sci. 54:1465–1474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morgan L, Nicholson RI and Hiscox S: SRC
as a therapeutic target in breast cancer. Endocr Metab Immune
Disord Drug Targets. 8:273–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anbalagan M, Moroz K, Ali A, Carrier L,
Glodowski S and Rowan BG: Subcellular localization of total and
activated Src kinase in African American and Caucasian breast
cancer. PLoS One. 7:e330172012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ito Y, Kawakatsu H, Takeda T, Sakon M,
Nagano H, Sakai T, Miyoshi E, Noda K, Tsujimoto M, Wakasa K, et al:
Activation of c-src gene product in hepatocellular carcinoma is
highly correlated with the indices of early stage phenotype. J
Hepatol. 35:68–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen ML, Chai CY, Yeh KT, Wang SN, Tsai
CJ, Yeh YT and Yang SF: Crosstalk between activated and inactivated
c-Src in hepatocellular carcinoma. Dis Markers. 30:325–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hunter T: A tail of two src's: Mutatis
mutandis. Cell. 49:1–4. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roskoski R: Src protein-tyrosine kinase
structure and regulation. Biochem Biophys Res Commun.
324:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|