Introduction
Hyperthermia is known to sensitize mammalian cells
to radiation treatment (1), and a
better understanding of the molecular mechanism(s) involved in
thermally induced radiosensitization may be beneficial for cancer
treatments that involve concurrent radiotherapy and chemotherapy.
Hyperthermia has four unique biological effects: i) cellular
lethality with a break point of ~42.5–43°C, ii) hypersensitivity
during late S phase (2), iii)
thermotolerance (3), and iv)
radiosensitization (4). Following
hyperthermia, there are large initial changes in cellular
metabolism and heat shock protein expression (5). Heat shock protein upregulation may
explain the observed thermotolerance during hyperthermia, but does
not provide an explanation for the remaining biological effects
(6). A previous study defined the
underlying mechanism of the classically accepted theory, that
hyperthermia induces radiosensitization in low linear energy
transfer (LET) photon radiation in a time- and
temperature-dependent manner (7). The
study utilized cell lines lacking homologous recombination (HR)
repair pathways, and demonstrated that there was no
radiosensitization when these cells were subjected to hyperthermia.
Previous studies using wild-type cells have focused on the
formation of Rad51 foci, which are involved in DNA repair and their
presence correlates with cell survival following exposure to
radiation. These studies have demonstrated a significant decrease,
and even disappearance of radiation-induced Rad51 foci formation
following hyperthermia (7,8). These previous studies support the
hypotheses that HR repair is suppressed by hyperthermia, and that
Rad51 foci disruption or re-formation may be a fundamental
molecular mechanism for sensitization to chemotherapeutic agents
and ionizing radiation (7,8).
Hadron particle radiotherapy, such as proton and
carbon-ion radiotherapy is one of the latest radiotherapy treatment
strategies (9). There are 36 proton
therapy facilities and six carbon ion facilities in operation
worldwide, and ~30 more proton facilities and several other carbon
ion facilities under construction or being planned. Proton and
carbon-ion radiotherapy is known to have reduced latent effects in
normal tissues and at least similar to greater tumor control, when
compared to current photon radiation therapy (10). Proton and carbon-ion radiotherapy can
be improved when combined with effective hyperthermia treatment
(11–14).
A limited number of studies have investigated
combined hyperthermia and particle radiation therapy. A previous
study using human squamous cell carcinoma cells lines with 44°C for
10–20 min post irradiation hyperthermia demonstrated that
accelerated carbon-ions with a LET of ≥100 keV/µm exhibited no
hyperthermia-induced hypersensitization (11). Another previous study, using a Chinese
Hamster Ovary cell line, CHO-SC1 wild-type cells exposed to
accelerated neon ions with a LET <80 keV/µm demonstrated
successful hypersensitization using 41.5°C for 4 h post-irradiation
treatment. The study revealed a similar thermal enhancement ratio
(TER) for 42°C for 1.5 h treatment, which is as equitoxic as 41.5°C
for 4 h (12). In another study,
glioblastoma cell lines exposed to thermal neutron radiation were
sensitized by treatment with hyperthermic conditions (44°C for
15–40 min). However, no sensitization was observed when sufficient
amount of boron was added, as neutron-captured boron releases
lithium and α particle, which are very high LET radiation particles
(13). The previous studies showed
that decreased hyperthermia induced radiosensitization in high LET
radiation. While the potential molecular mechanisms of
hyperthermia-induced radiosensitivity are unclear, previous studies
have confirmed that p53 is involved in hyperthermia-induced high
LET radiosensitivity by regulating apoptosis (11,14).
The aims of the present study were to elucidate the
molecular mechanisms of hyperthermia-induced sensitization
following exposure to hadron radiation, to clarify whether cells
exposed to proton and carbon ion particles with different LET can
be sensitized by hyperthermia, and to determine the repair
mechanisms that may be responsible for this sensitization. To
address these questions, wild-type Chinese hamster ovary (CHO)
cells and two different DNA repair pathway-deficient cell lines
were used.
Materials and methods
Cell culture
CHO wild-type (CHO 10B2) cells and the DNA
repair-deficient CHO mutants, irs20 [DNA-dependent protein kinase
catalytic subunit, (DNA-PKcs] (15)
and xrs5 (Ku80) (16) were kindly
supplied by Dr Joel Bedford of Colorado State University (Fort
Collins, CO, USA). CHO wild-type (CHO AA8) cells and the DNA
repair-deficient CHO mutants, V3 (DNA-PKcs) (17), 51D1 (Rad51D) and irs1SF (XRCC3)
(18) were kindly supplied by Dr
Larry Thompson of Lawrence Livermore National Laboratory
(Livermore, CA, USA) (19). CHO cells
were maintained in Alpha MEM (GE Healthcare Life Sciences, Logan,
UT, USA) with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO,
USA), antibiotics (Antibiotic-Antimycotic; Life Technologies, Grand
Island, NY, USA) and were cultured in incubators at 37°C with 5%
CO2 and humidity.
Hyperthermia and radiation
conditions
X-ray irradiations were performed using a TITAN
X-ray generator (Shimadzu Corp., Tokyo, Japan) using 5-mm Al and Cu
filters at 200 kVp and 20 mA. The dose rate was ~1 Gy/min at room
temperature. Hadron irradiations were conducted at the National
Institute of Radiological Sciences (NIRS) in Chiba, Japan. Carbon
and iron ions were accelerated to 290 and 500 MeV/n, respectively,
using the Heavy Ion Medical Accelerator in Chiba (HIMAC), and
protons were accelerated to 70 MeV using the NIRS-930 cyclotron.
The dose rates for carbon ions and protons were set at 3 Gy/min.
Monoenergetic 290 MeV/n carbon ions have a LET value of 13 keV/µm
on entrance. Different LET values were achieved by varying the
thickness of poly(methyl methacrylate) (20). Monoenergetic 70 MeV protons have a LET
value of 1 keV/µm on entrance. Monoenergetic 500 MeV/n iron ions
have a LET value of 200 keV/µm on entrance. For hyperthermia
experiments, tightly sealed cell culture containers were immersed
in a pre-heated temperature-controlled water bath (Lauda, Delran,
NJ, USA) at a temperature of 42.5°C for 1 h. Hyperthermia treatment
was carried out immediately following radiation exposure.
Cell survival colony formation
assay
For the radiation and hyperthermia experiment,
following exposure to radiation, the cells were kept at either 37°C
or hyperthermia of 42.5°C for 1 h, and subsequently trypsinized and
plated to form colonies. For the radiation experiment with the
additional cell lines, the cells were immediately plated following
irradiation. Colonies were fixed and stained 7–10 days later using
100% ethanol, followed by 0.1% crystal violet. Macroscopic colonies
containing >50 cells were marked as survivors (21). Cell survival curves were constructed
from cell survival fraction data using Graphpad Prism 6 (GraphPad
Software Inc., La Jolla, CA, USA) and a linear quadratic regression
model. D10 values (radiation dose to achieve 10% cell survival)
were determined, and the relative biological effectiveness (RBE)
and thermal enhancement ratio (TER) were calculated from the D10
values (7,20). X-ray was the standard radiation for
RBE calculations.
Statistics
Experiments were performed at least three times and
error bars indicated standard error of the means. Data analysis
using the Student's t-test was performed with Prism 6. A
statistically significant difference was defined as P<0.05.
Results
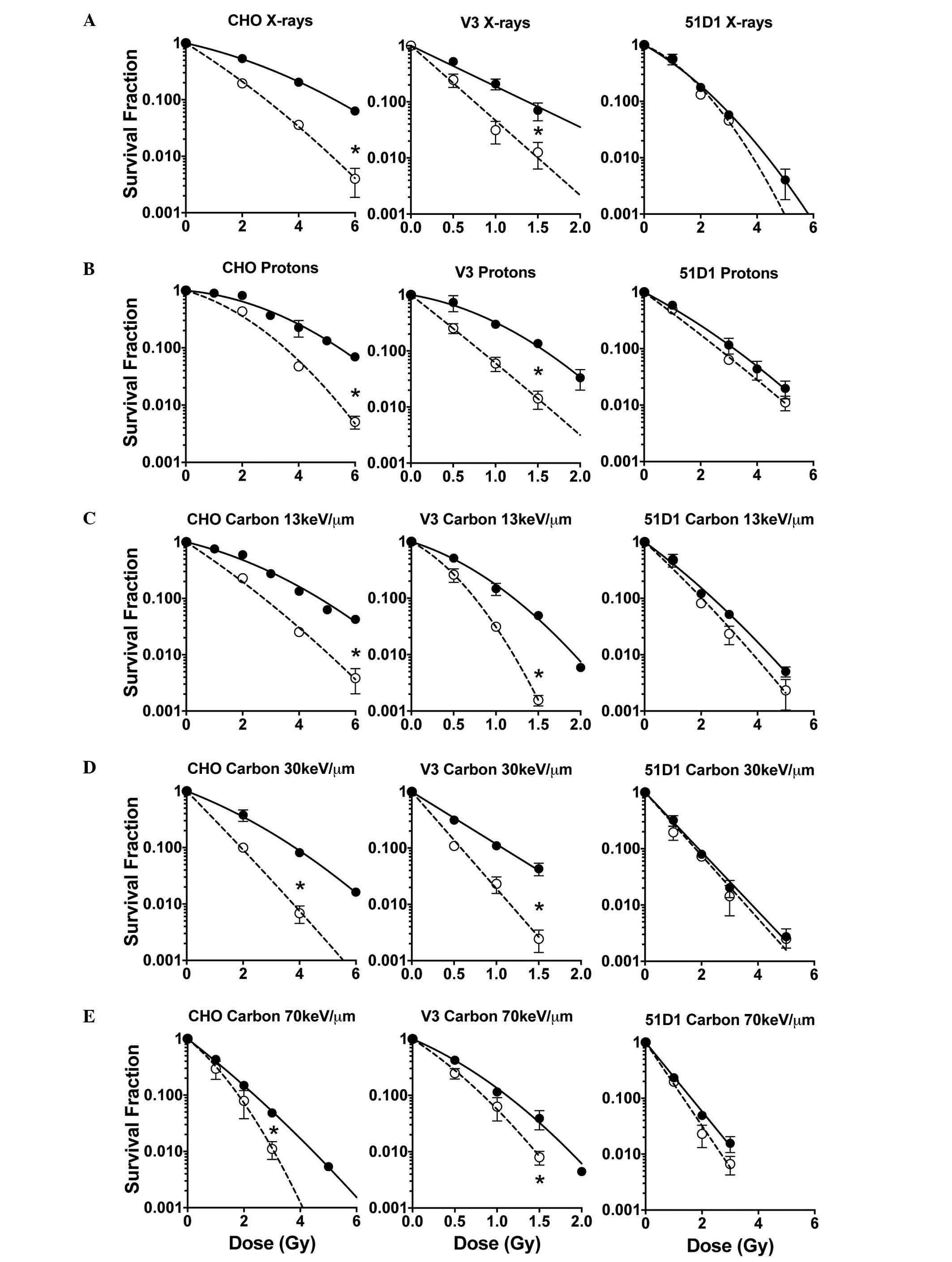
Cell survival in response to
hyperthermia combined with different types of radiation
In line with previous studies (7), cell survival curves demonstrated that
hyperthermia treatment of 42.5°C for 1 h post irradiation,
sensitized CHO wild-type cells and V3 cells but not 51D1 cells
(Fig. 1). In low LET radiation
including proton, X-ray and carbon 13 keV/µm, the cell survival
curves of CHO wild-type and V3 [non-homologous end-joining (NHEJ)
repair deficient] cells demonstrated significant and large
differences between the control group (radiation only) and the
combined group (radiation plus hyperthermia). However, the cells
exposed to carbon 30 and 70 keV/µm LET showed significant but small
differences in the cell survival curves between the control and
combined groups. HR repair-deficient 51D1 cells exhibited no
radiosensitization from the hyperthermia treatment in all the
radiation conditions used in this study.
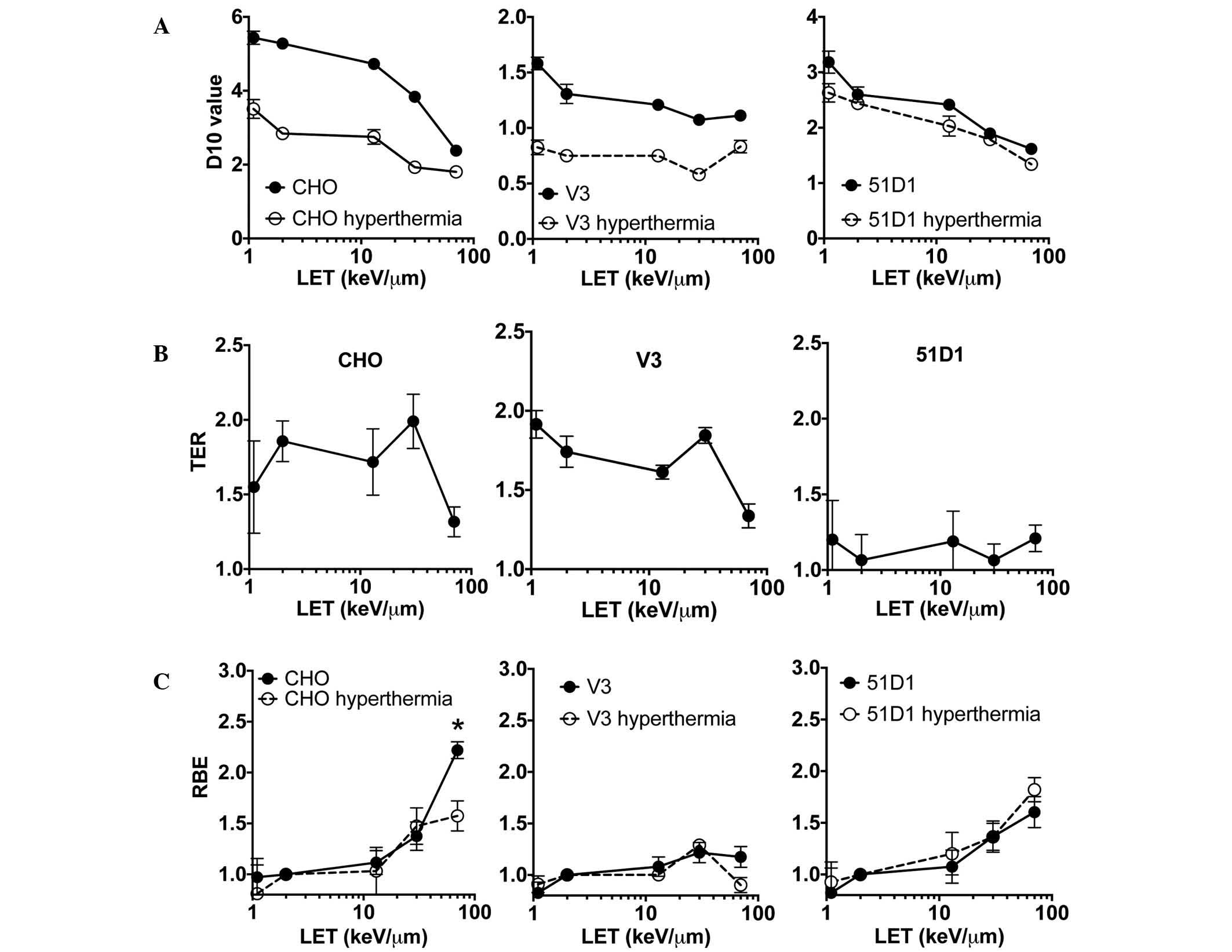
D10, RBE, and TER analysis
Analysis of the D10 values taken from the CHO
wild-type and 51D1 cell survival data (Fig. 2A) revealed a clear trend in reduction
of D10 values in the higher LET radiation for the control group
(radiation only) and combined group (radiation plus hyperthermia).
For the V3 cells, the control group had very small reductions in
D10 values when LET increased. The combined group exhibited no
observable changes in D10 values for the different values of LET.
Comparison of the D10 values between the control and combined
groups revealed the differences of each D10 were greatest in low
LET radiation such as proton and X-rays, and at high LET radiation,
these differences were smaller. Hyperthermia-induced
radiosensitization in HR-deficient (51D1) cells (Fig. 1) resulted in no statistically
significant differences in the D10 values of the control and
combined groups. The D10 values were used to analyze the thermal
sensitization. TER was obtained from D10 values of the control
group of cells and the radiation plus hyperthermia (combined) group
of cells (Fig. 2B). This analysis
revealed that the data from the CHO wild-type and V3 cells followed
a similar trend. In the LET range 1–30 keV/µm, TER was calculated
as 1.5–2.0. At the highest LET used in the present study, 70
keV/µm, TER reduced to 1.3. By contrast, there was no LET-dependent
TER decrease for the HR repair mutant cells. The 51D1 group had a
TER range of 1.0–1.2. Wild-type and NHEJ mutants have HR repair
capacity, and the high TER values suggested that cells have a high
HR repair capacity in low LET <30 keV/µm. RBE was also
calculated from D10 values (Fig. 2C),
and no statistically significant differences in RBE values were
detected between control and hyperthermia-exposed cells among the
three cell lines, with the exception of wild-type CHO cells exposed
to carbon LET 70 keV/µm (high LET; P<0.05, t-test). The
wild-type combined group RBE value at LET 70 keV/µm showed a
significant reduction compared to the control group. This result
suggested hyperthermia does not inhibit repair of the DNA damage
produced by high LET radiation, or the DNA repair pathway targeted
by hyperthermia does not contribute to the repair of high LET
radiation-induced DNA damage.
RBE values of NHEJ repair mutants and
HR repair mutants exposed to high LET radiation
Additional DNA repair-deficient CHO mutants were
used to further investigate the capacity of DNA repair following
high LET radiation exposure (Fig.
3A). The radiosensitive NHEJ mutants, xrs5 cells did not
exhibit a difference in RBE when LET was increased. The DNA-PKcs
null mutant V3 showed a slight increase in RBE when LET was
increased. By contrast, the intermediately radiosensitive irs20
cells exhibited a notable but intermediate RBE increase at LET 70
and 200 keV/µm. Furthermore, 51D1 cells and the HR repair mutant
irs1SF had a moderate increase in RBE, but this change was not as
large as that of the CHO wild-type cells (Fig. 3B).
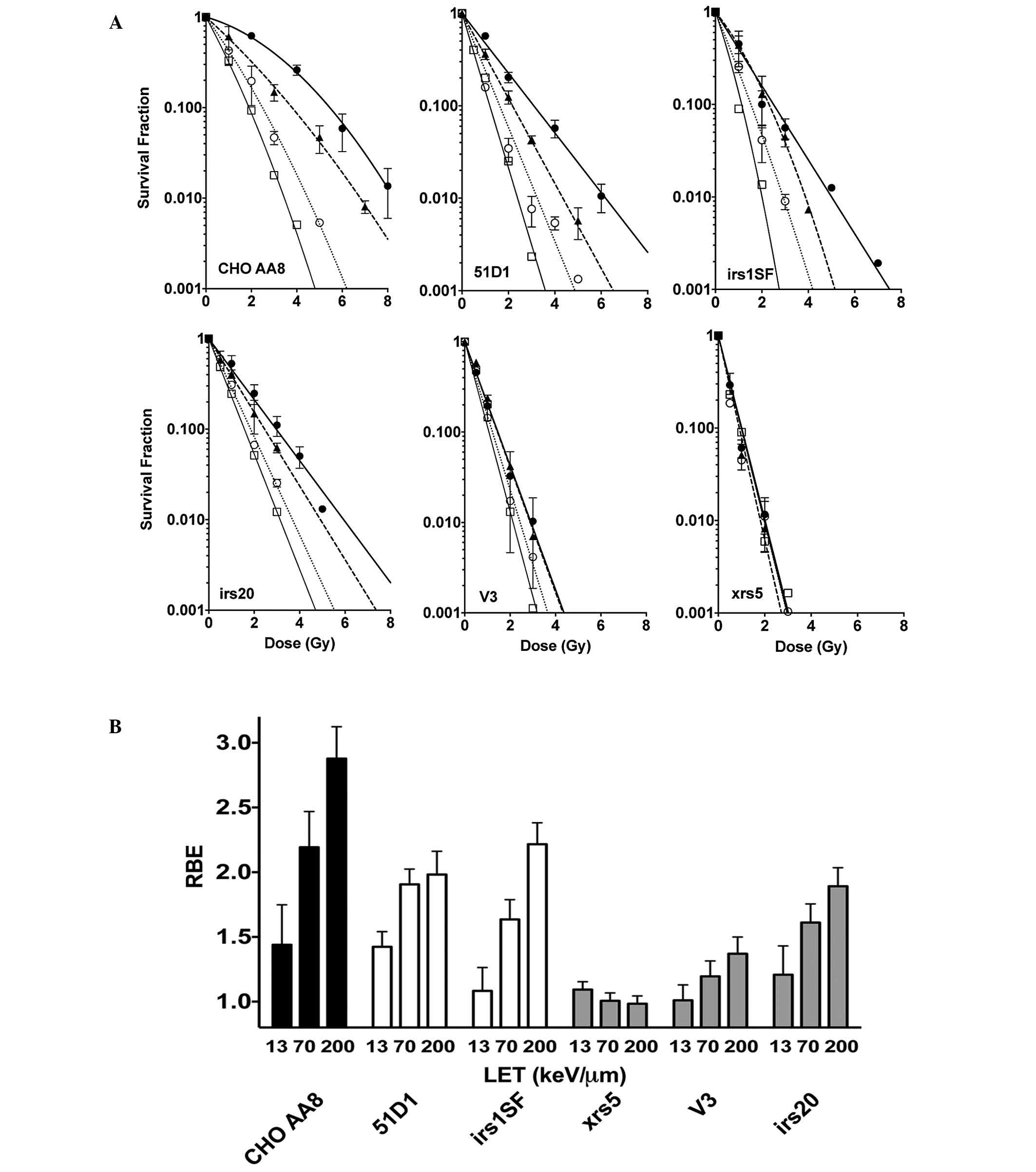 | Figure 3.(A) Cell survival curves for CHO
wild-type cells, NHEJ repair-deficient cells and HR
repair-deficient cells. Closed circles indicate X-rays, closed
triangles indicate carbon ion LET 13 keV/µm, open circles indicate
carbon ion LET 70 keV/µm, and open squares indicate iron ion LET
200 keV/µm. Error bars are the standard error of the mean values of
at least three independent experiments. Lines were constructed
using GraphPad Prism 6 with linear quadratic regression. (B) RBE
values of CHO wild-type, xrs5, V3, irs20, 51D1, and irs1SF cells.
CHO, chinese hamster ovary NHEJ, non-homologous end joining; HR,
homologous recombination; LET, linear energy transfer; RBE,
relative biological effectiveness. |
Discussion
Hyperthermia treatment enhances radiation-induced
cell death (1) and the present study
set out to investigate the mechanisms underlying this effect. To
the best of our knowledge, the present study is the first to use
two different hadron radiations to directly compare the effects of
proton and carbon ion radiation on cell survival following
hyperthermia-induced radiosensitization. The results of the present
study were in concordance with the results from previous studies
that examined particle radiation and hyperthermia. Considering the
results from the previous literature and the current study, it is
clear that hyperthermia-induced radiosensitization is dependent on
the LET of ionizing radiation. This applies to all of the previous
studies and the present study, using thermal neutron, proton,
helium, lithium, carbon, and neon ions (11–13).
The significantly large difference in RBE at the
carbon LET 70 keV/µm between the control (radiation only) and
radiation plus hyperthermia group may help to understand the
induction and repair of DNA damage by high LET radiation. The
results of the present study and previous studies indicate that the
main target of hyperthermia is the HR repair pathway, as HR
repair-deficient cells did not show any sensitization when combined
with hyperthermia (7,8). In cells where HR repair was active and
properly functional, the cells were more sensitive to DNA damaging
agents when exposed to hyperthermia than when no hyperthermia was
used. We observed this hypersensitization even in V3 NHEJ mutant
cells, which are particularly radiosensitive. Previous findings
have demonstrated that NHEJ proteins, especially DNA-PKcs, are
important for hyperthermia-induced radiosensitization (22–26). The
hyperthermia condition caused hypersensitization in all types of
radiation tested in the DNA-PKcs mutant V3 cell. Therefore,
DNA-PKcs may be a target of hyperthermia-induced
radiosensitization, however, its contribution to cellular lethality
may be minor.
On comparison of the RBE at carbon LET 70 keV/µm in
the different cell types, NHEJ mutants had the lowest RBE among the
CHO cell lines. Thus, it is obvious that NHEJ is the primary repair
pathway that counters DNA damage produced by any types of ionizing
radiation. NHEJ mutant cells exhibited similar survival against low
LET and high LET radiation (27).
Loss of NHEJ prevents the effective repair of DNA damage produced
by any type of ionizing radiation. By contrast, the RBE values of
the HR mutants were similar to those of the CHO wild type, except
at high LET radiation exposures. This result suggests that HR
repair contributes to DNA repair, when DNA is damaged by low LET
radiation (proton and low LET carbon ions), however, its
contribution is minor for DNA damage caused by high LET radiation.
Therefore, we conclude that HR repairs some DNA damage induced by
high LET radiation, but its repair capacity is lower in high LET
radiation-induced damage compared with low LET radiation.
High LET radiation is known to produce complex DNA
damage that is difficult to repair. There are several previous
studies concerning the limited contribution of HR repair to the
damage induced by high LET radiation exposure (28,29). If HR
repair is more important in repairing high LET-induced DNA damage
than low LET-induced damage, HR mutants should have larger RBE
values when exposed to high LET radiation. The results of the
present study nor our previous study (30) support this hypothesis.
Based on the results of the present and previous
studies, it is clear that HR repair was responsible for the
hyperthermia-induced radiosensitization for photon and hadron
radiation. The lower TER in high LET radiation may be explained by
the limited capacity of HR repair following high LET radiation
exposure. However, there is a good possibility that alternative
radiation damage-associated pathways to DNA double-strand break
repair, can be inhibited or altered by hyperthermia and affect only
certain types of ionizing radiation. Several good examples of such
alternatives include heat shock proteins and p53 (11). ATM, the Fanconi Anemia signal pathway,
and nucleotide excision repair pathway can be excluded as targets
of hyperthermia based on previous studies (31,32). The
current study used photon radiation, two forms of hadron radiation,
proton and carbon ion (low to high LET) radiation. Hyperthermia was
unable to cause hypersensitivity in 51D1 cells exposed to hadron
radiation and X-rays.
In conclusion, the results of the present study
suggest that hyperthermia-induced radiosensitization for hadron
radiation is dependent on HR inhibition, and these effects are
significant for low LET hadron radiation. Synergistic cellular
lethality effects may be expected from NHEJ inhibition.
Acknowledgements
The authors of the current study appreciate the
technical support from the NIRS cyclotron and HIMAC. This study was
partially funded by the Colorado State University start-up fund,
the Dr Akiko Ueno Radiobiology research fund and the NIRS
International Open Laboratory.
References
|
1
|
Dewey WC: Arrhenius relationships from the
molecule and cell to the clinic. Int J Hyperther. 25:3–20. 2009.
View Article : Google Scholar
|
|
2
|
Gerweck LE, Gillette EL and Dewey WC:
Effect of heat and radiation on synchronous Chinese hamster cells:
Killing and repair. Radiat Res. 64:611–623. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holahan EV, Highfield DP, Holahan PK and
Dewey WC: Hyperthermic killing and hyperthermic radiosensitization
in Chinese hamster ovary cells: Effects of pH and thermal
tolerance. Radiat Res. 97:108–131. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dewey WC and Miller HH: Effect of
temperature on x-ray induced cell lethality and chromosomal
aberrations. Int J Radiat Biol Relat Stud Phys Chem Med. 18:91–93.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee YJ and Dewey WC: Induction of heat
shock proteins in Chinese hamster ovary cells and development of
thermotolerance by intermediate concentrations of puromycin. J Cell
Physiol. 132:1–11. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YJ and Dewey WC: Effect of
cycloheximide or puromycin on induction of thermotolerance by heat
in Chinese hamster ovary cells: Dose fractionation at 45.5 degrees
C1. Cancer Res. 47:5960–5966. 1987.PubMed/NCBI
|
|
7
|
Genet SC, Fujii Y, Maeda J, Kaneko M,
Genet MD, Miyagawa K and Kato TA: Hyperthermia inhibits homologous
recombination repair and sensitizes cells to ionizing radiation in
a time- and temperature-dependent manner. J Cell Physiol.
228:1473–1481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krawczyk PM, Eppink B, Essers J, Stap J,
Rodermond H, Odijk H, Zelensky A, van Bree C, Stalpers LJ, Buist
MR, et al: Mild hyperthermia inhibits homologous recombination,
induces BRCA2 degradation, and sensitizes cancer cells to poly
(ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA.
108:9851–9856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamada T, Tsujii H, Tsuji H, Yanagi T,
Mizoe JE, Miyamoto T, Kato H, Yamada S, Morita S, Yoshikawa K, et
al: Working Group for the Bone and Soft Tissue Sarcomas: Efficacy
and safety of carbon ion radiotherapy in bone and soft tissue
sarcomas. J Clin Oncol. 20:4466–4471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsujii H and Kamada T: A review of update
clinical results of carbon ion radiotherapy. Jpn J Clin Oncol.
42:670–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi A, Ohnishi K, Wang X, Kobayashi
M, Matsumoto H, Tamamoto T, Aoki H, Furusawa Y, Yukawa O and
Ohnishi T: The dependence of p53 on the radiation enhancement of
thermosensitivity at different let. Int J Radiat Oncol Biol Phys.
47:489–494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang PY, Tobias CA and Blakely EA:
Protein synthesis modulates the biological effectiveness of the
combined action of hyperthermia and high-LET radiation. Radiat Res.
129:272–280. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinashi Y, Masunaga SI, Suzuki M, Ono K
and Ohnishi T: Hyperthermia enhances thermal-neutron-induced cell
death of human glioblastoma cell lines at low concentrations of
10B. Int J Radiat Oncol Biol Phys. 40:1185–1192. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi A, Ohnishi K, Ota I, Asakawa I,
Tamamoto T, Furusawa Y, Matsumoto H and Ohnishi T: p53-dependent
thermal enhancement of cellular sensitivity in human squamous cell
carcinomas in relation to LET. Int J Radiat Biol. 77:1043–1051.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stackhouse MA and Bedford JS: An ionizing
radiation-sensitive mutant of CHO cells: Irs-20. I. Isolation and
initial characterization. Radiat Res. 136:241–249. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeggo PA and Kemp LM: X-ray-sensitive
mutants of Chinese hamster ovary cell line. Isolation and
cross-sensitivity to other DNA-damaging agents. Mutat Res.
112:313–327. 1983.PubMed/NCBI
|
|
17
|
Whitmore GF, Varghese AJ and Gulyas S:
Cell cycle responses of two X-ray sensitive mutants defective in
DNA repair. Int J Radiat Biol. 56:657–665. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuller LF and Painter RB: A Chinese
hamster ovary cell line hypersensitive to ionizing radiation and
deficient in repair replication. Mutat Res. 193:109–121.
1988.PubMed/NCBI
|
|
19
|
Hinz JM, Tebbs RS, Wilson PF, Nham PB,
Salazar EP, Nagasawa H, Urbin SS, Bedford JS and Thompson LH:
Repression of mutagenesis by Rad51D-mediated homologous
recombination. Nucleic Acids Res. 34:1358–1368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kato TA, Tsuda A, Uesaka M, Fujimori A,
Kamada T, Tsujii H and Okayasu R: In vitro characterization of
cells derived from chordoma cell line U-CH1 following treatment
with X-rays, heavy ions and chemotherapeutic drugs. Radiat Oncol.
6:1162011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maeda J, Roybal EJ, Brents CA, Uesaka M,
Aizawa Y and Kato TA: Natural and glucosyl flavonoids inhibit
poly(ADP-ribose) polymerase activity and induce synthetic lethality
in BRCA mutant cells. Oncol Rep. 31:551–556. 2014.PubMed/NCBI
|
|
22
|
Zeng ZC, Jiang GL, Wang GM, Tang ZY,
Curran WJ and Iliakis G: DNA-PKcs subunits in radiosensitization by
hyperthermia on hepatocellular carcinoma hepG2 cell line. World J
Gastroenterol. 8:797–803. 2002.PubMed/NCBI
|
|
23
|
Ihara M, Takeshita S, Okaichi K, Okumura Y
and Ohnishi T: Heat exposure enhances radiosensitivity by
depressing DNA-PK kinase activity during double strand break
repair. Int J Hyperthermia. 30:102–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomita M: Involvement of DNA-PK and ATM in
radiation- and heat-induced DNA damage recognition and apoptotic
cell death. J Radiat Res (Tokyo). 51:493–501. 2010. View Article : Google Scholar
|
|
25
|
Umeda N, Matsumoto Y, Yin HL, Tomita M,
Enomoto A, Morita A, Mizukoshi T, Sakai K, Hosoi Y and Suzuki N:
Difference in the heat sensitivity of DNA-dependent protein kinase
activity among mouse, hamster and human cells. Int J Radiat Biol.
79:671–680. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Woudstra EC, Konings AW, Jeggo PA and
Kampinga HH: Role of DNA-PK subunits in radiosensitization by
hyperthermia. Radiat Res. 152:214–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weyrather WK, Ritter S, Scholz M and Kraft
G: RBE for carbon track-segment irradiation in cell lines of
differing repair capacity. Int J Radiat Biol. 75:1357–1364. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olsson G, Czene S, Jenssen D and
Harms-Ringdahl M: Induction of homologous recombination in the hprt
gene of V79 Chinese hamster cells in response to low- and high-LET
irradiation. Cytogenet Genome Res. 104:227–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zafar F, Seidler SB, Kronenberg A, Schild
D and Wiese C: Homologous recombination contributes to the repair
of DNA double-strand breaks induced by high-energy iron ions.
Radiat Res. 173:27–39. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Genet SC, Maeda J, Fujisawa H, Yurkon CR,
Fujii Y, Romero AM, Genik PC, Fujimori A, Kitamura H and Kato TA:
Comparison of cellular lethality in DNA repair-proficient or
-deficient cell lines resulting from exposure to 70 MeV/n protons
or 290 MeV/n carbon ions. Oncol Rep. 28:1591–1596. 2012.PubMed/NCBI
|
|
31
|
Mitchel RE, Smith BP, Wheatly N, Chan A,
Child S and Paterson MC: Sensitivity of hyperthermia-treated human
cells to killing by ultraviolet or gamma radiation. Radiat Res.
104:234–241. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitchel RE, Chan A, Smith BP, Child SD and
Paterson MC: The effects of hyperthermia and ionizing radiation in
normal and ataxia telangiectasia human fibroblast lines. Radiat
Res. 99:627–635. 1984. View
Article : Google Scholar : PubMed/NCBI
|