Introduction
Pituitary adenomas (PAs) are clinically relevant
endocrine tumors that account for ~10% of all intracranial
neoplasms. The majority of PAs are benign, however, 30–55% are
locally invasive, and a number infiltrate the dura, bone and
sinuses, and are thus considered to be highly aggressive (1,2). The
surgical treatment of aggressive PAs is often incomplete, leading
to the high recurrence rate (3). A
potential treatment strategy for aggressive PAs is the targeting of
tumor invasion and metastasis-associated genes.
In humans, anterior gradient 2 (AGR2) encodes the
human homologue of a secreted protein that was first identified in
Xenopus laevis (4). AGR2 has
been reported to be overexpressed in several adenocarcinomas,
including breast (5), colorectal
(6), esophageal (7), lung (8),
pancreatic (9) and prostate (10,11)
carcinomas. ARG2 is considered to promote cell proliferation, cell
survival and the metastasis of cancer cells. Salmans et al
demonstrated that AGR2 is a marker of breast cancer metastasis and
that its overexpression in estrogen receptor-positive breast cancer
is associated with a poor prognosis, particularly in tumors that
evade anti-hormone therapies (5).
This indicates that AGR2 may participate in the process of cell
metastasis in hormone-associated tumors. Since PAs are associated
with multiple hormones in humans and as no studies have mentioned
the role of AGR2 in PA, the present study investigated the
expression profile of AGR2 in 117 PAs of different histological
subtype by immunohistochemistry and western blotting.
Materials and methods
PA sample collection
A total of 117 PAs of different histological subtype
were randomly selected from patients aged 17–69 year-old who
underwent endoscopic or microscopic total resection between May
2013 and June 2014 in the Department of Neurosurgery, Jinling
Hospital (School of Medicine, Nanjing University, Nanjing, Jiangsu,
China). The study was approved by the ethics committee of Jinling
Hospital and written informed consent was obtained from all
patients. All resected PA tumor tissues were formalin-fixed and
paraffin-embedded, and then pathologically diagnosed. The tissues
consisted of 24 prolactin-secreting adenomas, 24 growth hormone
(GH)-secreting adenomas, 5 adrenocorticotropic hormone
(ACTH)-secreting adenomas, 8 follicle-stimulating hormone
(FSH)-secreting adenomas and 56 non-functioning adenomas.
Immunohistochemical staining
A streptavidin-peroxidase method was used for
immunostaining, as previously described (12). Briefly, slides were deparaffinized
with xylene three times (for 5–10 min each), dehydrated three times
in a gradient series of ethanol (100, 95 and 75%), and rinsed with
phosphate-buffered saline (PBS). Each slide was treated with 3%
H2O2 for 15 min to quench endogenous
peroxidase activity. Non-specific binding was blocked by treating
the slides with normal goat serum for 20 min. The slides were first
incubated with rabbit anti-human monoclonal anti-AGR2 (#13062;
1:500; Cell Signaling Technology, Inc., Danvers, MA, USA) overnight
at 4°C, and then rinsed twice with PBS. The slides were then
incubated with secondary antibody (goat anti-rabbit horseradish
peroxidase-conjugated IgG; #BS13278; 1:5,000; Beyotime Institute of
Biotechnology, Shanghai, China) for 15 min at 37°C, followed by
treatment with streptavidin-peroxidase reagent for 15 min, and were
rinsed twice with PBS. The slides were visualized with
3,3′-diaminobenzidine for 3 min, counterstained with hematoxylin
and mounted for microscopy.
Evaluation of staining
The slides were independently evaluated by two
investigators under a light microscope (TE200; Nikon, Tokyo,
Japan). As described previously (12), the staining intensity was scored as
follows: 0, negative; 1, weak; 2, medium; and 3, strong. The extent
of staining was scored as follows: 0, 0%; 1, 1–25%; 2, 26–50%; 3,
51–75%; and 4, 76–100%, according to the percentages of the
positive staining area in relation to the whole carcinoma area. The
sum of the intensity and extent scores was used as the final
staining score (range, 0–7). Tumors with a final staining score of
>2 were considered to be positive.
Western blotting
For western blot analysis, the lysates were
separated by SDS-PAGE followed by being transferred to an
Immobilon-P Transfer membrane (Millipore Corporation, Bedford, MA,
USA). The membranes were probed with the anti-AGR2 and rabbit
anti-mouse polyclonal GAPDH (#AP0063; 1:5,000; Bioworld Technology,
Inc., St. Louis Park, MN, USA) primary antibodies, followed by
incubation with secondary antibody. Proteins were visualized with
chemiluminescence luminol reagents (Beyotime Institute of
Biotechnology, Shanghai, China).
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS Inc., Chicago, IL, USA). The positive expression rate of AGR2
in the different subtypes of PA was compared using χ2
tests. The association between the expression and clinical
parameters was analyzed using a χ2 test, or Fisher's
exact probability test when appropriate. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of AGR2 in PA tissues
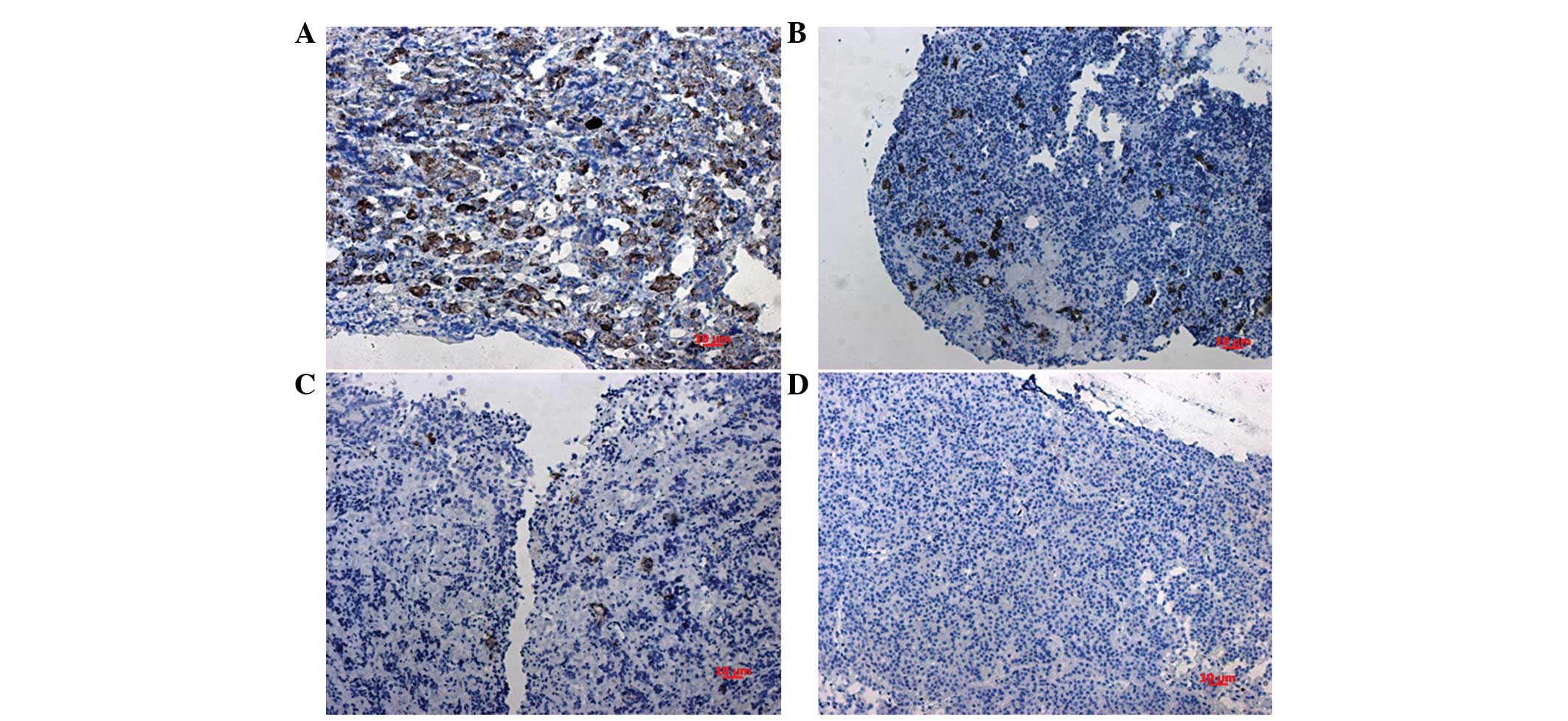
The location of AGR2 in the nuclei was considered
for scoring (Fig. 1A–D). The positive
expression of AGR2 was detected in 60 tissues. The proportions of
negative (score of ≤2) or positive (score of >2) expression in
the different subtypes of PAs are shown in Table I. In total, 51.3% of all PAs exhibited
AGR2 positive expression. The positive expression rate of AGR2
showed no significant differences in the PA subtypes
(χ2=6.537; P=0.162, P<0.05), thus, the expression of
AGR2 cannot be considered as discrepant in the different subtypes
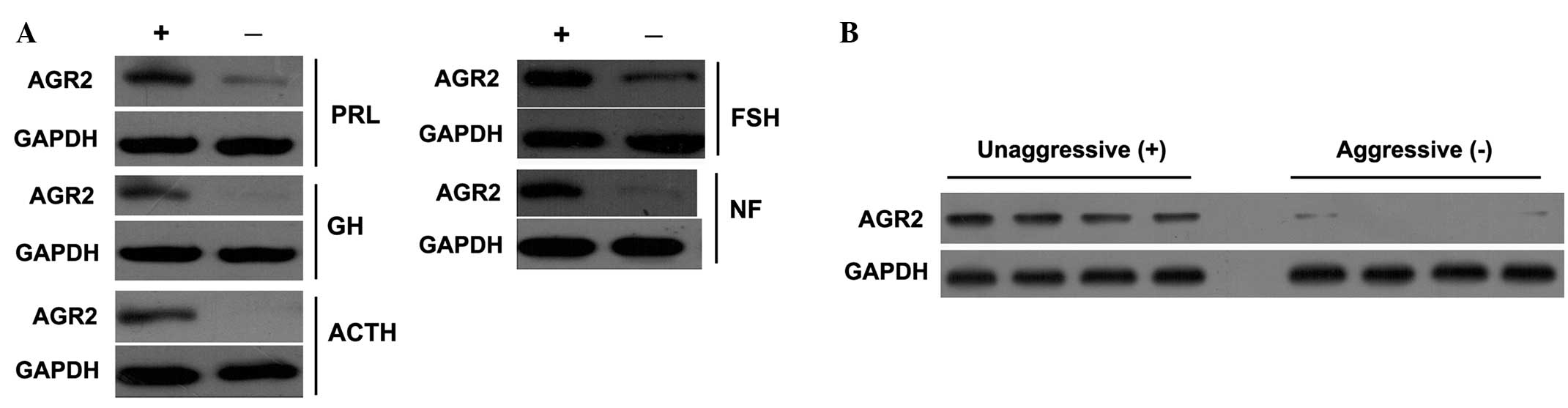
of PAs. The western blotting data supported and confirmed the
immunohistochemistry results, including high and negative
expression of AGR2 in the different subtypes of PA (Fig. 2A).
 | Table I.Expression profile of AGR2 in
different subtypes of PA. |
Table I.
Expression profile of AGR2 in
different subtypes of PA.
|
|
| AGR2 |
|---|
|
|
|
|
|---|
| PA subtypes | No. of patients | Negative, n | Positive, n | Positive rate, % |
|---|
| PRL | 24 | 14 | 10 | 41.7 |
| GH | 24 | 9 | 15 | 62.5 |
| ACTH | 5 | 1 | 4 | 80.0 |
| FSH | 8 | 2 | 6 | 75.0 |
| NF | 56 | 31 | 25 | 44.6 |
| Total | 117 | 57 | 60 | 51.3 |
Association of AGR2 expression with
clinical features of PAs
In the 117 cases, 65 patients were male and 52 were
female, with 60 patients >50 years old. The tumors were defined
as follows: 70 aggressive and 47 non-aggressive PAs (according to
Knosp's classification) (13); 12
recurrent and 105 primary PAs; and 13 microadenoma (diameter, ≤10
mm) and 104 macroadenoma (diameter, >10 mm). The associations
between clinical variables and AGR2 expression are showed in
Table II. Only the aggressiveness of
PA was found to be associated with AGR2 expression (P=0.0003,
P<0.001). The majority of aggressive PAs were negative for AGR2
expression. The western blotting data also supported this result
(Fig. 2B).
 | Table II.Association of AGR2 expression with
clinicopathological characteristics from patients with pituitary
adenomas. |
Table II.
Association of AGR2 expression with
clinicopathological characteristics from patients with pituitary
adenomas.
|
|
| AGR2, n |
|
|---|
|
|
|
|
|
|---|
| Parameters | No. of patients | − | + | P-value |
|---|
| Cases | 117 | 57 | 60 |
|
| Gender |
|
|
| 0.901 |
| Male | 65 | 32 | 33 |
|
|
Female | 52 | 25 | 27 |
|
| Age, years |
|
|
| 0.323 |
| ≤50 | 67 | 30 | 37 |
|
>50 | 60 | 27 | 23 |
| Aggressive |
|
|
| <0.001 |
| Yes | 70 | 46 | 24 |
|
| No | 47 | 11 | 36 |
|
| Recurrence |
|
|
| 0.482 |
| Yes | 12 | 7 | 5 |
|
| No | 105 | 50 | 55 |
|
| Tumor size, mm |
|
|
| 0.170 |
| ≤10 | 13 | 4 | 9 |
|
|
>10 | 104 | 53 | 51 |
|
Discussion
AGR2 is well studied in malignant tumors, and to the
best of our knowledge, it has never been mentioned with regard to
PAs. In the present study, the expression profile was detected in
117 PA tissues of different histological subtypes for the first
time. Aberrant AGR2 expression has been reported in primary breast,
lung and prostate carcinomas (14–17). AGR2
overexpression in breast epithelial cell lines results in the
development of metastases in an animal model (18). However, overexpression is not the only
manner in which AGR2 contributes to tumor development (19). The loss of AGR2 expression has also
been demonstrated to be associated with the dysplasia-to-carcinoma
sequence in colonic polyps (20). The
present study data demonstrated that in 117 different histological
subtypes of PA, 51.3% exhibited AGR2-positive expression. The
positive expression rate of AGR2 showed no significant differences
in the PA subtypes, although its expression occurred more
frequently in PAs secreting GH (62.5%), ACTH (80.0%) and FSH
(75.0%). Next, the association between AGR2 expression and clinical
parameters was analyzed. Notably, the result showed that the
aggressiveness of PA was associated with AGR2 expression, and that
in the majority of aggressive PAs, AGR2 expression was negative.
This suggested that AGR2-negative expression may be an indication
for PA aggressiveness.
The AGR2 protein contains a canonical cleavable
N-terminal signal peptide that targets it to the secretory pathway
(21). The secreted proteins
metastasis-associated GPI-anchored C4.4A protein and the
extracellular domain of α-dystroglycan, have been reported to
directly interact with AGR2, indicating potential mechanisms for
AGR2 in the promotion of tumor metastasis via the regulation of
receptor adhesion and the interaction with the extracellular matrix
(17,22). Thus, AGR2 interacts with the cell
surface in order to modulate adhesion and promote tumor cell
dissemination. However, by contrast, Riener et al (23) demonstrated that the loss of AGR2
expression occurred in colorectal cancer cell lines and tissue
samples, which was significantly associated with a higher tumor
grade and metastasis. Thus, the study suggested that AGR2 was an
independent prognostic factor in primary colorectal carcinoma.
These results may partly support our suggestion that AGR2-negative
expression may be an indication for PA aggressiveness. Numerous PAs
present with aggressive characteristics, although PA is considered
as a benign tumor. Aggressive PAs are usually hard to totally
resect and show a tendency to recur, even after initially
successful treatment (24).
Post-operative radiotherapy is always recommended to treat residual
tumors and to prevent recurrence (25). Identifying the aggressiveness of
pituitary tumors is important for the selection of appropriate
treatment and prognostic evaluation (26). The present study results showing that
AGR2 is negative in the majority of aggressive PAs indicated that
the loss of AGR2 expression may also associate with aggressiveness,
similar to the dysplasia-to-carcinoma sequence in colonic polyps
(20). To clarify the role of AGR2 in
PA aggressiveness or maybe canceration, further cellular in
vitro and animal in vivo experiments are necessary.
In conclusion, in the present study, 117 cases of PA
of different subtypes were detected. PAs secreting GH, ACTH and FSH
were found to present with more frequent AGR2 expression; however,
this association was not statistically significant. The
aggressiveness of PA is associated with AGR2 expression, and in the
majority of aggressive PAs, AGR2 expression was negative. AGR2 may
be a target for the study of PAs aggressiveness, and a potential
index for the diagnosis or prognosis of PAs.
Acknowledgements
The authors would like to thank the Department of
Pathology of Jinling Hospital for providing technical support. This
study was supported by the National Natural Science Foundation of
China (no. 30801178).
References
|
1
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma
cells via ROS/MAPKs-mediated pathway. J Neurooncol. 116:221–230.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu JK, Patel SK, Gillespie DL, Whang K
and Couldwell WT: R-flurbiprofen, a novel nonsteroidal
anti-inflammatory drug, decreases cell proliferation and induces
apoptosis in pituitary adenoma cells in vitro. J Neurooncol.
106:561–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng J, Hong L, Wu Y, Li C, Wan H, Li G,
Sun Y, Yu S, Chittiboina P, Montgomery B, et al: Identification of
a subtype-specific ENC1 gene related to invasiveness in human
pituitary null cell adenoma and oncocytomas. J Neurooncol.
119:307–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brychtova V, Vojtesek B and Hrstka R:
Anterior gradient 2: A novel player in tumor cell biology. Cancer
Lett. 304:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salmans ML, Zhao F and Andersen B: The
estrogen-regulated anterior gradient 2 (AGR2) protein in breast
cancer: A potential drug target and biomarker. Breast Cancer Res.
15:2042013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao H, Xu X, Chen B, Wang F, Zhang W, Geng
H and Wang Y: Anterior gradient 2: A new target to treat colorectal
cancer. Med Hypotheses. 80:706–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DiMaio MA, Kwok S, Montgomery KD, Lowe AW
and Pai RK: Immunohistochemical panel for distinguishing esophageal
adenocarcinoma from squamous cell carcinoma: A combination of p63,
cytokeratin 5/6, MUC5AC, and anterior gradient homolog 2 allows
optimal subtyping. Human Pathol. 43:1799–1807. 2012. View Article : Google Scholar
|
|
8
|
Pizzi M, Fassan M, Balistreri M,
Galligioni A, Rea F and Rugge M: Anterior gradient 2 overexpression
in lung adenocarcinoma. Appl Immunohistochem Mol Morphol. 20:31–36.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen R, Pan S, Duan X, Nelson BH, Sahota
RA, de Rham S, Kozarek RA, McIntosh M and Brentnall TA: Elevated
level of anterior gradient-2 in pancreatic juice from patients with
pre-malignant pancreatic neoplasia. Mol Cancer. 9:1492010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Forootan SS, Liu D, Barraclough
R, Foster CS, Rudland PS and Ke Y: Increased expression of anterior
gradient-2 is significantly associated with poor survival of
prostate cancer patients. Prostate Cancer Prostatic Dis.
10:293–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kani K, Malihi PD, Jiang Y, Wang H, Wang
Y, Ruderman DL, Agus DB, Mallick P and Gross ME: Anterior gradient
2 (AGR2): Blood-based biomarker elevated in metastatic prostate
cancer associated with the neuroendocrine phenotype. Prostate.
73:306–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Li J, Tohti M, Hu Y, Wang S, Li W,
Lu Z and Ma C: The expression profile of Dopamine D2 receptor, MGMT
and VEGF in different histological subtypes of pituitary adenomas:
A study of 197 cases and indications for the medical therapy. J Exp
Clin Cancer Res. 33:562014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Ieva A, Rotondo F, Syro LV, Cusimano MD
and Kovacs K: Aggressive pituitary adenomas - diagnosis and
emerging treatments. Nat Rev Endocrinol. 10:423–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Hao Y and Lowe AW: The
adenocarcinoma-associated antigen, AGR2, promotes tumor growth,
cell migration and cellular transformation. Cancer Res. 68:492–497.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fritzsche FR, Dahl E, Dankof A, Burkhardt
M, Pahl S, Petersen I, Dietel M and Kristiansen G: Expression of
AGR2 in non small cell lung cancer. Histol Histopathol. 22:703–708.
2007.PubMed/NCBI
|
|
16
|
Fritzsche FR, Dahl E, Pahl S, Burkhardt M,
Luo J, Mayordomo E, Gansukh T, Dankof A, Knuechel R, Denkert C, et
al: Prognostic relevance of AGR2 expression in breast cancer. Clin
Cancer Res. 12:1728–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang JS, Gong A, Cheville JC, Smith DI
and Young CY: AGR2, an androgen-inducible secretory protein
overexpressed in prostate cancer. Genes Chromosomes Cancer.
43:249–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu D, Rudland PS, Sibson DR,
Platt-Higgins A and Barraclough R: Human homologue of cement gland
protein, a novel metastasis inducer associated with breast
carcinomas. Cancer Res. 65:3796–3805. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vivekanandan P, Micchelli ST and Torbenson
M: Anterior gradient-2 is overexpressed by fibrolamellar
carcinomas. Hum Pathol. 40:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S, Bang S, Song K and Lee I:
Differential expression in normal-adenoma-carcinoma sequence
suggests complex molecular carcinogenesis in colon. Oncol Rep.
16:747–754. 2006.PubMed/NCBI
|
|
21
|
Adam PJ, Boyd R, Tyson KL, Fletcher GC,
Stamps A, Hudson L, Poyser HR, Redpath N, Griffiths M, Steers G, et
al: Comprehensive proteomic analysis of breast cancer cell
membranes reveals unique proteins with potential roles in clinical
cancer. J Biol Chem. 278:6482–6489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao F, Edwards R, Dizon D, Afrasiabi K,
Mastroianni JR, Geyfman M, Ouellette AJ, Andersen B and Lipkin SM:
Disruption of Paneth and goblet cell homeostasis and increased
endoplasmic reticulum stress in Agr2-/- mice. Dev Biol.
338:270–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Riener MO, Thiesler T, Hellerbrand C,
Amann T, Cathomas G, Fritzsche FR, Dahl E, Bahra M, Weichert W,
Terracciano L and Kristiansen G: Loss of anterior gradient-2
expression is an independent prognostic factor in colorectal
carcinomas. Eur J Cancer. 50:1722–1730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turner HE, Nagy Z, Esiri MM, Harris AL and
Wass JA: Role of matrix metalloproteinase 9 in pituitary tumor
behavior. J Clin Endocrinol Metab. 85:2931–2935. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Z, Lee Vance M, Schlesinger D and
Sheehan JP: Hypopituitarism after stereotactic radiosurgery for
pituitary adenomas. Neurosurgery. 72:630–637, 636-637. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sattler MG, Vroomen PC, Sluiter WJ, Schers
HJ, van den Berg G, Langendijk JA, Wolffenbuttel BH, van den Bergh
AC and van Beek AP: Incidence, causative mechanisms and anatomic
localization of stroke in pituitary adenoma patients treated with
postoperative radiation therapy versus surgery alone. Int J Radiat
Oncol Biol Phys. 87:53–59. 2013. View Article : Google Scholar : PubMed/NCBI
|