Introduction
Dermatofibrosarcoma protuberans (DFSP) is a
localized, low-grade malignant fibrosarcoma originating in the skin
and extending into the subcutaneous tissue (1). It is a rare tumor, accounting for only
0.1% of all cutaneous malignancies, with an overall incidence of
0.8–5.0 cases per million individuals in the USA (2). It is associated with a low capacity for
infiltration, rare occurrences of metastasis and a high incidence
of local recurrence. Clinically, this tumor often presents as a
protuberant and hard solid lump. The diagnosis depends on the
histopathological and immunohistochemical examination (3). Surgical resection is the first choice
for treatment and the prognosis is relatively good, with a
five-year survival rate of >90% (4). However, the local recurrence rate is
high, ranging between 0 and 60% depending on the surgical technique
used and tumor location (5).
Therefore, early diagnosis and complete resection are extremely
important. To the best our knowledge, no case of pit-like DFSP has
previously been reported. The present study reports one such case
of pit-like DFSP in a female patient.
Case report
A 27-year-old female presented to the Second
Affiliated Hospital of Xi'an Jiaotong University (Xi'an, Shaanxi,
China) with subcutaneous nodules on the left side of the neck,
which had been present for 5 years. The nodules had gradually begun
caving in during the last year. The patient had incidentally
noticed a nodule the size of a soybean and of normal skin color
that was not elevated above the skin surface. Since there were no
other symptoms, the nodule was not considered to be serious.
Thereafter, the patient often habitually touched and pressed the
nodule, which slowly increased in size. At ~1 year prior to
presentation, a mild depression was noted on the nodule surface and
the area became pink in color. As the depression progressively
deepened, the patient visited the hospital. The nodule was
surgically resected and submitted for pathological examination. The
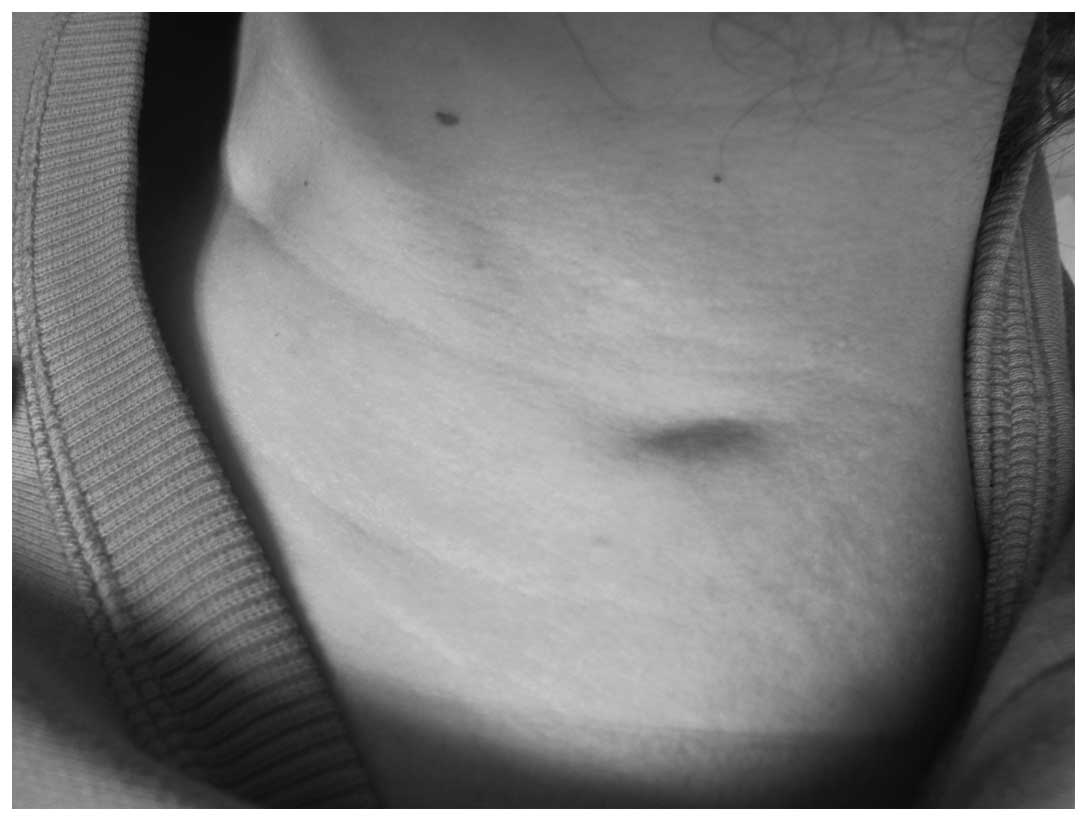
nodule appearance is shown in Fig. 1.
On the left side of the neck, an irregular depression (2.5 cm in
length, 1.0 cm in width and ~0.3 cm in depth) with an unclear
boundary was observed (Fig. 1). A
subcutaneous nodule with a rubbery consistency was palpable; it was
not tender, showed little activity and adhered to the subcutaneous
tissue.
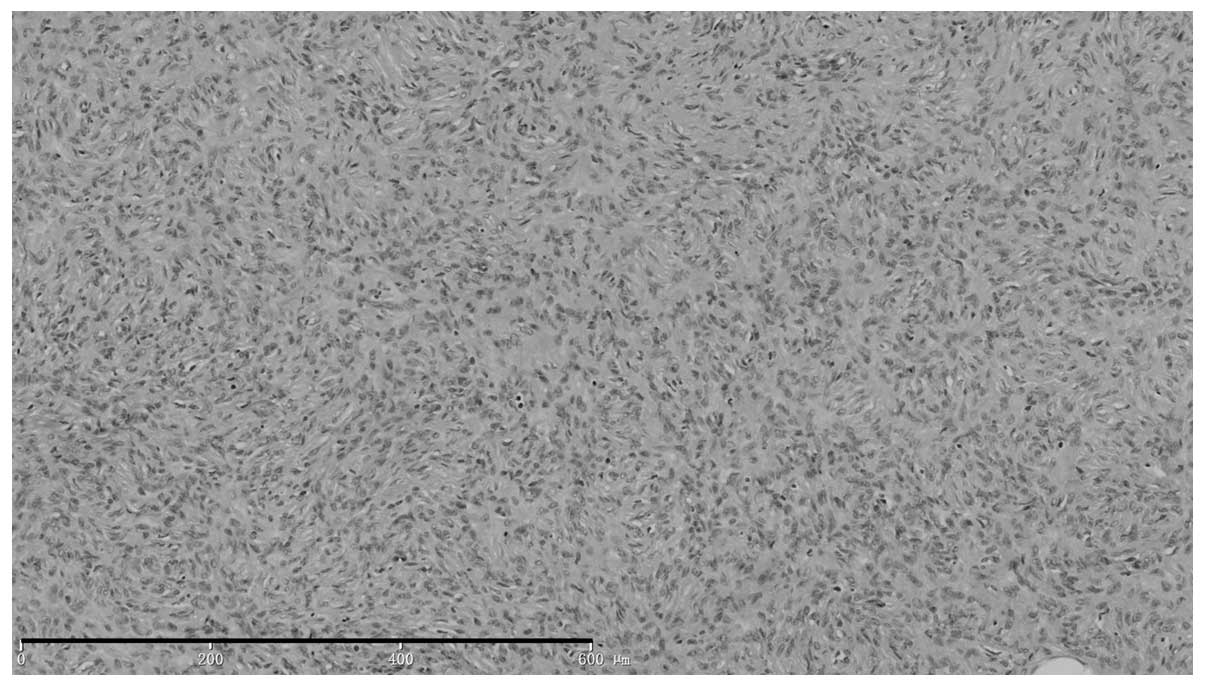
Histopathological examination (Fig. 2) revealed visible depression in the
skin lesions, with normal tissue on the right side of the neck
raised higher than the nodular tissue on the left side. The
epidermis appeared normal. Tissue sections from the tumor located
between the sunken dermis and subcutaneous tissue showed the
presence of a non-capsulated tumor composed of fusiform cells with
large nuclei that were interwoven into a whirlpool pattern, with
little nuclear mitotic activity, but with fat segmentation.
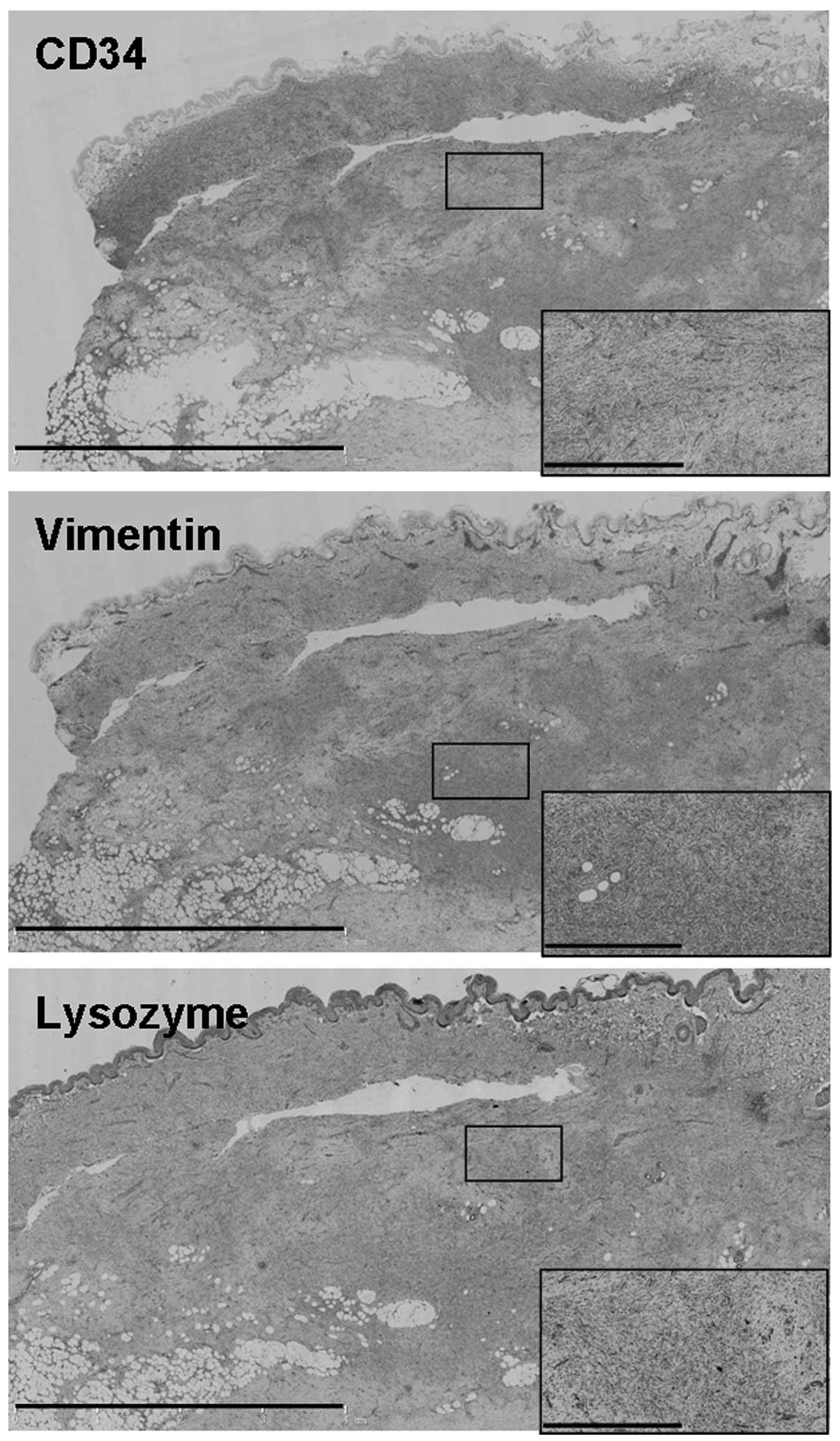
Immunohistochemical studies revealed positive
staining for cluster of differentiation (CD)34, vimentin, and
lysozyme (Fig. 3), but negative
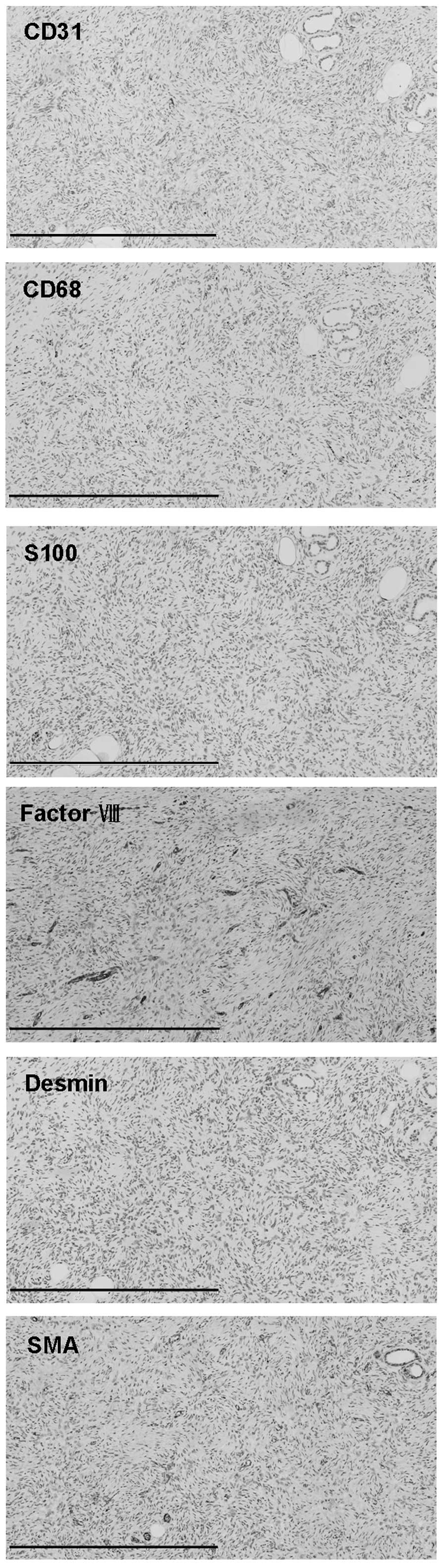
staining for CD31, CD68, S100, factor VIII, desmin and smooth
muscle actin (SMA) (Fig. 4). The
diagnosis of DFSP was based on the aforementioned findings. The
patient then underwent an extended resection of the tumor, followed
by adjacent skin repair. The resected tumor was further examined
histopathologically, and no tumor involvement was detected at the
surgical margins. Following surgery, the patient achieved good
results, with no relapse during the 8-month clinical follow-up
period.
Discussion
DFSP was first described in 1890 as a rare tumor
originating from fibroblasts and myofibroblasts with a keloid-like
appearance and a tendency for recurrence (6). The etiology of DFSP remains unclear and
may be associated with certain genetic characteristics, a history
of trauma and the extent of radiation exposure. Recent studies on
the molecular basis of DFSP development have reported that >90%
of DFSP patients present with the t(17;22)(q22;q13.1) chromosomal
translocation, which causes the fusion of the collagen type 1 α1
chain (COL1A1) gene on 17q22 and the platelet-derived growth factor
β chain (PDGFB) gene on 22q13.1 (7–10). This
results in the formation of the COL1A1-PDGFB fusion protein. The
sustained activation of fusion protein-related receptors by protein
tyrosine kinase causes abnormal cell proliferation, thereby
resulting in DFSP (11).
Although DFSP can occur at any age, it is more
common in adults between 20 and 50 years of age, regardless of
gender (12). The tumor often
presents in the torso, particularly the chest, followed by the
proximal extremities, but rarely occurs in the head and neck region
(13). The clinical manifestations of
this slow-growing tumor vary, depending on the developmental stage,
presenting as pink- or skin-colored nodules with diameters ranging
from a few millimeters to a few centimeters (14). The tumor is usually hard to touch and
initially adheres to the epidermis or subcutaneous tissue. The
disease further progresses with the occurrence of a single nodule
or multiple nodules of different sizes and has a protruding
appearance. The growth of these nodules can be suddenly accelerated
until their surface ruptures (7).
DFSP is usually asymptomatic, except for the presence of mild to
moderate pain in certain patients. Recurrence following tumor
resection is common, and disseminated nodules can occur around the
incision (15). However, tumor
metastasis is rare. In the advanced stages, the disease may
occasionally metastasize to the lungs, abdomen, brain, bones or
nearby lymph nodes, often due to repeated local recurrence. DFSP
can also present as atrophic lesions, sclerotic plaques and
localized scleroderma, and hence, it is termed atrophic DFSP (or
non-eminent DFSP) (16,17). Such a presentation could easily lead
to a misdiagnosis. To the best of our knowledge, a nodule with
depressed lesions has not previously been reported.
Typical DFSP are characterized as a tumor without a
capsule, located in the dermis and separated from the epidermis by
normal narrow bands. Diffuse irregular invasion into the
subcutaneous fat, presenting as a lace-like and branch-shaped beams
in parallel with epidermal cells, is also typically observed. Tumor
histology reveals spindle cells of uniform size with few areas of
dual-colored cytoplasm, and stained oval- or spindle-shaped nuclei.
The cells appear similar to naive fibroblasts with good
differentiation and little mitosis. Furthermore, tumor cells are
generally arranged around small blood vessels or fiber axes in a
radial, spoke- or whirlpool-like pattern. Such an arrangement is of
diagnostic significance. Diffused and positive staining for CD34
with a sensitivity as high as 84–100% detected by
immunohistochemistry is specific for DFSP with diagnostic value
(18). Focal positive staining for
vimentin, lysozyme, CD68 and SMA has been observed in certain
cases, whereas the tumor stains negative for factor VIII, factor
XIIIa, S-100 and desmin (18–20). The pathological and
immunohistochemical features of the present sturdy were consistent
with the aforementioned typical features of DFSF. However, to the
best of our knowledge, the pit-like appearance observed in the
presence case has not been previously reported.
DFSP is often misdiagnosed as a benign tumor and
thus is excised by a routine process, which easily leads to tumor
recurrence and even progression to malignant fibrosarcoma or
malignant fibrous histiocytoma. Therefore, an early diagnosis of
DFSP is extremely important. DFSP diagnosis mostly relies on the
histopathological examination. A diagnosis of DFSP should be
considered in patients with slowly-growing, painless, single or
multiple fused hard nodules in the torso or limbs, and
histopathological and immunohistochemical studies should be
performed (21). DFSP should also be
distinguished from skin fibroma, fibrosarcoma, malignant fibrous
histiocytoma and neurofibromatosis (22).
DFSP is generally treated with surgery, particularly
with extended resection and Mohs micrographic surgery. Since DFSP
easily recurs and presents on the body surface, radiation therapy
should not cause serious damage to vital organs and tissues.
Certain studies have therefore suggested treating DFSP with a
combination of surgery and radiotherapy. However, the tumor should
be treated in a timely manner, instead of waiting until repeated
recurrences occur, as recurrence can complicate surgery and
radiotherapy, and affect the outcome (23,24).
Recent studies on targeted therapy have demonstrated
that imatinib mesylate (Gleevec) may be useful in the treatment of
DFSP at the molecular level (21,25,26).
Imatinib mesylate is a tyrosine kinase inhibitor that can
specifically inhibit the expression of PDGF receptor β, as well as
ATP-binding cassette and tyrosine protein kinase (27).
DFSP is a low-grade malignancy with a slow growth
rate and good prognosis. The 5- and 15-year survival rates of DFSP
can be as high as 99.2 and 97.2%, respectively (28). However, its recurrence rate is high
and this usually occurs after 3 years of treatment.
In the present study, pathological and
immunohistochemical analyses confirmed that the patient had classic
DFSP; The tumor was composed of spindles cells exhibiting a
whirlpool-like pattern without epidermal atrophy. However, in the
present case, the tumor manifested with a pit-like appearance,
instead of the commonly observed protuberant lump. Since skin
atrophy was not observed, a diagnosis of atrophic DFSP was
eliminated. To the best of our knowledge, DFSP with such
manifestations has never been reported. Whether the current case
presents an early manifestation of DFSP or a novel type of DFSP
warrants confirmation by future studies.
Acknowledgements
The current study was supported by Program for
Changjiang Scholars and Innovative Research Team in University
(grant no. 1171).
References
|
1
|
Hegde U, Shetty SK, Sreeshyla HS and
Doddawada VG: Dermatofibrosarcoma protuberans - a recurrent lesion
with unusual presentation in the parotid region. J Clin Diag Res.
8:130–131. 2014.
|
|
2
|
Zheng Z, Piao J, Lee JH, Kim SE, Kim SC,
Chung KY and Roh MR: Dermatofibrosarcoma protuberans: A study of
clinical, pathologic, genetic, and therapeutic features in Korean
patients. Yonsei Med J. 56:440–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saiag P, Grob JJ, Lebbe C, et al:
Diagnosis and treatment of dermatofibrosarcoma protuberans.
European consensus-based interdisciplinary guideline. Eur J Cancer.
Jul 16–2015.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
Erdem O, Wyatt AJ, Lin E, Wang X and
Prieto VG: Dermatofibrosarcoma protuberans treated with wide local
excision and followed at a cancer hospital: Prognostic significance
of clinicopathologic variables. Am J Dermatopathol. 34:24–34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim M, Huh CH, Cho KH and Cho S: A study
on the prognostic value of clinical and surgical features of
dermatofibrosarcoma protuberans in Korean patients. J Eur Acad
Dermatol Venereol. 26:964–971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suit H, Spiro I, Mankin HJ, Efird J and
Rosenberg AE: Radiation in management of patients with
dermatofibrosarcoma protuberans. J Clin Oncol. 14:2365–2369.
1996.PubMed/NCBI
|
|
7
|
Llombart B, Serra-Guillén C, Monteagudo C,
López Guerrero JA and Sanmartíin O: Dermatofibrosarcoma
protuberans: A comprehensive review and update on diagnosis and
management. Semin Diagn Pathol. 30:13–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McArthur G: Molecularly targeted treatment
for dermatofibrosarcoma protuberans. Semin Oncol. 31:30–36. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sjöblom T, Shimizu A, O'Brien KP, Pietras
K, Dal Cin P, Buchdunger E, Dumanski JP, Ostman A and Heldin CH:
Growth inhibition of dermatofibrosarcoma protuberans tumors by the
platelet-derived growth factor receptor antagonist STI571 through
induction of apoptosis. Cancer Res. 61:5778–5783. 2001.PubMed/NCBI
|
|
10
|
Kiuru-Kuhlefelt S, El-Rifai W,
Fanburg-Smith J, Kere J, Miettinen M and Knuutila S: Concomitant
DNA copy number amplification at 17q and 22q in dermatofibrosarcoma
protuberans. Cytogenet Cell Genet. 92:192–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao J, Patel KU, López-Terrada D and Fang
H: Atrophic dermatofibrosarcoma protuberans: Report of a case
demonstrated by detecting COL1A1-PDGFB rearrangement. Diagn Pathol.
7:1662012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loss L and Zeitouni NC: Management of
scalp dermatofibrosarcoma protuberans. Dermatol Surg. 31:1428–1433.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia C, Clark RE and Buchanan M:
Dermatofibrosarcoma protuberans. Int J Dermatol. 35:867–871. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Llombart B, Monteagudo C, Sanmartin O,
López-Guerrero JA, Serra-Guillén C, Poveda A, Jorda E,
Fernandez-Serra A, Pellíin A, Guillén C and Llombart-Bosch A:
Dermatofibrosarcoma protuberans: A clinicopathological,
immunohistochemical, genetic (COL1A1-PDGFB) and therapeutic study
of low-grade versus high-grade (fibrosarcomatous) tumors. J Am Acad
Dermatol. 65:564–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maki RG, Awan RA, Dixon RH, Jhanwar S and
Antonescu CR: Differential sensitivity to imatinib of 2 patients
with metastatic sarcoma arising from dermatofibrosarcoma
protuberans. Int J Cancer. 100:623–626. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanabusa M, Kamo R, Harada T and Ishii M:
Dermatofibrosarcoma protuberans with atrophic appearance at early
stage of the tumor. J Dermatol. 34:336–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin L, Piette F, Blanc P, Mortier L,
Avril MF, Delaunay MM, Dréno B, Granel F, Mantoux F, Aubin F, et
al: Clinical variants of the preprotuberant stage of
dermatofibrosarcoma protuberans. Br J Dermatol. 153:932–936. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haycox CL, Odland PB, Olbricht SM and
Piepkorn M: Immunohistochemical characterization of
dermatofibrosarcoma protuberans with practical applications for
diagnosis and treatment. J Am Acad Dermatol. 37:438–444. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dominguez-Malagón HR, Ordóňez NG and
Mackay B: Dermatofibrosarcoma protuberans: Ultrastructural and
immunocytochemical observations. Ultrastruct Pathol. 19:281–289.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sachdev R and Sundram U: Expression of
CD163 in dermatofibroma, cellular fibrous histiocytoma and
dermatofibrosarcoma protuberans: Comparison with CD68, CD34 and
Factor XIIIa. J Cutan Pathol. 33:353–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bakry O and Attia A: Atrophic
dermatofibrosarcoma protuberans. J Dermatol Case Rep. 6:14–17.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marque M, Bessis D, Pedeutour F, Viseux V,
Guillot B and Fraitag-Spinner S: Medallion-like dermal dendrocyte
hamartoma: The main diagnostic pitfall is congenital atrophic
dermatofibrosarcoma. Br J Dermatol. 160:190–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bowne WB, Antonescu CR, Leung DH, Katz SC,
Hawkins WG, Woodruff JM, Brennan MF and Lewis JJ:
Dermatofibrosarcoma protuberans: A clinicopathologic analysis of
patients treated and followed at a single institution. Cancer.
88:2711–2720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JK, Malik MM and Egan CA: Atrophic
dermatofibrosarcoma protuberans: An uncommon and misleading
variant. Australas J Dermatol. 45:175–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Labropoulos SV, Fletcher JA, Oliveira AM,
Papadopoulos S and Razis ED: Sustained complete remission of
metastatic dermatofibrosarcoma protuberans with imatinib mesylate.
Anticancer Drugs. 16:461–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rutkowski P, Debiec-Rychter M, Nowecki Z,
Michej W, Symonides M, Ptaszynski K and Ruka W: Treatment of
advanced dermatofibrosarcoma protuberans with imatinib mesylate
with or without surgical resection. J Eur Acad Dermatol Venereol.
25:264–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Price VE, Fletcher JA, Zielenska M, Cole
W, Viero S, Manson DE, Stuart M and Pappo AS: Imatinib mesylate: An
attractive alternative in young children with large, surgically
challenging dermatofibrosarcoma protuberans. Pediatr Blood Cancer.
44:511–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kohli N and Srivastava D:
Dermatofibrosarcoma protuberans. Dermatology Atlas for Skin of
Color. Jackson-Richards D and Pandya AG: (Berlin). Springer.
269–272. 2014.
|