Introduction
Hydatidiform mole with a coexistent fetus (HMCF) is
a rare condition, with an incidence of 1/22,000-1,000,000
pregnancies (1). There have been a
number of cases of HMCF previously reported (2–4). The
majority are complete HMCF cases, which possess a significant risk
(19.5–62.5%) of inducing persistent trophoblastic disease (PTD)
(5). By contrast, persistent or
metastatic trophoblastic disease following a partial mole is rare,
with an estimated risk of ~1–5.6% (6). However, to the best of our knowledge,
there have been no previous reports investigating the risk of
persistent or metastatic trophoblastic disease following partial
HMCF (PHMCF), due to the rarity of this condition. The management
of such pregnancies creates a dilemma for the physician and
parents, particularly when PHMCF occurs during the second trimester
of pregnancy. Medical termination is effective during the second
trimester; however, this technique increases the risk of the
occurrence of PTD when used to terminate molar pregnancies
(7). The aim of this study was to
investigate the safety of medical termination for patients with
PHMCF. The present study reports a case of PHMCF in which the
pregnancy was terminated and delivery was induced via
intra-amniotic administration of Rivanol, followed by aspiration
curettage, which resulted in the occurrence of PTD and pulmonary
metastases. Written informed consent was obtained from the
patient.
Case report
In November 2013, a 22-year-old female (gravida 2,
para 0) was referred to the Department of Gynecology at the Women's
Hospital of Zhejiang University School of Medicine (Zhejiang,
China) at 16 weeks of gestation, with suspected molar pregnancy.
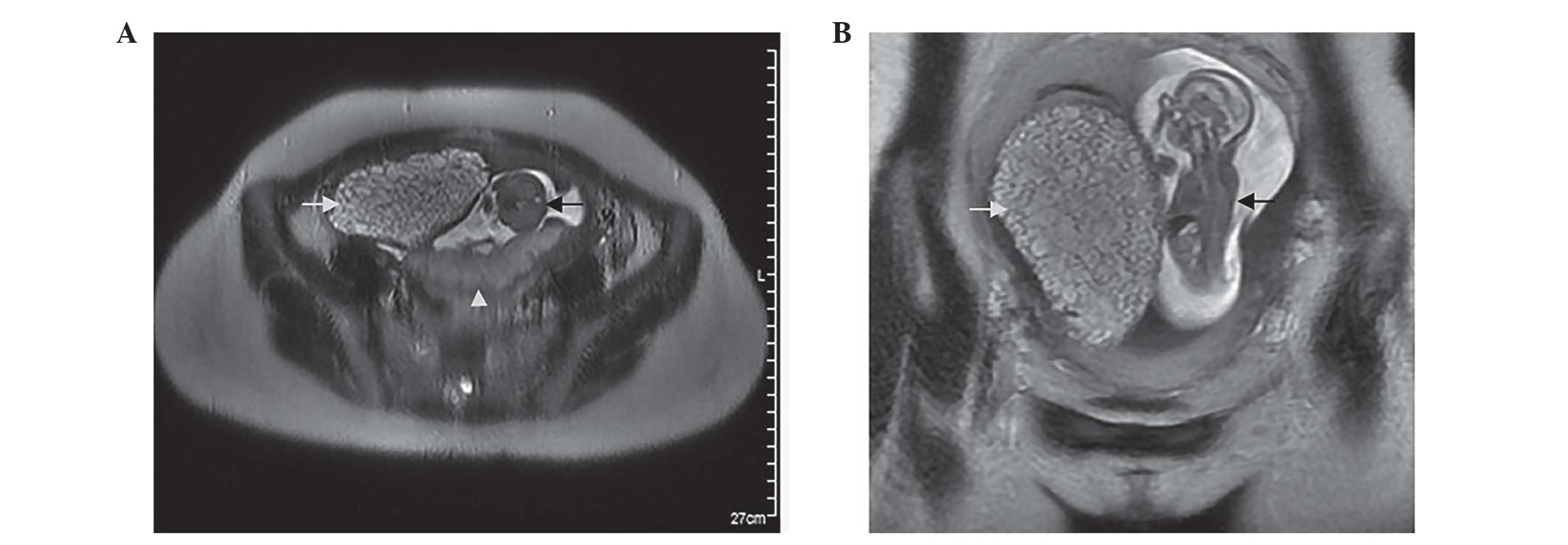
Sonography and magnetic resonance imaging (MRI) revealed a fetus
and placenta, which appeared normal, in the posterior portion of
the uterus. However, an additional cystic placenta with a
degenerated twin was identified in the right anterior portion of
the uterus (Fig. 1). In addition, the
patient's serum β-human chorionic gonadotropin (β-HCG) levels were
markedly increased (449,078 IU/l). The couple decided to terminate
the pregnancy following consideration of the risks. Delivery was
induced via intra-amniotic administration of 80 mg Rivanol (Guangxi
Hefeng Pharmaceutical Co., Ltd., Hechi, China). Thirty-six hours
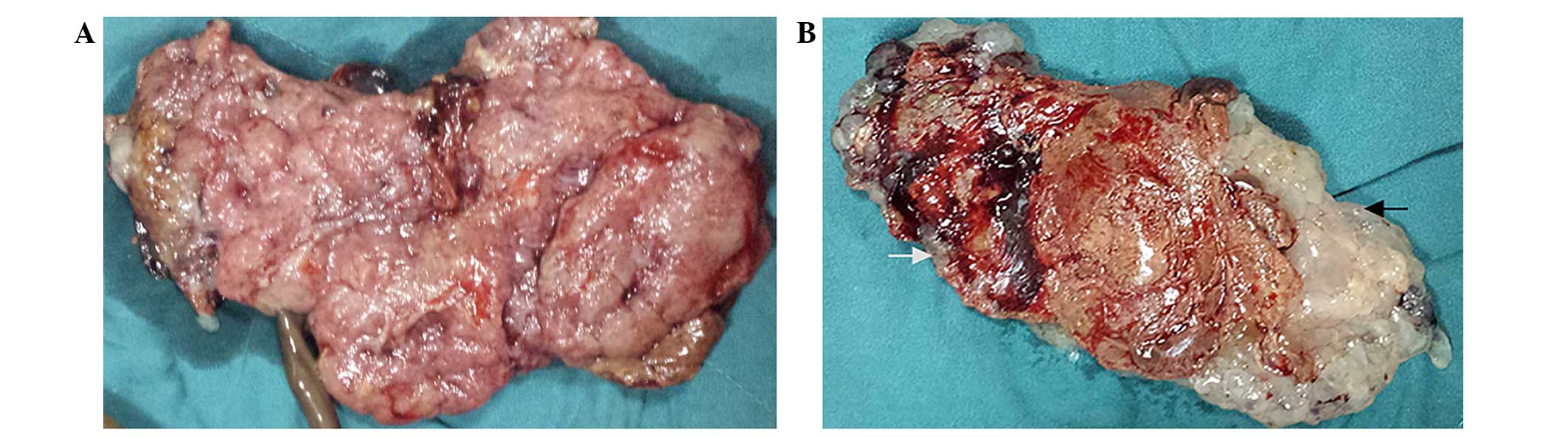
later, a fetus with a normal placenta, as well as a partially
cystic placenta were delivered (Fig.
2). Suction curettage was performed due to incomplete
evacuation. There were no fetal gross abnormalities and no genetic
abnormalities were identified by DNA Array (Affymetrix, Inc., Santa
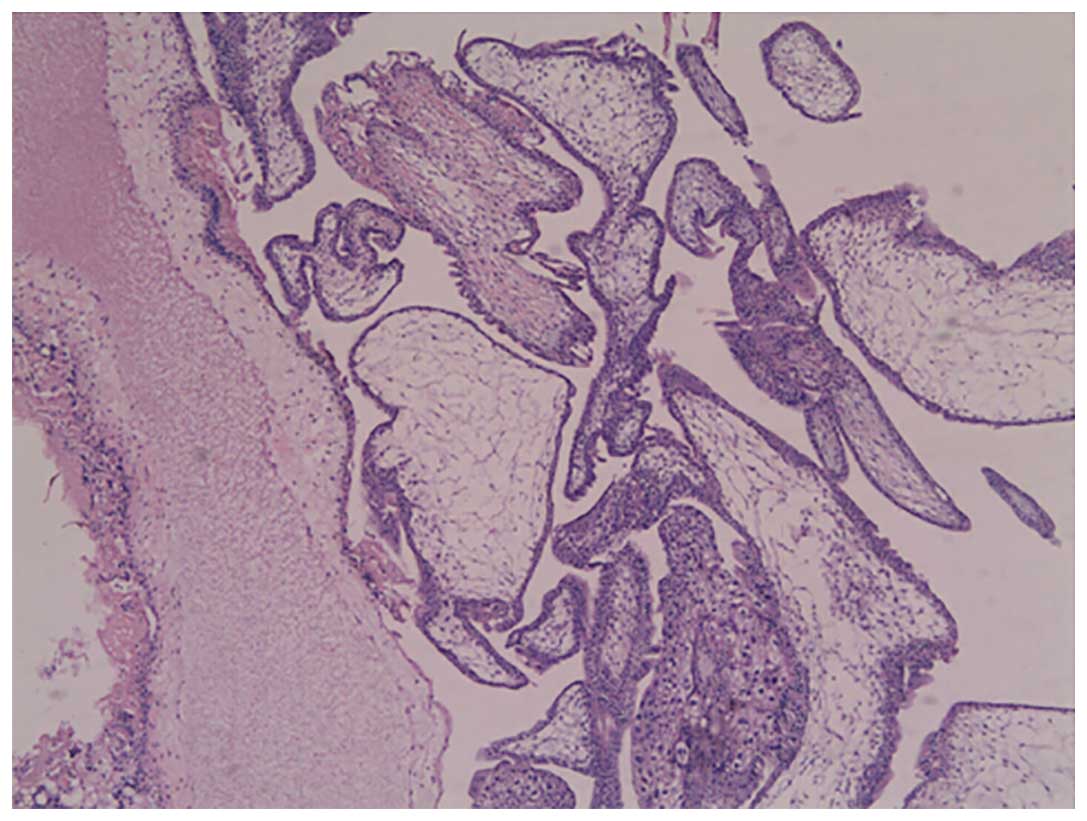
Clara, CA, USA). Histopathological analysis of the molar tissue
revealed chorionic villi of varying size and shape, with focal
edema and functioning villous circulation, as well as focal
trophoblastic hyperplasia (Fig. 3).
DNA Array (Affymetrix, Inc.) of the molar tissue revealed a
triploid cell line, and thus confirmed the diagnosis of PHMCF.
Serum β-HCG levels were monitored for three weeks
following the termination of pregnancy (TOP), and initially reduced
to 3,845 IU/l; however, 1 week later, β-HCG levels had increased to
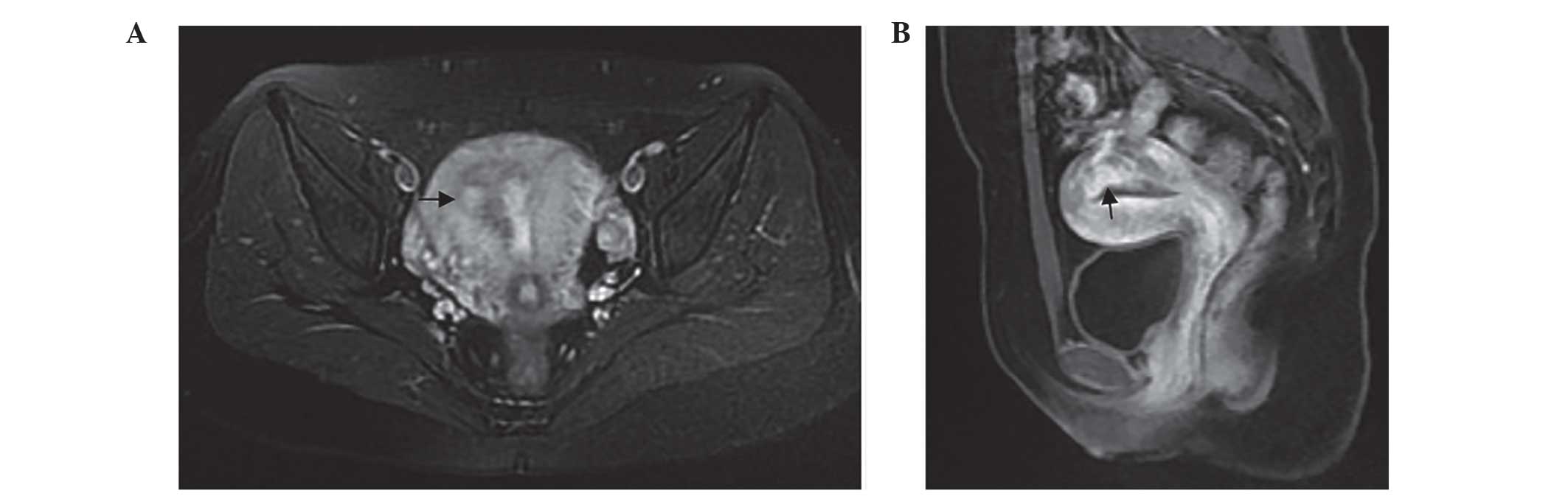
3,947 IU/l. An MRI study revealed abnormal signs in the right
posterior portion of the myometrium and uterine cavity, which
indicated the presence of an invasive mole (Fig. 4). Serum β-HCG levels were observed to
have plateaued (~1,706–2,603 IU/l) at the next two-week follow-up
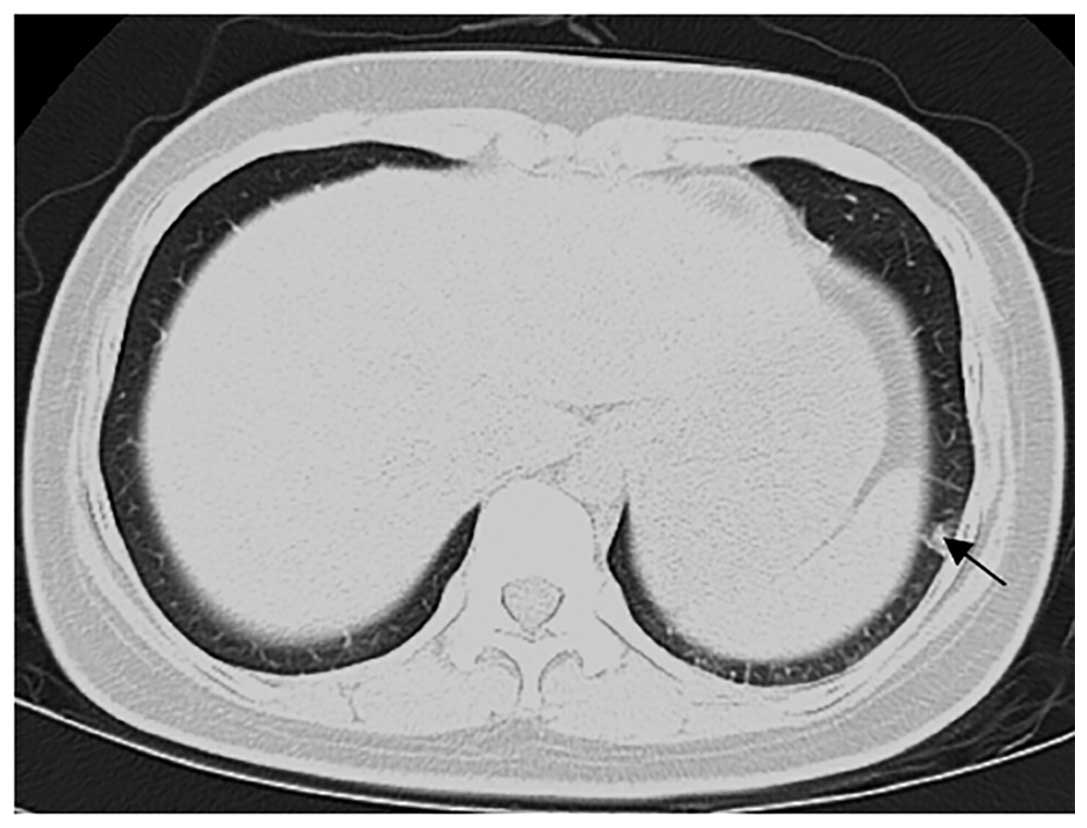
appointment. A follow-up chest computed tomography (CT) scan
revealed a number of small, ill-defined nodules of varying sizes in
the right and left lower lungs, indicative of lung metastases
(Fig. 5). An MRI of the head and a CT
scan of the abdomen were normal. The patient was admitted for
chemotherapy, and following three courses (3-week cycles) of
methotrexate (0.4 mg/kg, days 1–5), the metastases receded and
β-HCG levels reduced to within the normal range. The patient
demonstrated no disease recurrence during 1 year of follow-up.
Discussion
Multiple pregnancy involving a partial hydatidiform
mole and normal fetus is rare. The management of such pregnancies
presents a dilemma for physicians and parents between expectant
management and immediate intervention; particularly in pregnancies
involving assisted reproductive technology (8–10). A small
number of cases of PHMCF where pregnancy was allowed to continue
until 28 weeks of gestation or later have previously been reported,
and delivery was achieved via Caesarean section, resulting in a
number of healthy babies (11,12).
However, parents must also be aware of the risk of potential
maternal complications associated with molar pregnancy, including
early-onset preeclampsia, hyperemesis gravidarum, PTD and
metastases (13).
To the best of our knowledge, following an extensive
search of the relevant medical literature since the year 2000, 10
PHMCF cases have previously been reported (Table I) (8–12,14–18). Two of these cases of
PHMCF were treated by termination of pregnancy during the first
trimester, via dilatation and suction curettage, resulting in one
case of PTD and no metastases (8,14). In 3/5
cases (60%) that were terminated during the second trimester, two
by medically induced labor and the other via unspecified
methodology, the patient subsequently developed PTD and lung
metastases (9,10,15).
Finally, three cases that were terminated by caesarean section in
the third trimester did not develop PTD or metastases, and resulted
in the delivery of live infants (although one infant succumbed 30
days subsequent to delivery) (11,12,18). In
the present case, the PHMCF pregnancy was terminated by the
induction of labor via intra-amniotic administration of Rivanol at
17 weeks of gestation. PTD and pulmonary metastases were
subsequently detected, which required further treatment with
chemotherapy. Certain investigators have hypothesized that the risk
of PTD in HMCF pregnancies is not associated with the length of
gestation at termination (5). The
literature cited in the present study also supports this
conclusion, as none of the three cases where pregnancy was
terminated later than 28 weeks of gestation developed PTD.
 | Table I.Previously reported cases of partial
hydatidiform mole and coexisting fetus in the literature. |
Table I.
Previously reported cases of partial
hydatidiform mole and coexisting fetus in the literature.
| Study | Gestational age at
delivery or abortion, weeks | Method of
termination | Live neonate | PTD | Metastases | Ref |
|---|
| Ingec et
al | 10 | D&E | No | Yes | No | (11) |
| Tay | 11 | D&E | No | No | No | (12) |
| Kim et al | 14 | Medical | No | Yes | Yesa | (13) |
| Zhou et
al | 16 | Medical | No | Yes | Yesa | (14) |
| Shiina et
al | 20 | Unknown | No | Yes | Yesa | (15) |
| Sánchez-Ferrer et
al | 21 | Spontaneous
abortion | No | No | No | (16) |
| Chu et al | 24 | Caesarean
section | Yes | No | No | (17) |
| Copeland and
Stanek | 28 | Caesarean
section | Yes | No | No | (8) |
| Navarro Amezcua et
al | 29 | Caesarean
section | No | No | No | (18) |
| Sun et al | 35 | Caesarean
section | Yes | No | No | (9) |
| Present case | 17 | Medical | No | Yes | Yes |
|
Although advances in transvaginal ultrasound and MRI
may assist with the diagnosis of HMCF in the first trimester
(8,19), HMCF is typically diagnosed at ~15–20
weeks of gestation or later (20).
The empirical management of pregnancy in the second trimester is
more difficult (10). Suction
curettage is the typical treatment for females exhibiting molar
pregnancy, however normal fetal structures may preclude the use of
this method (7). Medical induction of
labor is an effective and safe way for the termination of normal
pregnancies during the second trimester (21). However, medical termination of molar
pregnancy is associated with an increased risk of a subsequent
requirement for chemotherapeutic treatment, due to the development
of increased intrauterine pressure in the first and second
trimesters (7). Performance of a
caesarean section is not recommended during the second trimester of
pregnancy, due to the increased risk of maternal morbidity and the
fact that the reduced gestational period means that the neonate is
too small to survive (7).
Evidence-based practice guidelines of the Royal
College of Obstetricians and Gynecologists recommend dilatation and
evacuation (D&E) as a safe and effective termination option for
pregnancies of >15 weeks gestation, when undertaken by
specialist practitioners with sufficient experience and skills
(22). A number of studies have
demonstrated that D&E is a rapid and well-tolerated method of
termination, demonstrating low complication rates when performed by
skilled physicians (23,24). However, to the best of our knowledge,
there have been no reports concerning the risk of PTD following
PHMCF termination by D&E. In a study conducted by Niemann et
al (5), 5/8 HMCF patients'
pregnancies were terminated by curettage during the second
trimester. One of these patients developed PTD, indicating a risk
factor of 20%.
Mid-trimester TOP due to PHMCF is challenging
(9,10). Due to the rarity of this condition,
minimal relevant data has been amassed, meaning that it is
difficult to formulate valid predictions for treatment and outcomes
of patients presenting with PHMCF during the second trimester. The
present study concluded that medical termination may not be a safe
method for patients with PHMCF, due to the high risk of PTD and
metastasis associated. D&E may be an effective alternative
method for termination of PHMCF in the second trimester. Pregnancy
may be allowed to continue empirically, provided that maternal
morbidity does not appear to be associated with the gestational age
at termination, and that the acquisition of healthy neonates is a
feasible outcome.
Acknowledgements
The authors would like to thank Professor Jian-Ming
Zhu of the University of North Carolina School of Medicine (Chapel
Hill, NC, USA) for his contribution in preparing this manuscript
and for his discussion.
References
|
1
|
Jones WB and Lauersen NH: Hydatidiform
mole with coexistent fetus. Am J Obstet Gynecol. 122:267–272.
1975.PubMed/NCBI
|
|
2
|
Steller MA, Genest DR, Bernstein MR, et
al: Natural history of twin pregnancy with complete hydatidiform
mole and coexisting fetus. Obstet Gynecol. 83:35–42.
1994.PubMed/NCBI
|
|
3
|
Sebire NJ, Foskett M, Paradinas FJ, et al:
Outcome of twin pregnancies with complete hydatidiform mole and
healthy co-twin. Lancet. 359:2165–2166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng M, Li L, Zheng J, Ding Y, Yu L and
Huang J: Termination of twin pregnancies with hydatidiform moles: A
case series of four patients. Iran J Public Health. 43:1000–1006.
2014.PubMed/NCBI
|
|
5
|
Niemann I, Sunde L and Petersen LK:
Evaluation of the risk of persistent trophoblastic disease after
twin pregnancy with diploid hydatidiform mole and coexisting normal
fetus. Am J Obstet Gynecol. 197:45.e1–45.e5. 2007. View Article : Google Scholar
|
|
6
|
Worley MJ Jr, Joseph NT, Berkowitz RS and
Goldstein DP: Women with a partial mole during their first
pregnancy and diagnosed earlier in gestation are at increased risk
of developing gestational trophoblastic neoplasia. Int J Gynecol
Cancer. 24:941–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tidy JA, Gillespie AM, Bright N, Radstone
CR, Coleman RE and Hancock BW: Gestational trophoblastic disease: A
study of mode of evacuation and subsequent need for treatment with
chemotherapy. Gynecol Oncol. 78:309–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tay ET: Partial hydatidiform mole and
coexisting viable twin pregnancy. Pediatr Emerg Care. 29:1298–1300.
2013.PubMed/NCBI
|
|
9
|
Kim CH, Kim YH, Kim JW, Kim KM, Cho MK,
Kim SM, Nam JH and Song TB: Triplet pregnancy with partial
hydatidiform mole coexisting with two fetuses: A case report. J
Obstet Gynaecol Res. 34:641–644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Chen Y, Li Y and Duan Z: Partial
hydatidiform mole progression into invasive mole with lung
metastasis following in vitro fertilization. Oncol Lett. 3:659–661.
2012.PubMed/NCBI
|
|
11
|
Copeland JW and Stanek J: Dizygotic twin
pregnancy with a normal fetus and a nodular embryo associated with
a partial hydatidiform mole. Pediatr Dev Pathol. 13:476–480. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun CJ, Zhao YP, Yu S, Fan L, Wu QQ, Li GH
and Zhang WY: Twin pregnancy and partial hydatidiform mole
following in vitro fertilization and embryos transfer: A novel case
of placental mosaicism. Chin Med J (Engl). 125:4517–4519.
2012.PubMed/NCBI
|
|
13
|
Vimercati A, de Gennaro AC, Cobuzzi I,
Grasso S, Abruzzese M, Fascilla FD, Cormio G and Selvaggi L: Two
cases of complete hydatidiform mole and coexistent live fetus. J
Prenat Med. 7:1–4. 2013.PubMed/NCBI
|
|
14
|
Ingec M, Borekci B, Altas S and Kadanali
S: Twin pregnancy with partial hydatidiform mole and coexistent
normal fetus. J Obstet Gynaecol. 26:379–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiina H, Oka K, Okane M, et al:
Coexisting true hermaphroditism and partial hydatidiform mole
developing metastatic gestational trophoblastic tumors. A case
report. Virchows Arch. 441:514–518. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sánchez-Ferrer ML, Ferri B, Almansa MT, et
al: Partial mole with a diploid fetus: Case study and literature
review. Fetal Diagn Ther. 25:354–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu W, Chapman J, Persons DL and Fan F:
Twin pregnancy with partial hydatidiform mole and coexistent fetus.
Arch Pathol Lab Med. 128:1305–1306. 2004.PubMed/NCBI
|
|
18
|
Navarro Amezcua ME, Castellanos Reyes J,
Cardona González O and Torres Gómez LG: Twin pregnancy with partial
hydatidiform mole and alive fetus: Case report. Ginecol Obstet Mex.
76:275–279. 2008.(In Spanish). PubMed/NCBI
|
|
19
|
Herek D and Karabulut N: The role of
magnetic resonance imaging in the diagnosis of complete
hydatidiform mole in a twin pregnancy. Int J Gynaecol Obstet.
123:772013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsui H, Sekiya S, Hando T, Wake N and
Tomoda Y: Hydatidiform mole coexistent with a twin live fetus: A
national collaborative study in Japan. Hum Reprod. 15:608–611.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mentula MJ, Niinimäki M, Suhonen S,
Hemminki E, Gissler M and Heikinheimo O: Immediate adverse events
after second trimester medical termination of pregnancy: Results of
a nationwide registry study. Hum Reprod. 26:927–932. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morrison J: Audit of the care of women
requesting induced abortion. J Obstet Gynaecol. 23:521–524. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panoskaltsis TA: Mid-trimester termination
of pregnancy by dilatation and evacuation. J Obstet Gynaecol.
21:280–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kent A: Second trimester termination of
pregnancy. Rev Obstet Gynecol. 4:952011.PubMed/NCBI
|