Introduction
The mitochondrial GTPase mitofusin-2 (Mfn2) gene,
which is also called the hyperplasia suppressor gene, was
originally identified in vascular smooth muscle cells from
spontaneously hypertensive rats, which exhibited markedly lower
expression than Wistar-Kyoto rats (1). Mfn2 localizes to the mitochondrial outer
membrane and plays an essential role in mitochondrial fusion, thus
regulating mitochondrial morphology and function. Further research
has indicated that Mfn2 has a potential apoptotic effect mediated
by the mitochondrial apoptotic pathway (1–3). Chen
et al (4) demonstrated that
Mfn2 notably suppresses cell growth and proliferation in a number
of tumor cell lines through the inhibition of the Ras-ERK MAPK
signaling pathway. Recently, Mfn2 has become a focal point in tumor
research. Several studies have investigated the function of Mfn2 in
various malignancies, including hepatocellular, urinary bladder and
gastric cancers, and Mfn2 is considered to perform pro-apoptotic
and anti-proliferative functions (5–7).
Clinical and epidemiological evidence reveals that
estrogens participate in the initiation and development of human
breast cancer (8,9). Understanding the role of estrogen
receptor (ER)α and β in the pathogenesis of breast cancer is
essential, since the effects of estrogen are mediated through these
two ERs (10). Although the function
of ERα has been established and this receptor remains the most
significant marker of the response to hormonal therapy in breast
cancer, the role of ERβ remains elusive as a result of a number of
conflicting studies (11). Our
previous study demonstrated that ERβ may inhibit the
estradiol-induced proliferation and migration of MCF-7 cells
through regulation of Mfn2 (12), but
the exact mechanism by which Mfn2 exerts its antitumor effects
remains unclear. Therefore, exploration of the function of Mfn2 may
also help us understand the role of ERβ in the pathogenesis of
breast cancer.
A previous study demonstrated that the PI3K/Akt
signaling pathway was involved in Mfn2-regulated smooth muscle cell
proliferation (13). However, the
correlation between them remains unclear in breast cancer. We
hypothesize that the outer-membrane protein Mfn2 participates in
the apoptotic process in association with the PI3K/Akt signaling
pathway. In the present study, we employed a plasmid to deliver
Mfn2 to MCF-7 cells, a human breast cancer cell line, in order to
evaluate the effect of Mfn2 on apoptosis and proliferation.
Furthermore, we investigated the mechanism of Mfn2-regulated
pro-apoptosis and the anti-proliferation effects of MCF-7 cells
in vitro.
Materials and methods
Cell lines and cell culture
MCF-7 cells were kindly donated by Professor
Mei-xiang Sang, Division of Scientific Research, the Fourth
Hospital of Hebei Medical University, Shijiazhuang, China. The
cells were cultured in growth medium consisting of Dulbecco's
modified Eagle's medium (DMEM; Gibco Life Technologies, Carlsbad,
CA, USA) containing 4.5 g/l glucose, 2 mM L-glutamine, 5000 IU/l
penicillin, 5 mg/l streptomycin, 125 U/l Fungizone, 2.2 g/l sodium
bicarbonate and 10% fetal bovine serum (FBS) pretreated with 5%
charcoal-dextran in an incubator at 37°C with a humidified
atmosphere of 5% CO2. For the experiments conducted in
serum-free conditions, the cells were induced to quiescence by
culturing in serum-free medium for 24 h. DMEM with antibiotics and
glutamine was supplemented with 0.5 g/l bovine serum albumin (BSA).
The cells of each experimental group were cultured for 48 h with
17β-estradiol (E2) at a dose of 10−6 mol/l, which was
confirmed in our previous study to have an optimal effect (12).
Expression vectors and transient
transfection
pEGFP-Mfn2 and its negative control vectors were
purchased from Yingrun Biotechnology Co. Ltd. (Changsha, China).
The pEGFP-Mfn2 plasmid carries the full-length Mfn2 gene. The
transient transfection of MCF-7 cells was performed using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer's instructions. Briefly, MCF-7
cells were cultured in six-well plates, and the medium was changed
every day until 80% confluence was achieved. The cells were
transfected with 4.0 µg vector DNA by 10 µl Lipofectamine 2000 in 2
ml serum-free DMEM. Six hours after transfection, the medium was
replaced by normal DMEM supplemented with 10% FBS, and the cells
were cultured for 24 h. The cells were then cultured for 48 h in
medium containing 10% FBS and E2 to detect the proliferation and
apoptosis of MCF-7 cells. The efficiency of transfection was ~70%
for all the experimental groups.
Cell proliferation
The cell proliferation was measured using methyl
thiazolyl tetrazolium (MTT) shade selection experiments. The cells
(5×103 per well) were plated in triplicate in 96-well
plates and cultured for 24 h. Then,
3–2,5-dihydro-1-methyl-5h-tetrazole-5-thion sodium salt was added
for 4 h, and the absorbance was determined at 490 nm (SpectraMax,
Molecular Devices, Sunnyvale, CA, USA).
Western blot analysis
The proteins extracted from MCF-7 cells were
separated on a 10% sodium dodecyl sulphate-polyacrylamide gel and
then transferred onto a polyvinylidene fluoride membrane
(Millipore, Billerica, MA, USA). The membrane was blocked for 1 h
at 37°C with 5% BSA in Tris-buffered saline containing 0.05%
Tween-20 (TBST). The membrane was then incubated at 4°C overnight
with primary antibodies for Mfn2 (1:200; Santa Cruz Biotechnology,
Santa Cruz, CA, USA), Akt (Cell Signaling Technology, MA, USA),
phospho-Akt (Cell Signaling Technology) and β-actin (1:1000; Santa
Cruz Biotechnology). Subsequently, the membrane was rinsed three
times with TBST containing secondary antibodies (1:5000) and
treated with enhanced chemiluminescence solution (Pierce, Rockford,
IL, USA), and the bands were detected by exposing the blots to
X-ray film. For quantitative analysis (i.e., normalized for
β-actin), the bands were evaluated with IPP 5.0 software (?). The
integrated optical density (IOD) of each band was measured, and the
relative IOD was calculated as the ratio of the target band IOD to
the IOD of the β-actin band.
Immunofluorescence
MCF-7 cells were plated on cover slides on six-well
plates. After fixation in 10% formalin at room temperature for 15
min, pretreatment with 0.3% Triton X-100 for 20 min at 37°C and
blocking with goat serum for 30 min at 37°C, the cells were
incubated with anti-Mfn2 (1:200) overnight at 4°C. After washing
three times with phosphate-buffered saline (PBS), the slides were
incubated with fluorescein isothiocyanate-conjugated secondary
antibody (1:200, Santa Cruz Biotechnology) for 2 h at 37°C. The
slides were then viewed after being rinsed three times with
PBS.
Bromodeoxyuridine (BrdU)
incorporation
MCF-7 cells were plated at 1×104
cells/well in 96-well plates, subjected to growth arrest for 24 h,
and exposed to E2 or treated with various agents in serum-free
DMEM. BrdU incorporation was measured using BrdU proliferation
assay kits (Millipore) according to the manufacturer's
instructions. Briefly, the cells were labeled with 10 ng/ml BrdU
during the incubation, washed three times with cold wash buffer,
fixed, air-dried and incubated for 1 h at room temperature with
mouse anti-BrdU monoclonal antibody (diluted 1:200). The antibody
was aspirated. The cells were washed three times and then incubated
with peroxidase goat anti-mouse IgG (1:2000) at room temperature
for 30 min. The cells were washed three times, and 100 µl of the
substrates was added to each well. The plate was then incubated for
30 min in the dark. Thereafter, the absorbance was measured at dual
wavelengths of 450 to 540 nm.
Flow cytometric analysis
MCF-7 cells were detached using trypsin for 48 h
following infection with pEGFP-Mfn2 and the control vector pEGFP.
The cells were washed three times with PBS. To detect the cell
cycle phases, the cells were treated with 50 µl DNA Prep LPR
(Beckman Coulter, Fullerton, CA, USA) for 30 min at room
temperature and 500 µl DNA Prep Stain (Beckman Coulter) for 30 min
at room temperature. To measure the apoptosis of MCF-7 cells
following treatment, an ApoScreen Annexin V apoptosis kit (Southern
Biotech, Birmingham, AL, USA) was used according to the
manufacturer's instructions. The cell cycle distribution and
apoptosis were determined using a flow cytometer (Beckman
Coulter).
Statistical analysis
Figure analysis was conducted using IPP software
(Media Cybernetics, Inc., Rockville, MD, USA). The quantitative
data are presented as the mean ± standard deviation. The
statistical analyses were performed using one-way analysis of
variance with the Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in protein expression levels
of Mfn2, Akt and phospho-Akt (p-Akt) in MCF-7 cells exposed to
E2
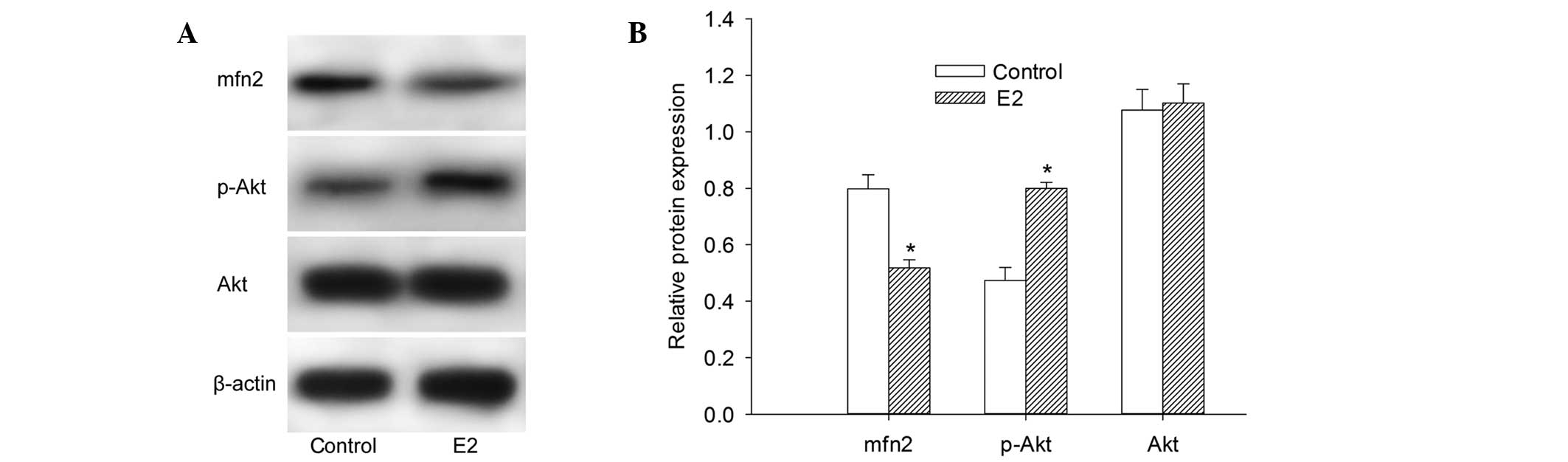
To determine the protein expression levels of Mfn2,
Akt and p-Akt, western blot analysis of the total proteins
extracted from MCF-7 cells was performed. It was observed that the
expression of Mfn2 protein was at a relatively high level in cells
cultured with 10% FBS. The cells pretreated with 10−6
mol/l E2 for 48 h demonstrated a 22.54% decrease in the protein
expression level of Mfn2 (Fig. 1A and
B). These findings demonstrated that E2 decreases the Mfn2
protein expression level. Notably, the protein expression of p-Akt
was elevated following culture with E2. However, there was no
significant change in the Akt protein expression level in our
experiment (Fig. 1A and B).
Mfn2 mediates E2-induced MCF-7 cell
proliferation
Plasmid transfection technology was used to
upregulate the expression of Mfn2, since it is a powerful
technology that allows the augmentation of cellular genes with
great specificity and potency. To assess the efficiency and
specificity of plasmid transfection, the expression of Mfn2 was
measured relative to that of β-actin by western blot analysis
(Fig. 2A and B). The empty pEGFR-N1
vector, as a green fluorescence-tagged negative control, also
demonstrated the efficiency of transfection (data not shown). As
shown in Fig. 3A and B, normal
cultured MCF-7 cells exhibited standard expression levels of Mfn2.
However, untransfected MCF-7 cells stimulated with 10−6
mol/l E2 and control vector-transfected cells stimulated with
10−6 mol/l demonstrated a notable decrease in Mfn2
expression. In comparison with MCF-7 cells transfected with control
vector, the Mfn2 levels were increased 1.89-fold in cells
transfected with the specific Mfn2 expression vector.
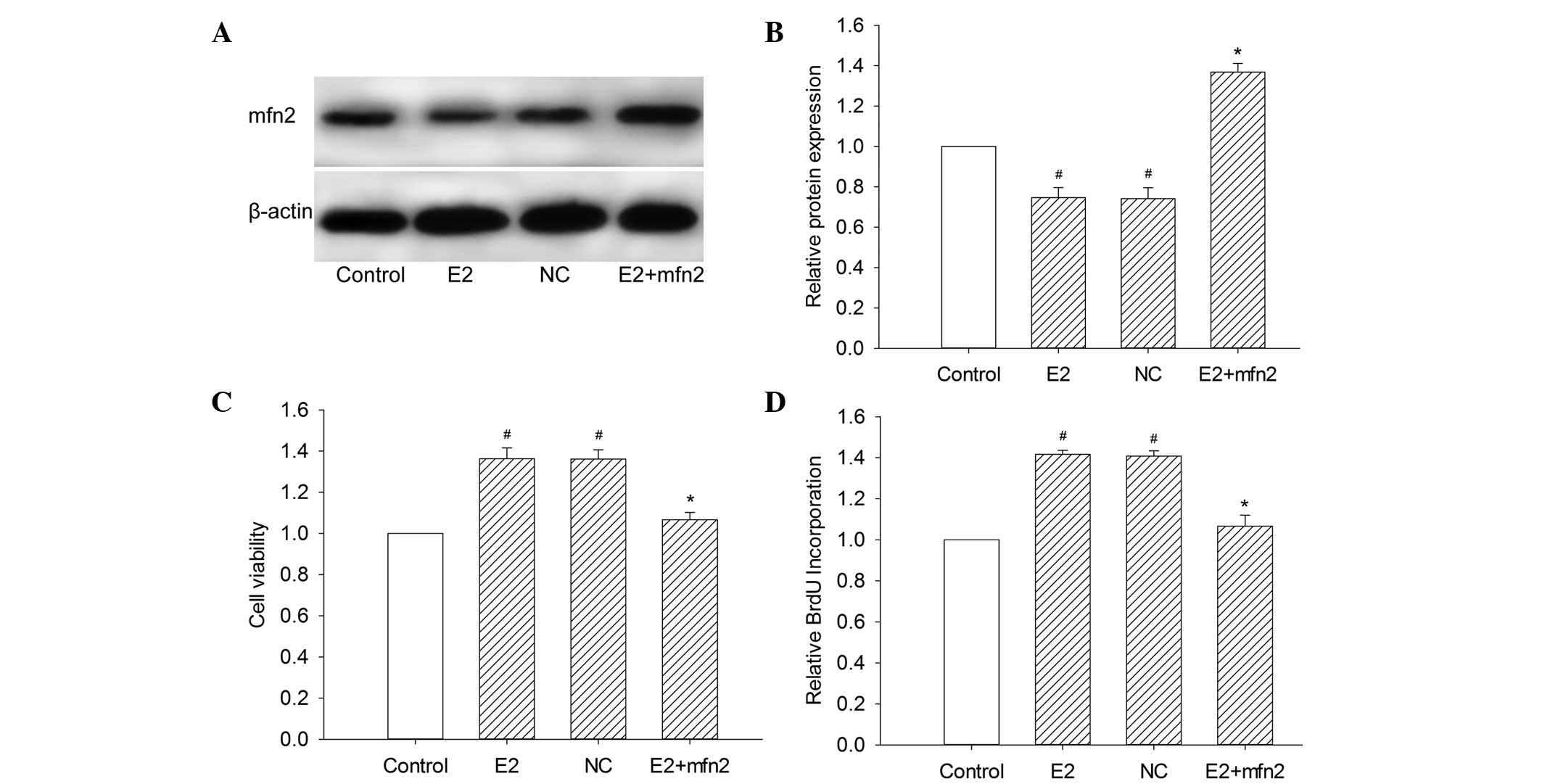
To investigate the role of Mfn2 on MCF-7 cell
proliferation, the cell viability was examined by measuring the
colorimetric conversion of MTT to formazan. The augmentation of
Mfn2 with a specific plasmid decreased the cell viability in the
presence of E2 compared with cells transfected with the control
vector (Fig. 2C). We also examined
the population of cells that were actively synthesizing DNA by
measuring the incorporation of BrdU. We observed that pEGFP-Mfn2
significantly suppressed BrdU incorporation and inhibited cell
proliferation (Fig. 2D). We further
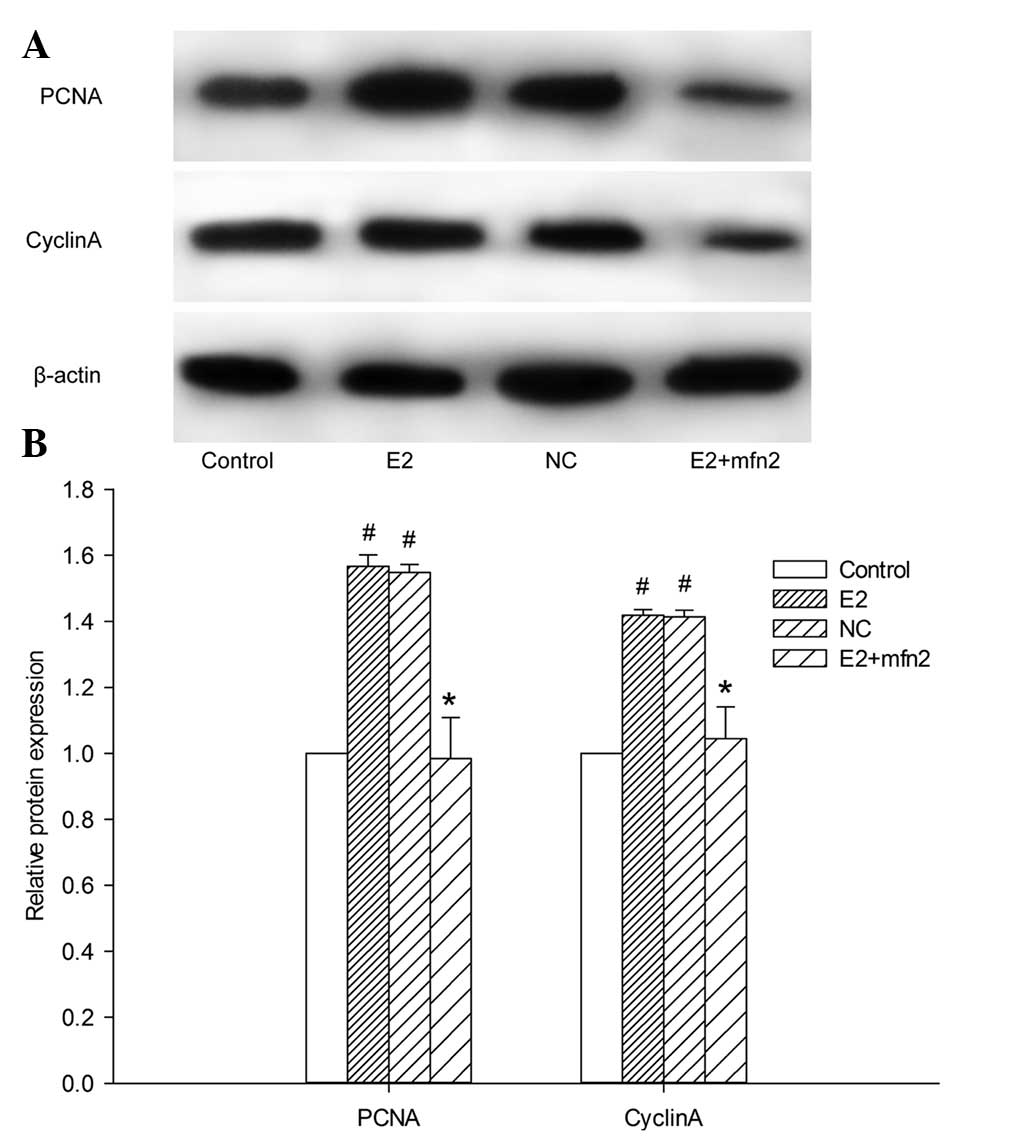
explored the role of Mfn2 in the E2-induced proliferation of MCF-7
cells by assaying the expression of proliferating cell nuclear
antigen (PCNA). The results revealed that, in comparison with cells
transfected with the control vector, PCNA expression in MCF-7 cells
was downregulated in the presence of E2 after the cells were
treated with pEGFP-Mfn2 (Fig. 3A and
B). The results indicated the negative role of Mfn2 in the
E2-mediated proliferation of MCF-7 cells.
Effect of Mfn2 on cell cycle
progression
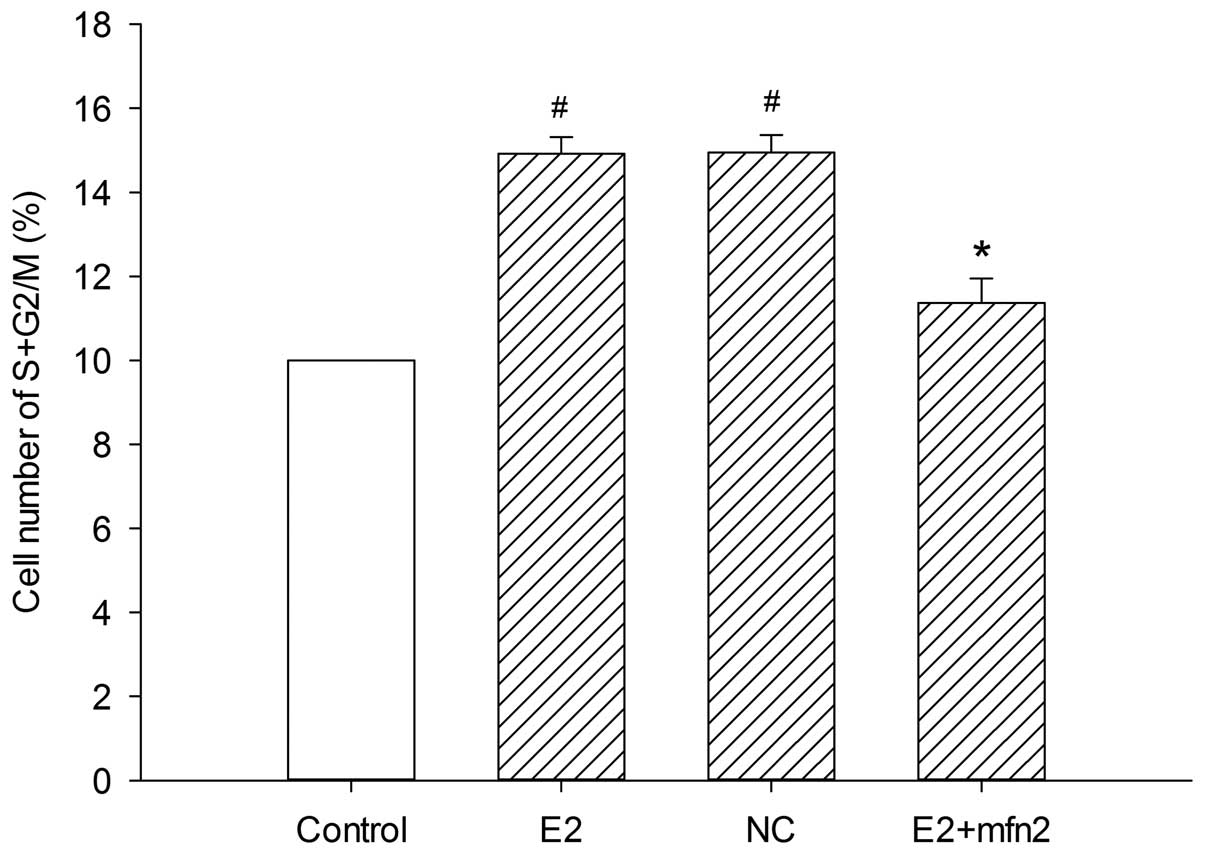
To further examine the mechanism underlying the cell
biological behavior in the presence of E2, we analyzed whether Mfn2
affects cell cycle progression. The number of cells in the various
cell cycle phases was counted by flow cytometry assay. It was
observed that E2 induced more cells to enter the S and G2/M phases
from the G0/G1 phase; in addition, pEGFP-Mfn2 suppressed cell cycle
progression and arrested MCF-7 cells at the G0/G1 phase (Fig. 4). Since cyclin A, a
proliferation-related protein, plays a significant role in the S
and G2/M phases, we analyzed the expression of cyclin A in MCF-7
cells. The results revealed that cyclin A protein expression was
decreased after the cells were treated with pEGFP-Mfn2 (Fig. 3A and B). These results suggested that
Mfn2 had a significant effect on cell cycle activity, which
inhibited MCF-7 cell proliferation.
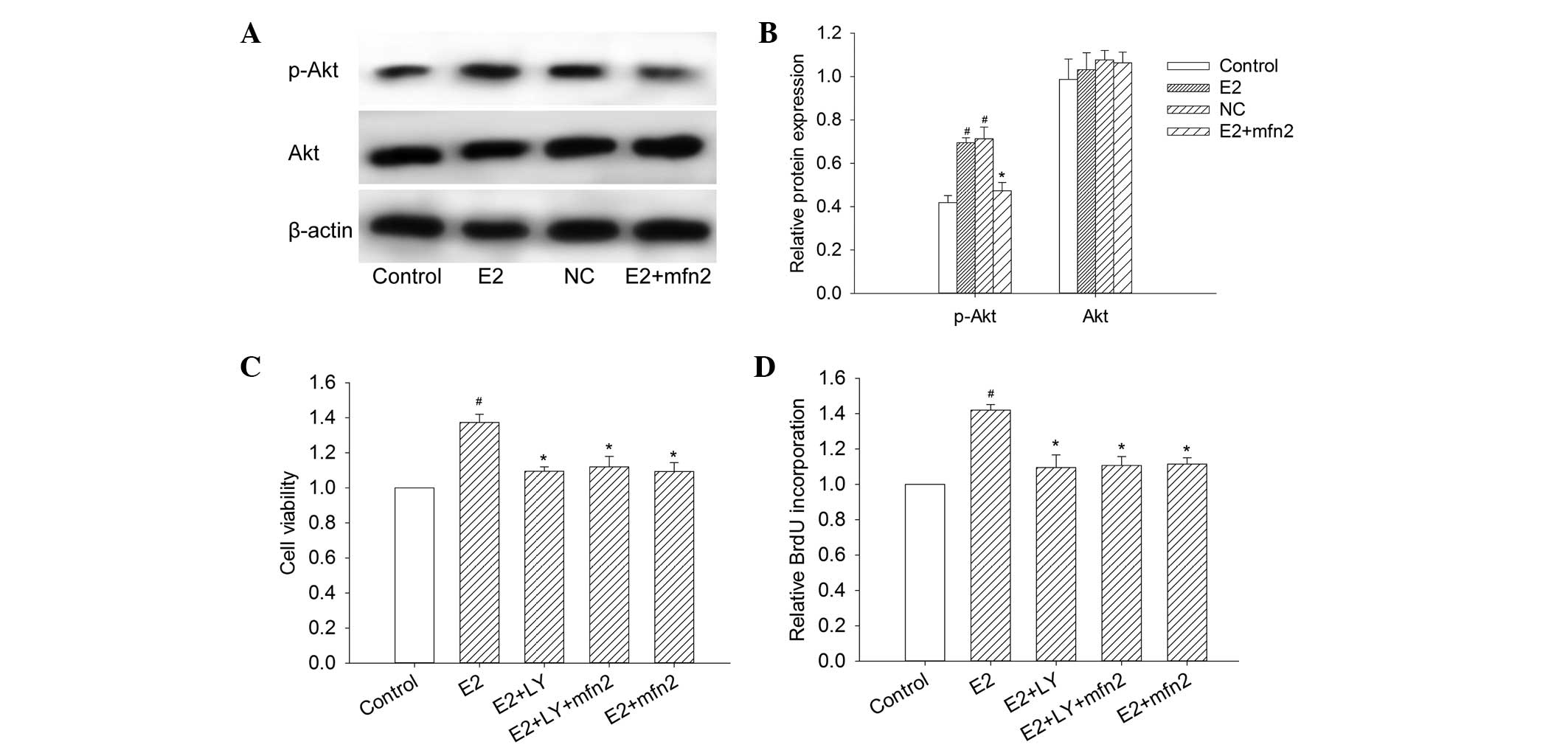
Mfn2 decreases Akt activity in MCF-7
cells
To determine whether the Mfn2-suppressed cell
proliferation was mediated by the activation of Akt, the Akt
activity and the expression of p-Akt at Ser473 were studied in
MCF-7 cells to determine the correlation between Mfn2 and the Akt
pathway. We observed a significant decrease in p-Akt 48 h following
the transfection of pEGFP-Mfn2 into MCF-7 cells (Fig. 5A and B). However, the change in Akt
protein expression was not notable. The results revealed that Mfn2
regulated p-Akt expression and that the Akt pathway was a
downstream target of Mfn2.
Mfn2 suppresses proliferation and
induces apoptosis in MCF-7 cells via the PI3K/Akt signaling
pathway
To examine the effects of Mfn2 and the PI3K/Akt
signaling pathway on cell viability, MCF-7 cells were transfected
with pEGFP-Mfn2 and stimulated with LY294002 (20 µM), a PI3K
inhibitor. The cell viability was determined by measuring MTT. As
shown in Fig. 5C, the Akt inhibitor
suppressed the cell viability as with MCF-7 cells transfected with
pEGFP-Mfn2. However, the cell viability was not further suppressed
in MCF-7 cells transfected with pEGFP-Mfn2 and treated with
LY294002, compared with the MCF-7 cells transfected with pEGFP-Mfn2
or treated with LY294002. The results also demonstrated that E2
increased the incorporation of BrdU and that this increase was
significantly suppressed by LY294002, whereas the cells transfected
with pEGFP-Mfn2 and treated with LY294002 exhibited almost no
change compared with the cells transfected with pEGFP-Mfn2 or
treated with LY294002 alone (Fig.
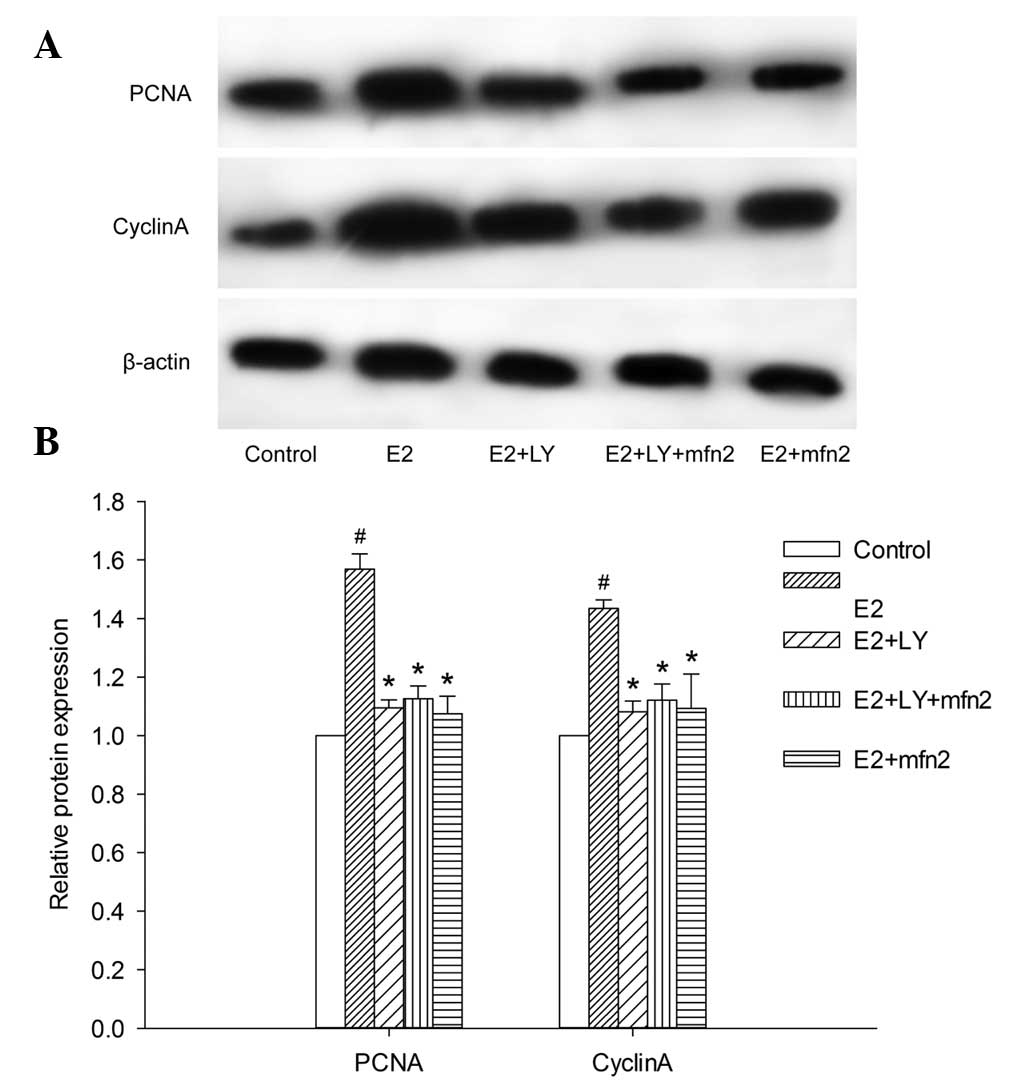
5D). To elucidate whether the PI3K/Akt pathway participates in
the pEGFP-Mfn2-induced downregulation of PCNA expression, we
blocked Akt with LY294002 and obtained results similar to those
obtained with the BrdU incorporation assay. The PCNA expression in
the cells transfected with pEGFP-Mfn2 and treated with LY294002
exhibited almost no change compared with the MCF-7 cells
transfected with pEGFP-Mfn2 or treated with LY294002 alone
(Fig. 6A and B).
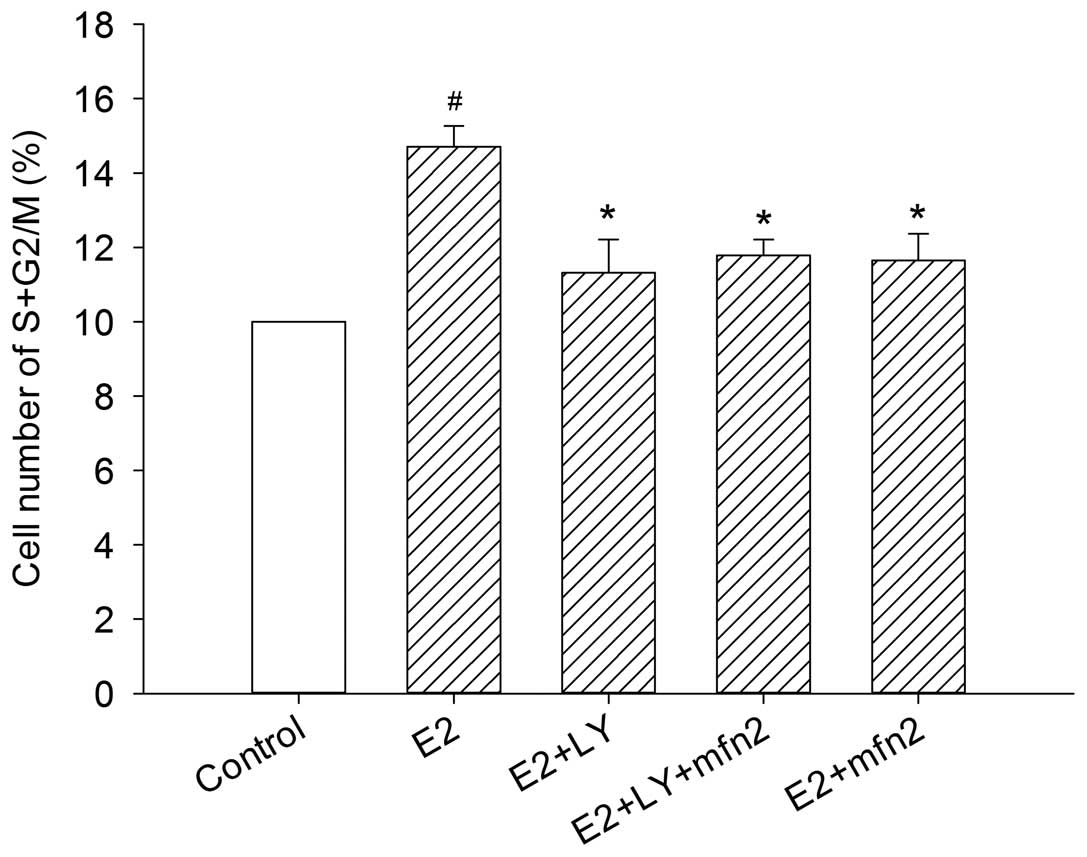
Mfn2 suppresses cell cycle progression
via the PI3K/Akt signaling pathway in MCF-7 cells
To further investigate whether the Akt pathway
participates in Mfn2-mediated cell cycle activity, we blocked Akt
with LY294002. In the presence of E2, the cells entered the S and
G2/M phases from the G0/G1 phase. However, the effect was abrogated
in the cells transfected with pEGFP-Mfn2 and in the cells in which
the Akt pathway was blocked by LY294002 alone, even in the presence
of E2. Notably, although the number of MCF-7 cells that progressed
into the S and G2/M phase of the cell cycle was suppressed
following transfection with pEGFP-Mfn2 or treatment with LY294002
alone, no major changes were observed in the cells after blocking
the Akt pathway with LY294002 and treatment with pEGFP-Mfn2
(Fig. 7). To further understand the
role of Akt in cell cycle progression, the expression of cyclin A
was examined in MCF-7 cells. The expression of cyclin A was
suppressed in the cells transfected with pEGFP-Mfn2 and in the
cells in which the Akt pathway was blocked with LY294002, and the
same results were detected in the cells in which the Akt pathway
was blocked with LY294002 and treated with pEGFP-Mfn2 (Fig. 6A and B). These findings suggested that
Mfn2 suppressed cell cycle progression via the PI3K/Akt signaling
pathway in MCF-7 cells.
Discussion
The specific mechanisms of breast carcinogenesis are
unclear, although estrogen and its receptors (ERα and ERβ) have
been considered essential factors for a long time. ERα was
established due to its function in the development and progression
of breast cancer, whereas the function of ERβ is unclear and
requires further studies. ERβ may inhibit estradiol-induced
proliferation and migration of MCF-7 cells through the regulation
of Mfn2, as demonstrated in our previous study (12). Thus, the penetration study of Mfn2 may
help us understand the exact role of ERβ in breast carcinogenesis.
In addition, dysregulation of the balance between proliferation and
apoptosis is essential in human carcinogenesis (14). The two main pathways involved in
apoptosis are the death receptor (extrinsic) pathway and the
mitochondrial (intrinsic) pathway (15). Mitochondria are the core organelles of
apoptosis, even in the extrinsic pathway. Thus, studies of the
exact functional mechanism of Mfn2, which has been confirmed to
exert a potential apoptotic effect via the mitochondrial apoptotic
pathway (5), are essential. In the
present study, we investigated Mfn2, which has been widely studied
in Charcot-Marie-Tooth disease (16).
Mfn2 is known to be correlated with antitumor activity in a number
of malignancies (5–7); however, its effect in breast cancers has
not been previously reported. The present study confirmed this
association in breast cancer cell lines, and we identified that the
pro-apoptotic and anti-proliferative effects of Mfn2 in breast
cancer cells occur via PI3K/Akt signaling.
The Mfn2 gene is located on the 1p36.22 chromosome
in humans. This chromosome region has been extensively studied, and
is considered to contain a number of tumor suppressor genes
(17). Mfn2, which controls
mitochondrial fusion, is a highly conserved GTPase (18). Similar proteins have been identified
in fruit flies and mammals (19).
Mfn2 possesses two trans-membrane domains which span the outer
mitochondrial membrane, a possible protein kinase A or G
phosphorylation site and a p21 (Ras) signature motif (amino acids
77–92), which plays an essential role in signaling (4,18)
In our previous study, we suggested that E2 induces
the proliferation of MCF-7 cells by downregulating the expression
of Mfn2 (12). In the present study,
we confirmed this finding. Notably, accompanied with the
downregulation of Mfn2, the protein expression of p-Akt was
elevated in the presence of E2. However, no significant change in
Akt protein expression was noted in our experiment. We also
demonstrated a negative role of Mfn2 in mediating the proliferation
of MCF-7 cells through the MTT proliferation assay, BrdU assay and
the detection of PCNA protein expression following transfection of
pEGFP-Mfn2, which specifically mediates Mfn2 overexpression. Taken
together, the data indicate that Mfn2 suppresses proliferation
through regulation of the PI3K/Akt signaling pathway.
Cell cycle retardation is a notable
anti-proliferation mechanism in cancer. In the present study, the
cell cycle distribution was analyzed 48 h after transfection with
pEGFP-Mfn2 and cells were observed to be accumulating at the G0/G1
phase. Cyclin A, a proliferation-related protein, plays a crucial
role in the S and G2/M phases. In this study, we observed that
cyclin A was significantly decreased in MCF-7 cells 48 h after
transfection with pEGFP-Mfn2 compared with cells transfected with
the control vector. All of these data indicate that Mfn2 blocks
cell cycle progression in the process of MCF-7 cell
proliferation.
Previous evidence has indicated that PI3K/Akt
signaling plays an essential role in numerous pathophysiological
events, including diabetes mellitus, neurodegenerative disease and
muscle hypotrophy (20). Akt is known
to regulate cell growth and survival (21). Zhang et al (13) previously reported that Mfn2 mediates
the proliferation of pulmonary artery smooth muscle cells via the
PI3K/Akt signaling pathway. Although there have been a number of
studies on the PI3K/Akt pathway and breast cancer in recent years
(21–23), none of these studies have demonstrated
that the PI3K/Akt signaling pathway is downstream of Mfn2. Our data
suggests that Mfn2 decreased Akt activity in the presence of E2,
and that Akt is downstream of Mfn2. LY294002 (an Akt inhibitor) was
employed to determine whether the PI3K/Akt pathway was involved in
Mfn2-decreased MCF-7 cell proliferation. The results revealed that
the expression of PCNA and cyclin A is suppressed in MCF-7 cells
following transfection with the pEGFP-Mfn2 plasmid and in cells in
which the Akt pathway is blocked with LY294002. The same results
were noted in the cells in which the Akt pathway was blocked with
LY294002 and treated with the pEGFP-Mfn2 plasmid. Similar results
were observed with the flow cytometry assay, the BrdU incorporation
assay and the MTT proliferation assay. The evidence suggests that
Mfn2 prevents cell cycle progression via the PI3K/Akt signaling
pathway in MCF-7 cells. The exact mechanisms underlying the
interaction between Mfn2 and the PI3K/Akt signaling pathway are
unclear. Mfn2 possesses two trans-membrane domains spanning the
outer mitochondrial membrane, and one of these domains is a p21
(Ras) signature motif (amino acids 77–92) (4,18). A
number of studies have suggested that Ras may act as an upstream
signaling pathway of PI3K/Akt in cancer carcinogenesis and
development (24–26). These studies partly explain our
findings. Further experimental studies, including in-depth promoter
analysis and chromatin immunoprecipitation, are required.
In conclusion, this study provides experimental
evidence confirming the role of Mfn2 as a tumor suppressor gene in
breast cancer. The Mfn2 gene significantly promotes apoptosis and
inhibits the proliferation of breast cancer cells, and Mfn2 may
induce apoptosis in breast cancer cells via the PI3K/Akt pathway.
These observations highlight a previously unexplored role of Mfn2
in cancer development, revealing Mfn2 as a potential therapeutic
target for the treatment of tumors and hyper-proliferative
diseases.
Acknowledgements
This study was supported by the Hebei Province
Natural Science Foundation of China (grant no. H2015206230).
References
|
1
|
Chen G, Liu N, Zhou A, Tang C, Ma D and
Tang J: The role of hypertension-related gene in aortic vascular
smooth muscle cells from mice and rats. Chin Med J (Engl).
114:833–836. 2001.PubMed/NCBI
|
|
2
|
Guo X, Chen KH, Guo Y, et al: Mitofusin 2
triggers vascular smooth muscle cell apoptosis via mitochondrial
death pathway. Circ Res. 101:1113–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karbowski M, Lee YJ, Gaume B, Jeong SY,
Frank S, Nechushtan A, Santel A, Fuller M, Smith CL and Youle RJ:
Spatial and temporal association of Bax with mitochondrial fission
sites, Drp1 and Mfn2 during apoptosis. J Cell Biol. 159:931–938.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D,
Li P, Qiu X, Wen S, Xiao RP and Tang J: Dysregulation of HSG
triggers vascular proliferative disorders. Nat Cell Biol.
6:872–883. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Lu J, Zhu F, Wei J, Jia C, Zhang
Y, Zhou L, Xie H and Zheng S: Pro-apoptotic and anti-proliferative
effects of mitofusin-2 via Bax signaling in hepatocellular
carcinoma cells. Med Oncol. 29:70–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin B, Fu G, Pan H, Cheng X, Zhou L, Lv J,
Chen G and Zheng S: Anti-tumour efficacy of mitofusin-2 in urinary
bladder carcinoma. Med Oncol. 28(Suppl 1): S373–S380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang GE, Jin HL, Lin XK, Chen C, Liu XS,
Zhang Q and Yu JR: Anti-tumor effects of mfn2 in gastric cancer.
Int J Mol Sci. 14:13005–13021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacGregor JI and Jordan VC: Basic guide to
the mechanisms of antiestrogen action. Pharmacol Rev. 50:151–196.
1998.PubMed/NCBI
|
|
9
|
Sommer S and Fuqua SA: Estrogen receptor
and breast cancer. Semin Cancer Biol. 11:339–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nilsson S, Mäkelä S, Treuter E, Tujague M,
Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M and
Gustafsson JA: Mechanisms of estrogen action. Physiol Rev.
81:1535–1565. 2001.PubMed/NCBI
|
|
11
|
Speirs V, Carder PJ, Lane S, Dodwell D,
Lansdown MR and Hanby AM: Oestrogen receptor beta: what it means
for patients with breast cancer. Lancet Oncol. 5:174–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Liu Y, Geng C, Qi X and Jiang J:
Estrogen receptor β inhibits estradiol-induced proliferation and
migration of MCF-7 cells through regulation of mitofusin 2. Int J
Oncol. 42:1993–2000. 2013.PubMed/NCBI
|
|
13
|
Zhang D, Ma C, Li S, Ran Y, Chen J, Lu P,
Shi S and Zhu D: Effect of Mitofusin 2 on smooth muscle cells
proliferation in hypoxic pulmonary hypertension. Microvasc Res.
84:286–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fabregat I, Roncero C and Fernandez M:
Survival and apoptosis: A dysregulated balance in liver cancer.
Liver Int. 27:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guicciardi ME and Gores GJ: Apoptosis: A
mechanism of acute and chronic liver injury. Gut. 54:1024–1033.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Braathen GJ, Sand JC, Lobato A, Høyer H
and Russell MB: Mfn2 point mutations occur in 3.4% of
Charcot-Marie-Tooth families. An investigation of 232 Norwegian CMT
families. BMC Med Genet. 11:482010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bagchi A and Mills AA: The quest for the
1p36 tumor suppressor. Cancer Res. 68:2551–2556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Brito OM and Scorrano L: Mitofusin 2: A
mitochondria-shaping protein with signaling roles beyond fusion.
Antioxid Redox Signal. 10:621–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Westermann B: Molecular machinery of
mitochondrial fusion and fission. J Biol Chem. 283:13501–13505.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirai K, Hayashi T, Chan PH, Zeng J, Yang
GY, Basus VJ, James TL and Litt L: PI3K inhibition in neonatal rat
brain slices during and after hypoxia reduces phospho-Akt and
increases cytosolic cytochrome c and apoptosis. Brain Res Mol Brain
Res. 124:51–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yi YW, Hong W, Kang HJ, Kim HJ, Zhao W,
Wang A, Seong YS and Bae I: Inhibition of the PI3K/AKT pathway
potentiates cytotoxicity of EGFR kinase inhibitors in
triple-negative breast cancer cells. J Cell Mol Med. 17:648–656.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pande S, Browne G, Padmanabhan S, Zaidi
SK, Lian JB, van Wijnen AJ, Stein JL and Stein GS: Oncogenic
cooperation between PI3K/Akt signaling and transcription factor
Runx2 promotes the invasive properties of metastatic breast cancer
cells. J Cell Physiol. 228:1784–1792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calleros L, Sánchez-Hernández I, Baquero
P, Toro MJ and Chiloeches A: Oncogenic Ras, but not (V600E) B-RAF,
protects from cholesterol depletion-induced apoptosis through the
PI3K/AKT pathway in colorectal cancer cells. Carcinogenesis.
30:1670–1677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signaling pathways: role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16(Suppl 2):
S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsubaki M, Satou T, Itoh T, Imano M, Ogaki
M, Yanae M and Nishida S: Reduction of metastasis, cell invasion
and adhesion in mouse osteosarcoma by YM529/ONO-5920-induced
blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathway. Toxicol Appl
Pharmacol. 259:402–410. 2012. View Article : Google Scholar : PubMed/NCBI
|