Introduction
Dendritic cells (DCs) are antigen-presenting cells
that are important in the initiation and regulation of immune
responses (1–3). DCs present antigenic peptides to
initiate primary T-cell responses, and additionally, DCs express
costimulatory molecules that drive quiescent T cells into the cell
cycle, promoting their differentiation (3,4). Previous
studies have demonstrated the expression levels of endothelial
(CD31, vWF and CD144) and dendritic precursor (CD205) cell surface
markers and the antigen-presenting ability of DCs decrease
significantly following their infiltration of tumors (5–8). However,
the mechanisms behind these observations remain to be
elucidated.
It has been reported previously that conditioned
medium from murine Lewis lung carcinoma cells redirects the
differentiation of CD34+ progenitor cells away from a DC
pathway to an endothelial cell (ECs) fate (9). In addition, DC precursors can
transdifferentiate into endothelial-like cells (ELCs) in mouse and
human ovarian carcinomas following the addition of vascular
endothelial growth factor-A (VEGF-A) and β-defensins (10). Furthermore, tumor-associated DCs
incubated with the pro-angiogenic factors VEGF-A and oncostain M
can transdifferentiate into ELCs, and this is suggested as an
alternative pathway of tumor angiogenesis (11). Additional reports have demonstrated
that DC progenitors or immature DCs (iDCs) have the ability to
transdifferentiate into ELCs, potentially contributing to
vasculogenesis in adult tissues. Therefore, DCs may be crucial to
the neovascularization process in a number of physiopathological
conditions (12,13).
STAT3 (signal transducer and activator of
transcription 3) is activated by JAK (janus tyrosine
kinase)-mediated tyrosine phosphorylation following receptor-ligand
binding. The JAK/STAT3 signaling pathway regulates cell growth,
proliferation, differentiation and apoptosis, and is important in
the signal transduction of cytokines and growth factors (14,15).
However, the function of the JAK/STAT3 signaling pathway on
endothelial-like differentiation (ELD) remains to be
elucidated.
Esophageal cancer (EC) is the sixth leading cause of
cancer-associated mortality and the eighth most frequently
diagnosed cancer worldwide (16).
China has one of the highest incidences of esophageal cancer, with
an estimate of >220,000 new detected cases and 200,000
mortalities every year (17). The
predominant form of esophageal cancer is esophageal squamous cell
carcinoma (ESCC), characterized by a poor prognosis and high
invasiveness (18). It has been
reported previously that tumor-associated factors derived from
homogenates of EC9706 human ESCC cells may induce iDCs to
differentiate into ELCs (19).
However, the impact of different tumor-differentiated degree ESCC
on the ELD of iDCs is unclear, and the function of JAK/STAT3 signal
in this process is unknown. In the present study, we investigated
the effect on ELD of iDCs using cell culture supernatant obtained
from the KYSE450 (high differentiation) and KYSE70 (poor
differentiation) ESCC cell line, and demonstrated the role of
JAK/STAT3 signal pathway therein.
Materials and methods
Preparation of KYSE450 and KYSE70 cell
line supernatant
The KYSE450 and KYSE70 ESCC cell lines (Nanjing
KeyGEN Biotech. Co., Ltd. (Nanjing, China) were cultured in
RPMI-1640 medium supplemented with 10% fetal calf serum. The cells
were replenished with fresh medium at 60–80% confluency. The cells
were used at passage 6–9. The supernatant was collected and
filtered following 24 h of incubation, and stored at −20°C.
Induction of ELCs from immature
DCs
Peripheral blood mononuclear cells (PBMCs) were
harvested from healthy adult volunteers (who provided written
informed consent) and isolated using density gradient
centrifugation with Ficoll-Paque. The purified cells were seeded in
12-well plates (11). Adherent cells
(monocytes) were induced towards a DC fate using rhGM-CSF (100
ng/ml; Amoytop Biotech, Xiamen, China) and rhIL-4, (5 ng/ml;
PeproTech China, Suzhou, China). 40% KYSE450 supernatant or 40%
KYSE70 supernatant and 60% DCs medium was added at the end of day
2. Following 7 days of induction (day 9), the cells were harvested
for use in experiments. As for controls, adherent cells (monocytes)
were similarly induced towards DC fate using rhGM-CSF and rhIL-4.
They were mature DCs when harvested for use in experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was extracted with Trizol
(Invitrogen), and converted to cDNA according to the protocol of
the one-step RT-PCR kit (TaKaRa). The sequences for the
oligo-nucleotide primer pairs are as follows: GAPDH, F 5′-GAA GGC
TGG GGC TCA TTT-3′ and R 5′-GAG GAG GCA TTG CTG ATG AT-3′; VEGF, F
5′-CCT CCG AAA CCA TGA ACT TT-3′ and R 5′-CCA CCT CGA TGA TTC
TGC-3′; IL6, F 5′-TAG TGA GGA ACA AGC CAG AGC-3′ and R 5′-TGG CAT
TTG TGG TTG GGT CA-3′; JAK2, F 5′-AGA ATG TCT TGG GAT GGC AG-3′ and
R 5′-TGA TAG TCT TGG ATC TTT GCT CG-3′; STAT3, F 5′-TGC TTC CCT GAT
TGT GAC TG-3′ and R 5′-CTG ACA GAT GTT GGA GAT CACC-3′; CD144, F
5′-AAA CAC CTC ACT TCC CCA TC-3′ and R 5′-ACC TTG CCC ACA TAT TCT
CC-3′; and vWF, F 5′-ATG AGT ATG AGT GTG CCT GC-3′ and R 5′-GTA GAT
GGT GCT TCG GTGG-3′. The PCR conditions used were: 95°C for 10 min,
40 × (95°C for 10 sec + 60°C for 30 sec). Each sample was run in
triplicate. Analysis of the RT-qPCR data was performed using
Applied Biosystems 7500 Fast Real-Time PCR System, v2.0.5 (Applied
Biosystems Life Technologies, Foster City, CA, USA).
Immunofluorescence
Control DCs, the KYSE450 cell group (induced by
KYSE450 cell supernatant) and the KYSE70 cell group (induced by
KYSE70 cell supernatant) were seeded in 12-well plates and
incubated for 24 h, then fixed with 4% paraformaldehyde for 30 min.
Antibodies were diluted in BSA-PBS (cat no. A8010; Solarbio,
Beijing, China). A rabbit anti-human von Willebrand factor (vWF)
antibody (1:100; cat no. sc-73268; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and a Cy3-conjugated goat anti-rabbit IgG
secondary antibody were used to detect vWF expression and
localization in the three cell groups. To visualize cluster of
differentiation 144 (CD144), a rabbit anti-human CD144 antibody
(1:200; cat no. 2500; CST Biological Reagents Company Ltd.,
Shanghai, China) and a 555-conjugated goat anti-rabbit secondary
antibody were used. The anti-p-JAK2 (Tyr 1007/Tyr 1008) primary
antibody (1:100; cat no. cc-16566-R; Santa Cruz Biotechnology,
Inc.) and a 555-conjugated goat anti-rabbit secondary antibody (cat
no. P0178-1; Beyotime Institute of Biotechnology, Shanghai, China)
were used to detect phosphorylated JAK2. The p-STAT3 (B-7) primary
antibody (1:100; cat no. sc-8059; Santa Cruz Biotechnology, Inc.)
and a 488-conjugated goat anti-mouse IgG antibody (cat no. P0188-1;
Beyotime Institute of Biotechnology) were used to detect
phosphorylated STAT3. Cells with fluorescent particles in the
cytoplasm were counted as positive expression.
Di1-labeled acetylated low-density
lipoprotein (LDL) uptake assay
Control DCs and the KYSE450 and KYSE70 cell groups
were seeded in 96-well plates. Following incubation for 36 h,
Di1-labeled acetylated-LDL (10 µg/ml; Biomedical Technologies,
Stoughton, MA, USA) was added to each well, and all the groups were
further incubated at 37°C for another 4 h. The medium containing
Dil-Ac-LDL was removed and all the cell samples were washed three
times with phosphate-buffered saline and then observed using a
IX2-IL100 fluorescence microscope (Olympus Corporation).
Statistical analysis
Data were presented as the mean ± standard deviation
(SD), taken from at least three separate experiments and analyzed
using one-way analysis of variance. A statistically significant
difference was defined as P<0.05.
Results
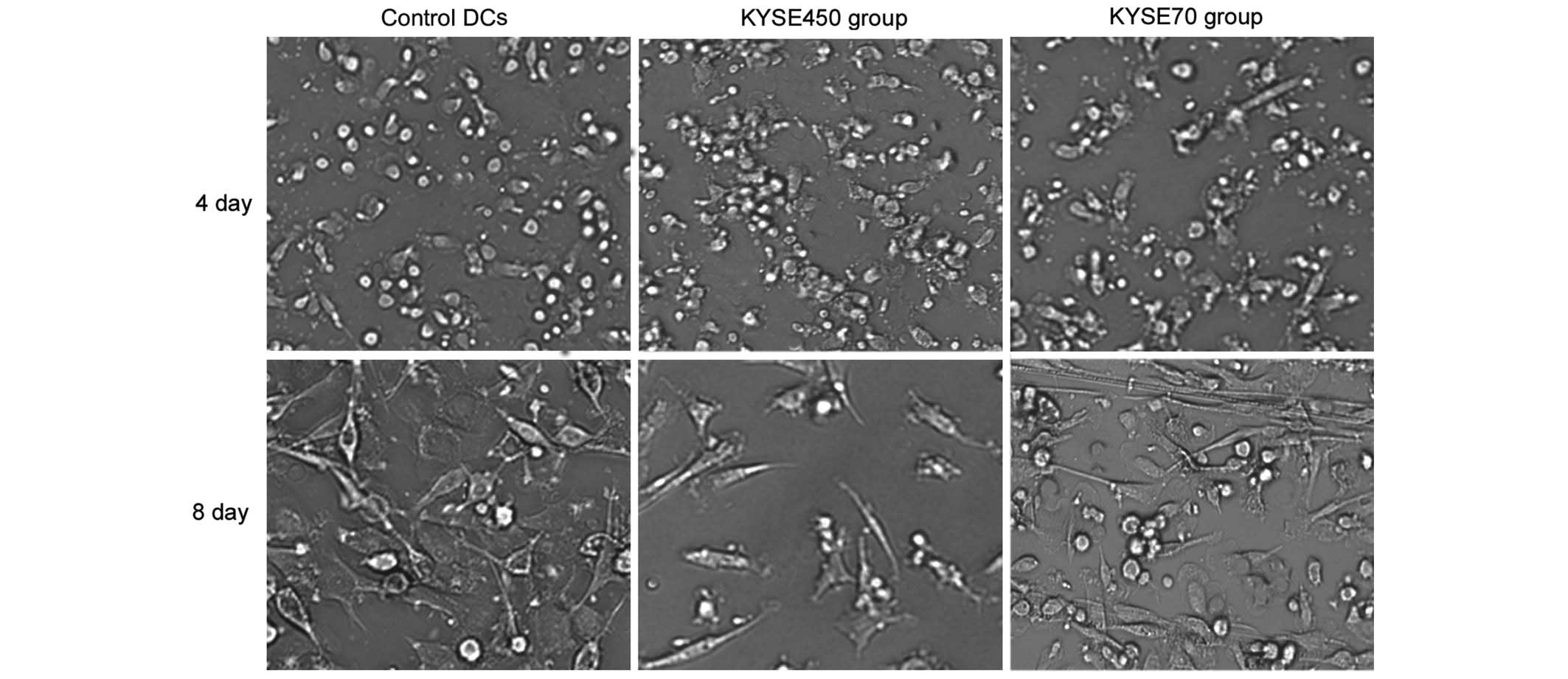
Changes in iDC morphology during the
ELD of iDCs, induced by KYSE450 and KYSE70 supernatant
Immature DCs induced by KYSE450 or KYSE70
supernatant for 4 days appeared round, with a similar form to
control DCs. However, following induction of 8 days, the KYSE450
group cells exhibited a fusiform shape, and a number of cells from
the KYSE70 group was arranged in cord-like structures, a typical
appearance of ECs (Fig. 1).
Protein and mRNA expression of the EC
markers vWF and CD144 were detected by immunofluorescence and
RT-qPCR
The protein expression levels of EC markers were
significantly increased in cells induced by KYSE450 or KYSE70
supernatant, compared with the control DCs (n=4, P<0.001). The
immunostaining intensity of the two EC markers was greater in the
poorly differentiated ESCC group (KYSE70) compared with the highly
differentiated group (KYSE450; Fig.
2A). RT-qPCR analysis demonstrated enhanced expression of vWF
and CD144 mRNA in the KYSE450 and KYSE70 groups compared to the
control DCs (n=3, P<0.01, P<0.001). Furthermore, the mRNA
expression levels of vWF and CD144 in the KYSE70 group were
enhanced significantly in comparison to the KYSE450 group (Fig. 2B; n=3, P<0.001). The uptake of
Dil-Ac-LDL is considered to be a characteristic function of ECs,
although this is performed by other cell types, such as macrophages
and monocytes (20,21). Previously, we demonstrated that PBMCs
had a weak uptake of Dil-Ac-LDL (19). By contrast, in the current study, the
cells induced by KYSE450 or KYSE70 supernatant exhibited a strong
uptake of Dil-Ac-LDL compared to the control DCs (n=4, P<0.001).
Additionally, the KYSE70 group cells demonstrated a stronger uptake
compared to the KYSE450 group (Fig.
2C; n=4, P<0.001). The above results demonstrated that iDCs
induced by KYSE450 and KYSE70 supernatant differentiate towards an
EC phenotype, and the KYSE70 supernatant induced a greater effect
during this transition.
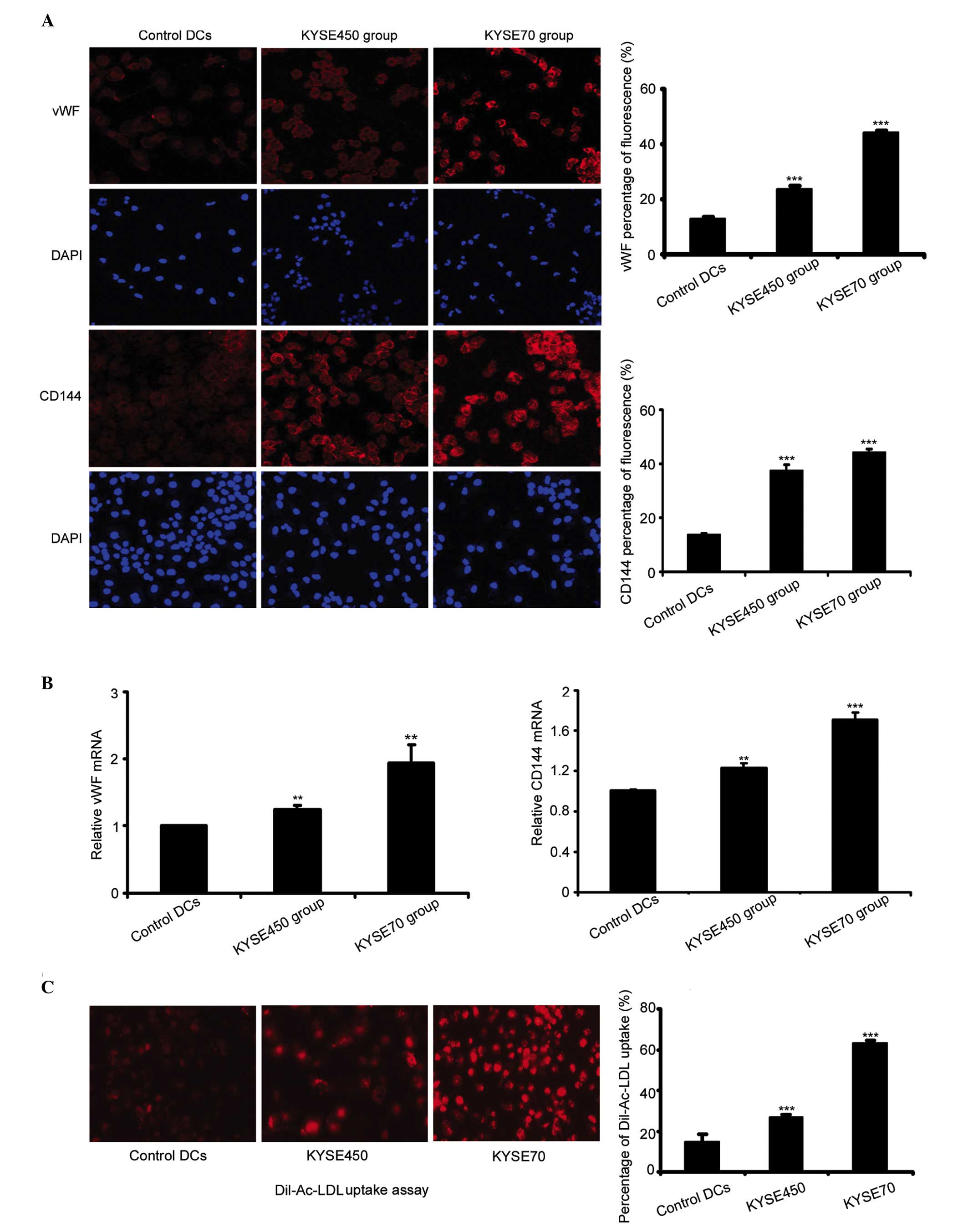 | Figure 2.Expression of endothelial cell markers
vWF and CD144 increased following induction by KYSE450 and KYSE70
supernatant. (A) Immunofluoresence was used to detect the
expression of vWF and CD144 in control DCs and the KYSE450 and
KYSE70 groups (magnification, ×200). Data are the mean ± standard
deviation (SD), n=4, ***P<0.001 vs. control DCs. (B) RT-qPCR
detection of mRNA levels of vWF and CD144 in control DCs and the
KYSE450 and KYSE70 groups. Data are the mean ± SD, n=3,
**P<0.01, ***P<0.001 vs. control DCs. (C) Dil-Ac-LDL uptake
was enhanced following induction by KYSE450 and KYSE70 supernatant
(magnification, ×200). Data are the mean ± SD, n=4, ***P<0.001
vs. control DCs. DCs, dendritic cells; vWF, von Willebrand factor;
CD144, cluster of differentiation 144; DiI-Ac-LDL, DiI-labeled
acetylated low-density lipoprotein; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
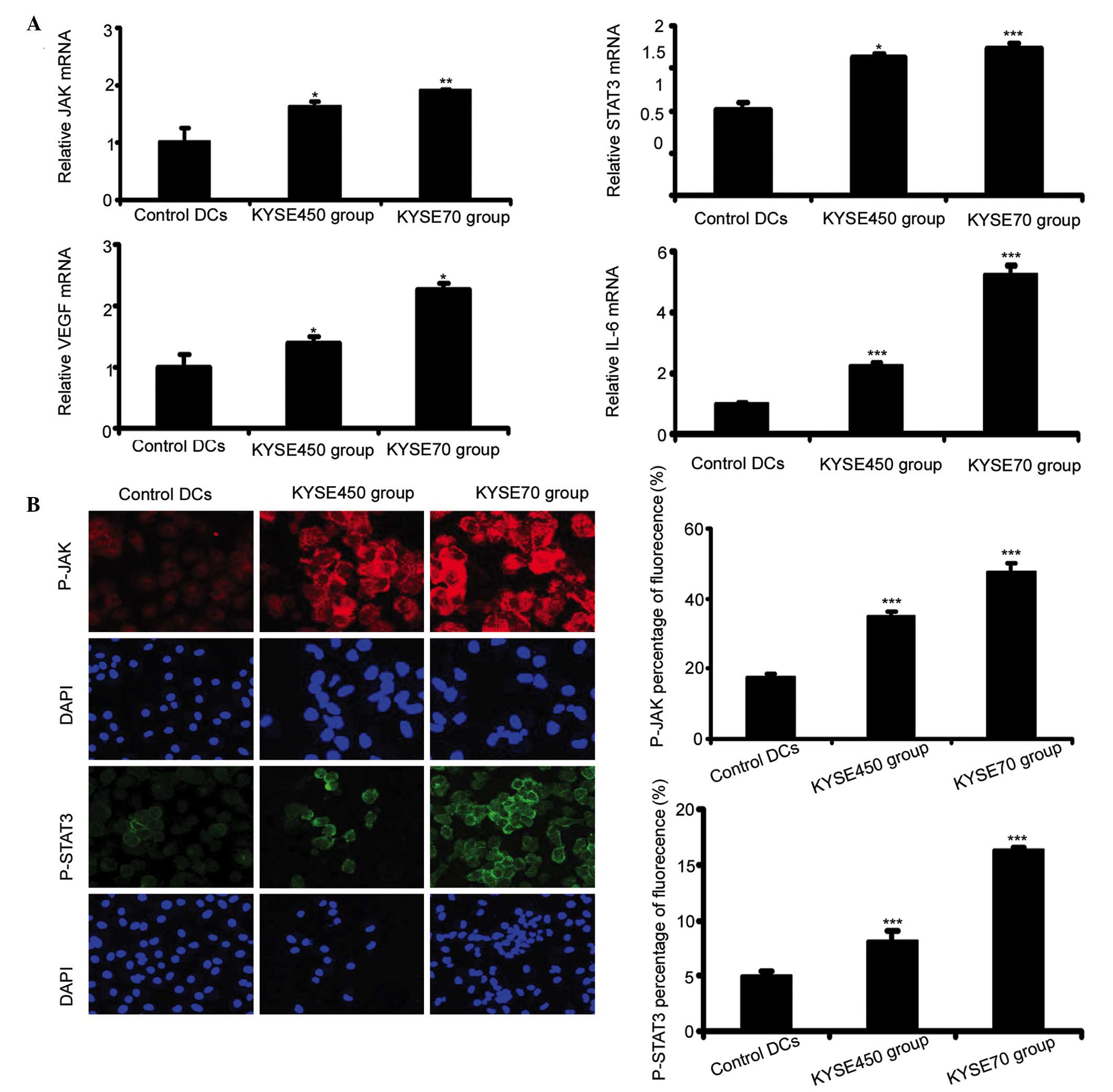
KYSE450 and KYSE70 supernatants induce
ELD and activate the JAK/STAT3 signaling pathway in iDCs
During the process of the ELD of iDC, JAK/STAT3
upregulation at the mRNA level was detected by RT-qPCR. The results
showed that the mRNA expression levels of JAK and STAT3 increased
in the KYSE450 and KYSE70 groups in comparison to the control DCs
(Fig. 3A; P<0.05, P<0.01,
P<0.001). Furthermore, the KYSE70 group exhibited slightly
increased mRNA levels of JAK and STAT3 compared with the KYSE450
group, however this difference was not statistically significant
(n=3, P=0.054, P=0.617, respectively). The mRNA expression levels
of VEGF-A and IL-6, cytokines downstream of JAK/STAT3 signaling
were determined. VEGF-A and IL-6 mRNA levels increased
significantly in the KYSE450 and KYSE70 groups compared to the
control DCs (P<0.01 for VEGF-A; P<0.001 for IL-6), with a
significantly higher level of expression in the KYSE70 group than
KYSE450 (Fig. 3A; P<0.01 for
VEGF-A; P<0.001 for IL-6). Immunocytochemical analysis
demonstrated that JAK and STAT3 were phosphorylated at higher
levels in the KYSE450 and KYSE70 groups compared with the control
DCs, and the fluorescence intensity in the KYSE70 group was
stronger than that in the KYSE450 group (Fig.3B; n=3; P<0.001).
Blocking JAK signaling in iDCs
inhibits ELD induced by KYSE450 and KYSE70 supernatants
AG490 (AG) is an inhibitor of JAK. To confirm
whether the JAK/STAT3 signaling pathway is involved in the ELD of
iDCs, we added 10 µmol AG490 to the KYSE450 and KYSE70
supernatant-treated cells at the end of day 2. Following 8 days of
incubation, the mRNA expression levels of vWF and CD144 had
significantly decreased in the KYSE450 + AG and KYSE70 + AG groups
(Fig. 4A; n=3, P<0.01,
P<0.001). Furthermore, the levels of these proteins were
significantly decreased, as demonstrated by a reduction in the
fluorescence intensity of CD144 and vWF in the KYSE450 + AG and
KYSE70+AG groups compared with the control KYSE450 group or KYSE70
group (Fig. 4B; n=3, P<0.001).
Additionally, Dil-Ac-LDL uptake was significantly reduced in the
KYSE450 and KYSE70 groups incubated with AG490 (Fig. 4C; n=4, P<0.001).
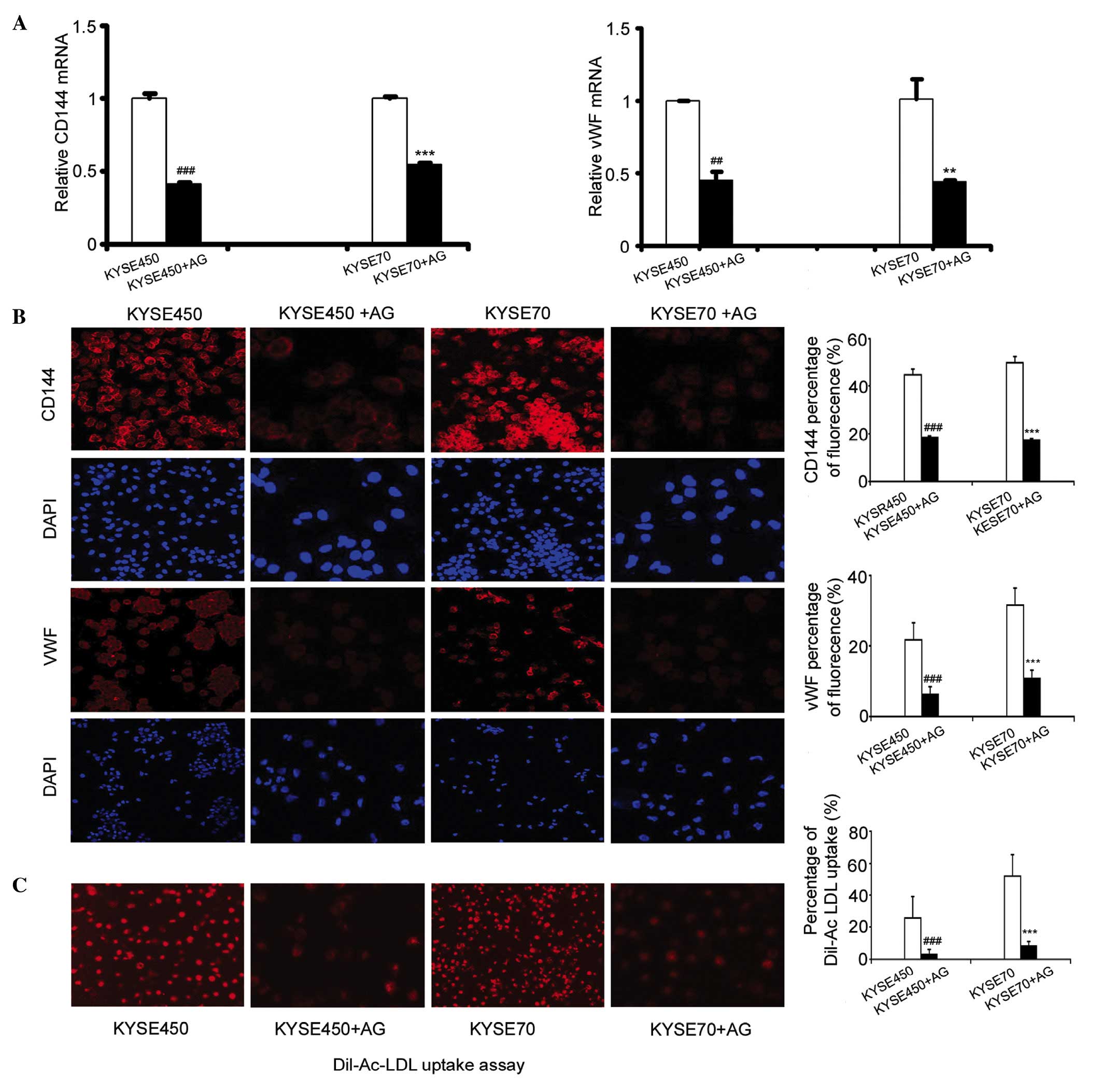 | Figure 4.Blocking the activation of JAK and
STAT3 inhibited endothelial-like differentiation of iDCs. (A)
Addition of 10 µmol AG490 at the end of day 2, decreased the
expression of vWF and CD144 in iDCs induced by KYSE450 and KYSE70
supernatant. RT-qPCR results shown are the mean ± SD (n=3).
**P<0.01, ***P<0.001 vs. KYSE450 group. **P<0.01,
***P<0.001 vs.KYSE70 group. (B) Immunofluorescence detection of
the protein expression of the EC markers CD144 and vWF in the
KYSE450, KYSE450 + AG, KYSE70, and KYSE70 + AG groups
(magnification, ×200). Data are the mean ± SD (n=3). ***P<0.001
vs. KYSE450 group. ***P<0.001 vs. KYSE70 group. (C) Dil-Ac-LDL
uptake decreased after induction by KYSE450 + AG490 and KYSE70 +
AG490. Original magnification, ×200. Results shown are the mean ±
SD (n=4). ***P<0.001 vs. KYSE450 group. ***P<0.001 vs. KYSE70
group. AG, AG490; iDCs, immature dendritic cells; vWF, von
Willebrand factor; CD144, cluster of differentiation 144;
DiI-Ac-LDL, DiI-labeled acetylated low-density lipoprotein;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
In summary, the results demonstrated the JAK/STAT3
signaling pathway promotes the ELD of iDCs induced by KYSE450 and
KYSE70 supernatant, and blocking JAK/STAT3 signaling inhibits the
ELD of iDCs.
Discussion
It has previously been reported that incubation of
tumor-associated DCs with VEGF and oncostatin M can direct
progenitor cell differentiation away from the DC pathway to an ELC
fate (11). Furthermore, the tumor
microenvironment can induce the ELD of iDCs, but not of mature DCs
(22–24). While these previous studies have
examined the effect of DC differentiation stage on the transition
to ELCs, to the best of our knowledge, there is no previous study
that addresses whether the degree of differentiation of tumor cells
can influence the ELD of iDCs. In the present study, the impact of
the microenvironment produced by ESCC cell line KYSE450 (highly
differentiated) and KYSE70 (poorly differentiated) supernatant on
the differentiation of iDCs derived from PBMCs, was investigated.
The results demonstrate that iDCs induced by the KYSE70 supernatant
appeared with fusiform shapes and were arranged into cord-like
structures in the KYSE70 and KYSE450 groups. Additionally, the
KYSE70- induced group expressed higher levels of the EC markers vWF
and CD144, and exhibited stronger Dil-Ac-LDL uptake in comparison
with the KYSE450-induced group. These results demonstrate that iDCs
can transdifferentiate into ELCs in an ESCC microenvironment, and
the supernatant from the poorly differentiated KYSE70 cells had a
greater effect on ELD than the supernatant from the highly
differentiated KYSE450 cells. This may be due to increased IL-6 and
VEGF protein expression in the KYSE70 group, a hypothesis that is
supported by our previous findings showing VEGF can induce the ELD
of iDCs (22,23). It is known that PBMCs are pluripotent
stem cells and can be induced to acquire macrophage, lymphocyte,
epithelial, endothelial, neuronal, and hepatocyte phenotypes in the
absence of fusion with pre-existing mature tissue cells (25). In the present study, iDCs retained
some features of PBMCs, thus, iDCs may differentiate into ELCs in
the ESCC microenvironment to varying degrees.
A large number of growth factors, cytokines and
vasoactive substances can induce cell differentiation through
cellular signal transduction. The JAK/STAT3 signaling pathway is
important in cell growth, differentiation, proliferation and
apoptosis, and involved in the abnormal differentiation of DCs
(26). Previous studies have
demonstrated that STAT3 is a key component of diverse signal
transduction pathways generated by a large number of cytokines and
growth factors, such as IL-6, epidermal growth factor and
platelet-derived growth factor. Furthermore, the receptors for
these ligands are associated with JAK (27). The results of the current study
revealed the JAK/STAT3 signaling pathway was activated during the
ELD of iDCs, and the cells in the KYSE70 group exhibited higher
levels of p-JAK and p-STAT3, compared with the cells in the KYSE450
group. AG490 is the inhibitor of the JAK/STAT3 signaling pathway,
and not only inhibited the activation of JAK signaling, but also
inhibited the differentiation of iDCs to ELCs.
In conclusion, the present study demonstrated that
iDCs may differentiate into ELCs in response to an ESCC
microenvironment, and the degree of differentiation of the ESCC
cells may affect the phenotype of the resulting ELC. Poorly
differentiated ESCCs had a greater effect on the ELD of iDCs than
highly differentiated ESCCs. Additionally, JAK/STAT3 signaling is
involved in this process.
Acknowledgements
The present authors would like to thank the healthy
peripheral blood donors and the staff in the Open Laboratory of the
Basic Medical College of Zhengzhou University. The current study
was supported by the National Natural Science Foundation of China
(grant no. 81101731), and Science Foundation of Zhengzhou
University for the Excellent Young Teacher (grant no. 1421328057),
and Henan University of Science and Technology Innovation Talents
(grant no. 15HASTIT038).
References
|
1
|
Milano F and Krishnadath KK:
Electroporation of dendritic cells with autologous total RNA from
tumor material. Methods Mol Biol. 1139:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benteyn D, Van Nuffel AM, Wilgenhof S and
Bonehill A: Single-step antigen loading and maturation of dendritic
cells through mRNA electroporation of a tumor-associated antigen
and a TriMix of costimulatory molecules. Methods Mol Biol.
1139:3–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aravindaram K, Wang PH, Yin SY and Yang
NS: Tumor- associated antigen/IL-21-transduced dendritic cell
vaccines enhance immunity and inhibit immunosuppressive cells in
metastatic melanoma. Gene Ther. 21:457–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torchia Giardino ML, Ciaglia E, Masci AM,
Vitiello L, Fogli M, la Sala A, Mavilio D and Racioppi L: Dendritic
cells/natural killer cross-talk: A novel target for human
immunodeficiency virus type-1 protease inhibitors. PLoS One.
5:e110522010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benencia F, Sprague L, McGinty J, Pate M
and Muccioli M: Dendritic cells the tumor microenvironment and the
challenges for an effective antitumor vaccination. J Biomed
Biotechnol. 2012:4254762012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farren MR, Carlson LM, Netherby CS,
Lindner I, Li PK, Gabrilovich DI, Abrams SI and Lee KP:
Tumor-induced STAT3 signaling in myeloid cells impairs dendritic
cell generation by decreasing PKCβII abundance. Sci Signal.
7:ra162014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu TC, Xu K, Banchereau R, Marches F, Yu
CI, Martinek J, Anguiano E, Pedroza-Gonzalez A, Snipes GJ,
O'Shaughnessy J, et al: Reprogramming tumor-infiltrating dendritic
cells for CD103+ CD8+ mucosal T-cell
differentiation and breast cancer rejection. Cancer Immunol Res.
2:487–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH
and Wang RF: Tumor-infiltrating gammadelta T cells suppress T and
dendritic cell function via mechanisms controlled by a unique
toll-like receptor signaling pathway. Immunity. 27:334–348. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Young MR and Cigal M: Tumor skewing of
CD34+ cell differentiation from a dendritic cell pathway
into endothelial cells. Cancer Immunol Immunother. 55:558–568.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Conejo-Garcia JR, Benencia F, Courreges
MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A,
Na H, Zhang L, et al: Tumor-infiltrating dendritic cell precursors
recruited by a beta-defensin contribute to vasculogenesis under the
influence of Vegf-A. Nat Med. 10:950–958. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gottfried E, Kreutz M, Haffner S, Holler
E, Iacobelli M, Andreesen R and Eissner G: Differentiation of human
tumour-associated dendritic cells into endothelial-like cells: An
alternative pathway of tumour angiogenesis. Scand J Immunol.
65:329–335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berger S, Dyugovskaya L, Polyakov A and
Lavie L: Short-term fibronectin treatment induces endothelial-like
and angiogenic properties in monocyte-derived immature dendritic
cells: Involvement of intracellular VEGF and MAPK regulation. Eur J
Cell Biol. 91:640–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sozzani S, Rusnati M, Riboldi E, Mitola S
and Presta M: Dendritic cell-endothelial cell cross-talk in
angiogenesis. Trends Immunol. 28:385–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osuka K, Usuda N, Aoyama M, Yamahata H,
Takeuchi M, Yasuda M and Takayasu M: Expression of the
JAK/STAT3/SOCS3 signaling pathway in herniated lumbar discs.
Neurosci Lett. 569:55–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zang Y, Yu LF, Pang T, Fang LP, Feng X,
Wen TQ, Nan FJ, Feng LY and Li J: AICAR induces astroglial
differentiation of neural stem cells via activating the JAK/STAT3
pathway independently of AMP-activated protein kinase. J Biol Chem.
283:6201–6208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Montgomery EA, Basman FT, Brennan P and
Malekzadeh R: Oesophageal Cancer. World Cancer Report 2014. Stewart
BW and Wild CP: (Lyon). IARC Press. 374–382. 2014.
|
|
18
|
Wang XX, Wang K, Li XZ, Zhai LQ, Qu CX,
Zhao Y, Liu ZR, Wang HZ, An QJ, Jing LW, et al: Targeted knockdown
of IQGAP1 inhibits the progression of esophageal squamous cell
carcinoma in vitro and in vivo. PLoS One. 9:e965012014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Zhao J, Liu K, Zhao J, Yang H, Huang
Y, Qin Z, Bai R, Li P, Ma J, et al: MAPK/ERK1/2 signaling mediates
endothelial-like differentiation of immature DCs in the
microenvironment of esophageal squamous cell carcinoma. Cell Mol
Life Sci. 67:2091–2106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aranguren XL, Luttun A, Clavel C, Moreno
C, Abizanda G, Barajas MA, Pelacho B, Uriz M, Araña M, Echavarri A,
et al: In vitro and in vivo arterial differentiation of human
multipotent adult progenitor cells. Blood. 109:2634–2642. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Wu JC, Sheikh AY, Kraft D, Cao F,
Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, et al:
Differentiation, survival, and function of embryonic stem cell
derived endothelial cells for ischemic heart disease. Circulation.
116(Suppl): I46–54. 2007.PubMed/NCBI
|
|
22
|
Lu J, Liu K, Zhao J, Zhao J, Ma J, Yang H,
Huang Y, Qin Z, Bai R, Jiang L, et al: VEGF-A not Ang2 mediates
endothelial-like differentiation of immature DCs by ERK1/2
signaling in the microenvironment of human colon adenocarcinoma.
Int J Oncol. 38:1579–1588. 2011.PubMed/NCBI
|
|
23
|
Lu J, Zhao J, Zhao J, Ma J, Liu K, Yang H,
Huang Y, Qin Z, Bai R, Li P, et al: VEGF-A-induced immature DCs not
mature DCs differentiation into endothelial-like cells through
ERK1/2-dependent pathway. Cell Biochem Funct. 29:294–302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El Deeb NM and Mehanna RA: Assessment of
maturation status of tumor-infiltrating dendritic cells in invasive
ductal carcinoma of the breast: Relation with vascular endothelial
growth factor expression. Turk Patoloji Derg. 29:193–200.
2013.PubMed/NCBI
|
|
25
|
Zhao Y, Glesne D and Huberman E: A human
peripheral blood monocyte-derived subset acts as pluripotent stem
cells. Proc Natl Acad Sci USA. 100:2426–2431. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang Y, Luo Y, Jiang Z, Lin CJ, Kim C,
Carter MG, Amano T, Park J, Kish S, et al: Jak/Stat3 signaling
promotes somatic cell reprogramming by epigenetic regulation. Stem
Cells. 30:2645–2656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nefedova Y and Gabrilovich DI: Targeting
of Jak/STAT pathway in antigen presenting cells in cancer. Curr
Cancer Drug Targets. 7:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|