Introduction
An invasive mole (IM), a form of gestational
trophoblastic neoplasia (GTN) (1), is
a pregnancy-associated disorder, which is caused by a molar
pregnancy. It has been reported that 0.5–1% of partial hydatidiform
mole cases and 15–29% of complete hydatidiform mole cases
progressed to become IMs (2,3). Irregular vaginal bleeding is the most
common symptom of IM, however, further symptoms caused by bleeding
in the metastases, such as hemoptysis and neurological symptoms,
may also be detected (2). Myometrial
invasion, swollen villi and hyperplastic trophoblast are often
considered to be the pathological features of IM (1). The clinical diagnosis of IM relies on
medical history, clinical symptoms, laboratory tests and
examination using imaging. Pathological results are necessary for
confirmed cases. Timely and comprehensive treatment based on
chemotherapy can result in a good prognosis (1). In China, the incidence rate of IM
following pregnancy is 0.94–1.30% (4,5).
Generally, IM is considered to be a disease with malignant
behavior, due to its potential to invade into the myometrium and
metastasize to other organs. The most common locations for IM
metastases are the vagina, lungs and brain (6,7).
Alternative sites of metastases, including the epidural space and
bladder, have been rarely reported (8,9). To the
best of our knowledge, there have been no cases of IM with
subsequent metastasis to the kidney reported in the literature. In
the present study, the case of a woman presenting with IM and
bilateral kidney metastases is reported.
Case report
A 42-year-old woman was admitted to the Third
Xiangya Hospital of Central South University (Changsha, Hunan,
China) on the 18th January 2013, presenting with pain in the left
waist and abdomen, which had persisted for 1 week. The patient had
undergone an induced abortion 3 months prior to this hospital
admittance, and since then had subsequently experienced irregular
vaginal bleeding. Therefore, dilation and curettage was performed
for incomplete abortion in the primary hospital, however no
embryonic tissue was obtained.
A comprehensive examination and evaluation of the
patient was performed. A large and pliant mass in the left upper
abdomen was palpated during physical examination. The patient
exhibited a rapid heart rate (105 bpm; normal range, 60–100 bpm)
and reduced blood pressure (85/53 mmHg; normal range, 90/60 to
140/90 mmHg), combined with markedly decreased levels of hemoglobin
(72 g/L; normal range, 110–150 g/L), and therefore received
supportive treatment in the form of blood transfusions (2 U packed
red blood cells).
The patient was also administered preventive
antibacterial therapy (2.0 g cefamandole nafate, b.i.d. for 2
weeks) and was advised to rest in bed due to the possibility of
kidney injury. Laboratory tests revealed markedly elevated levels
of human chorionic gonadotropin β (β-HCG; 462,047 mIU/ml; normal
range, 0–10 mIU/ml) in the blood, indicating a possible GTN.
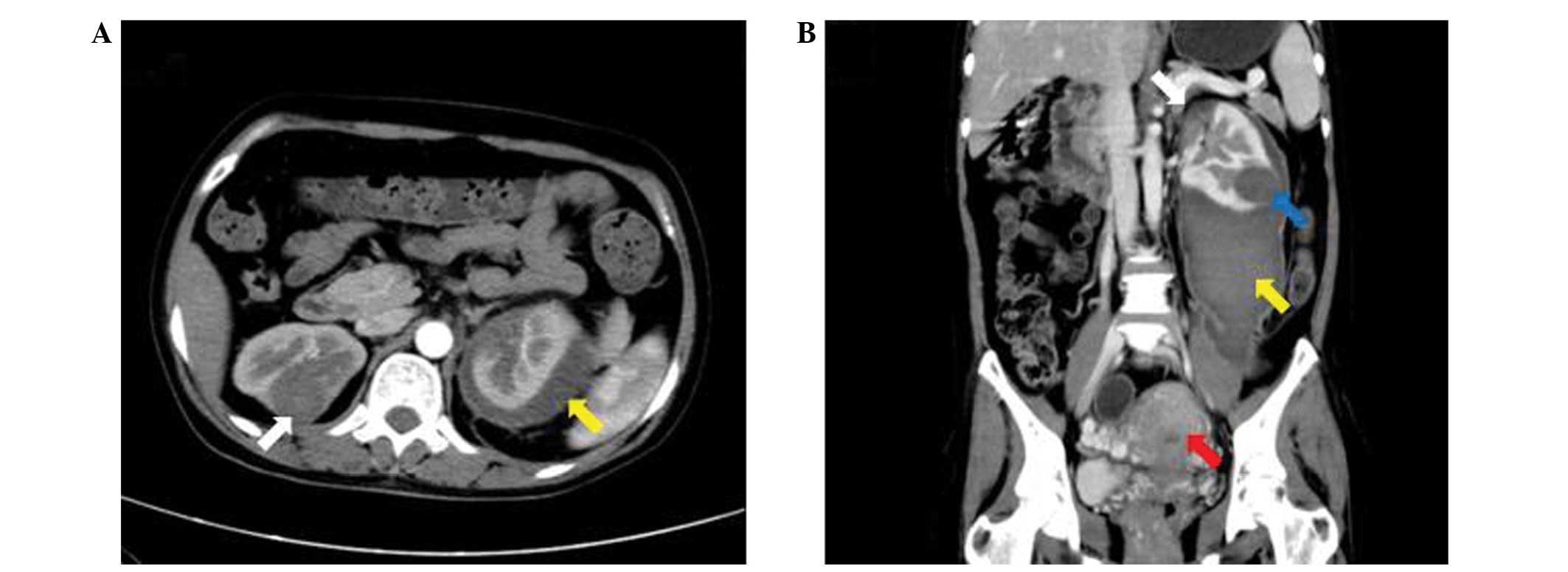
Computed tomography (CT) identified several bilateral masses in the
lungs and kidneys, as well as a large retroperitoneal hematoma,
caused by a ruptured mass in the left kidney, and an enlarged
uterus. Magnetic resonance imaging (MRI) of the brain also
indicated a mass in the right parietal lobe. A second CT scan was
performed 1 month later, which revealed that the retroperitoneal
hematoma had slightly reduced in size, however, the bilateral
masses and enlarged uterus demonstrated no marked alterations
compared with the initial CT scan (Fig.
1). The β-HCG levels in the blood were evaluated every week
during the period of conservative treatment (for avoidance of renal
damage) and were observed to be continuously increasing. Therefore,
the patient was clinically diagnosed with GTN [stage IV; score, 15;
according to the FIGO (the International Federation of Gynecology
and Obstetrics) staging system and FIGO prognostic scoring system]
(10). Furthermore, the masses
present in the kidneys, lungs and brain were considered to be
metastases from this primary GTN, as the patient possessed no
history of previously diagnosed primary tumors at these sites.
The patient was administered standard intravenous
EMA/CO chemotherapy every 3 weeks, consisting of: Etoposide 100
mg/m2, methotrexate 300 mg/m2 and actinomycin
D 0.5 mg on days 1 and 2 and cyclophosphamide 600 mg/m2,
and vincristine 1 mg/m2 (maximum dose of 2 mg) on day 8.
Simultaneously, a intrathecal injection of methotrexate was
administered (15 mg; twice in week 1, 10mg twice in week 2, every 3
weeks for 4 cycles). Following eight cycles of comprehensive EMA/CO
treatment, which lasted for ~4 months, the β-HCG levels in the
blood had decreased to within the normal range. Furthermore, a
second MRI scan of the brain, and a third CT scan, revealed that
the masses in the lungs, kidneys and brain had markedly reduced in
size. However, the retroperitoneal hematoma remained large and
unabsorbed, which increased the risk of infection and aggravated
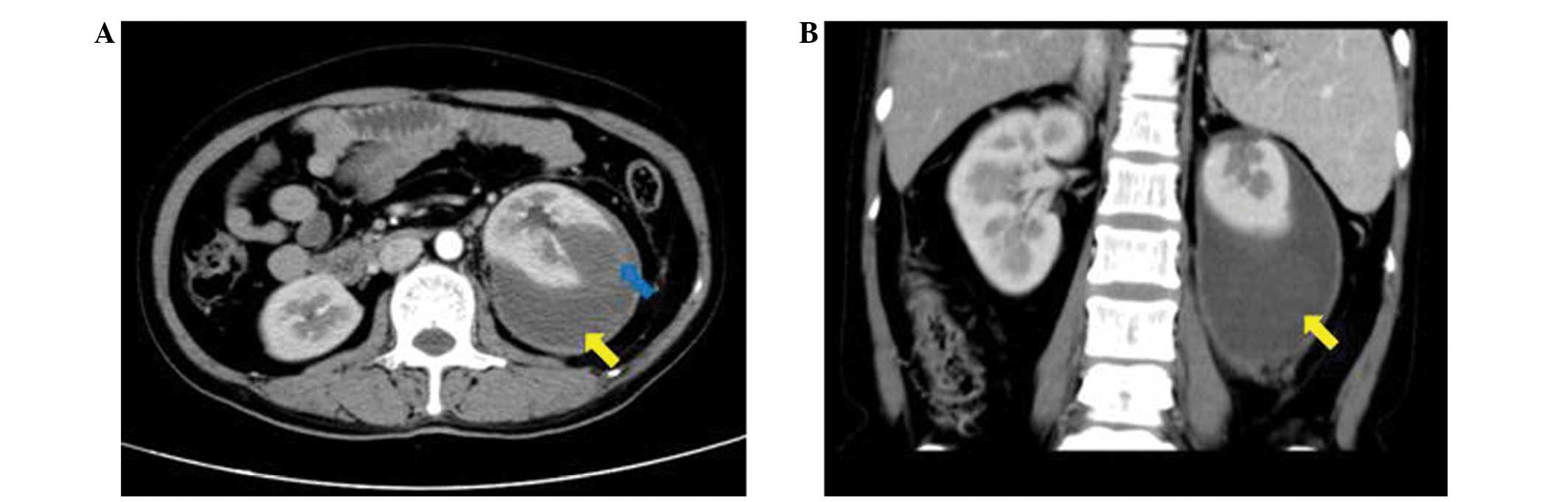
the compression symptoms. In addition, the left kidney was observed
to be ruptured (Fig. 2). Therefore,
the hematoma was removed and the left kidney was excised during
open surgery. It was also recommended that the patient undergo
surgery to remove the enlarged uterus, as a mass remained in this
area and the patient had no requirement for fertility, therefore a
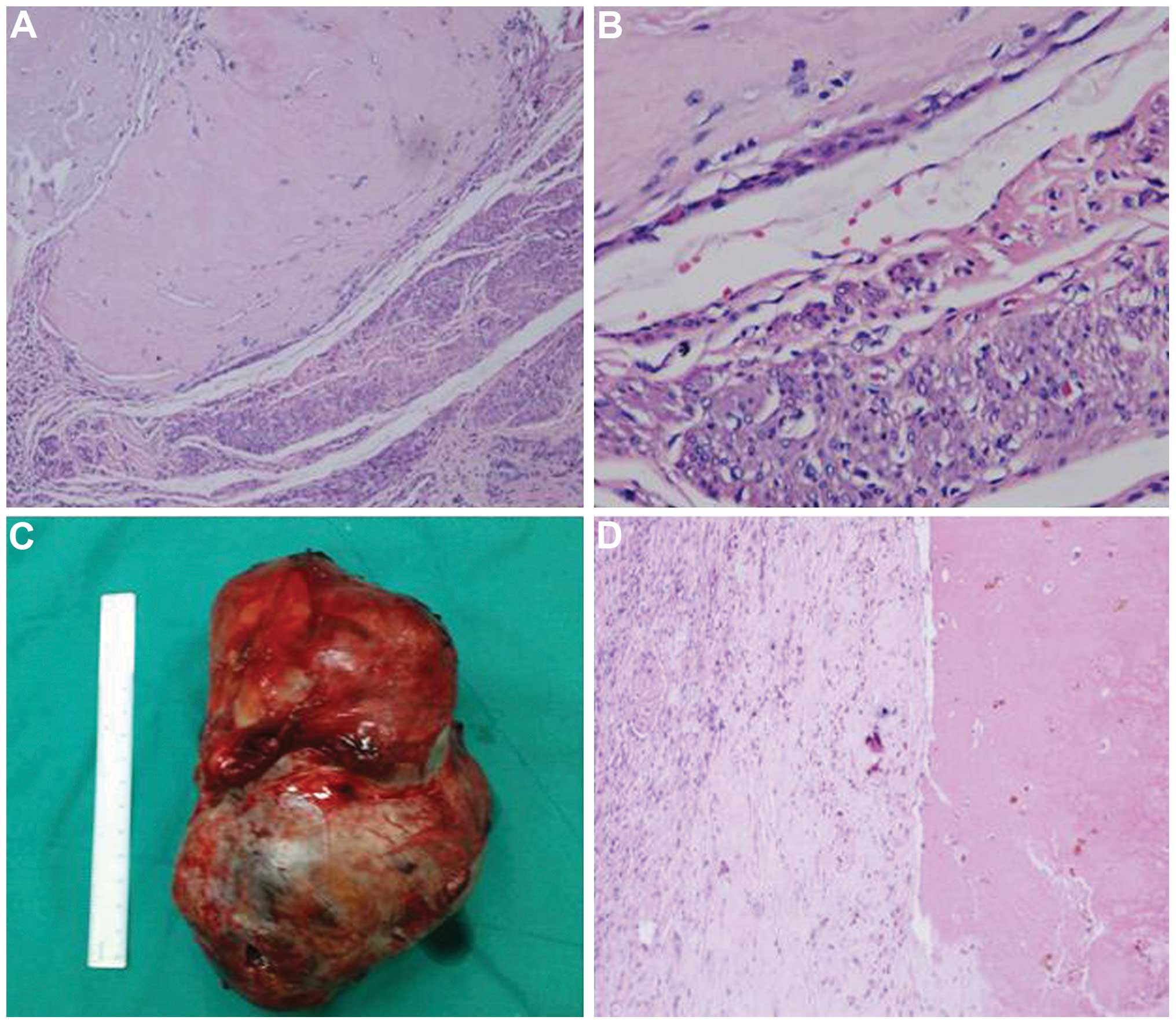
laparoscopic hysterectomy was performed. Histological analysis
identified degenerated villi and trophoblastic cells in the uterus,
as well as metastatic cells in the left kidney, which was
consistent with the clinical diagnosis and allowed further
classification of the primary tumor as an IM (Fig. 3). The post-operative recovery was
uneventful and an additional two cycles of chemotherapy (the same
chemotherapy regiments as before operation) were administered.
Currently, the patient's blood β-HCG levels and renal function
remain normal, and follow-up of the patient is ongoing.
Discussion
IM is a common form of GTN. The criteria for the
diagnosis of GTN following a hydatidiform mole are as follows: i) A
plateau of HCG lasting for four measurements over a period of 3
weeks or longer; ii) an increase in HCG levels following weekly
consecutive measurements, over a period of 2 weeks or more; iii)
HCG levels remaining elevated for 6 months or longer; and iv) a
histological diagnosis of choriocarcinoma (10). Myometrial invasion, swollen villi and
hyperplastic trophoblasts are frequently considered to be
pathological features of IM, however, the majority of IM cases are
diagnosed clinically rather than pathologically (11).
Chemotherapy is a main treatment approach for IM.
According to the FIGO staging system (10), patients classified as low risk (stage
II–III and score <7) may receive treatment with a single
chemotherapeutic agent, for example methotrexate or actinomycin D
(12,13). However, patients classified as high
risk (stage IV, or stage II–III and score ≥7) are recommended to be
treated with combination chemotherapy regimens; EMA/CO has been
considered to be the first choice treatment regimen for the last 10
years, and has demonstrated good patient responses and long-term
survival rates (14–16). Furthermore, patients exhibiting brain
metastases may be administered with systemic chemotherapy, as well
as simultaneous administration of one of the three following
treatment methods: Whole-brain or stereotactic radiotherapy,
intrathecal injection of methotrexate and surgical intervention,
for example, craniotomy (17,18). For the treatment of lung metastases,
systemic chemotherapy is the typical choice rather than surgery
(1).
To the best of our knowledge, a number of cases of
choriocarcinoma metastasis in the kidneys have been reported,
however, no cases of IM metastasis to the kidneys have been
reported (19,20). In the current case, the patient
presented with irregular vaginal bleeding following an induced
abortion, as well as an elevated β-HCG level for >3 months.
Furthermore, a pathological diagnosis of IM was confirmed following
the identification of degenerative villi in the primary lesions of
the uterus, and a metastatic tumor was additionally identified in
the patient's left kidney.
Based on our experience, we propose that patients
exhibiting steady renal metastases should be administered with a
systemic chemotherapy regimen and be monitored by strict follow-up
appointments. In the event that metastatic tumors rupture and cause
subsequent bleeding, patients should be administered a series of
conservative treatments for kidney trauma. In addition, essential
surgical treatments should be considered when vital signs are
unstable or the conservative treatments are deemed to have been
ineffective. Conservative and surgical treatments should be
accompanied by systemic chemotherapy.
In the present case, the patient was classified as
high risk according to FIGO criteria, and was treated with the
standard EMA/CO chemotherapeutic regimen, as well as an intrathecal
injection of methotrexate. Simultaneously, conservative treatment
was administered to remedy the damage to the patient's left kidney.
Following eight cycles of this standard chemotherapy, the
metastases in the bilateral kidneys had greatly reduced in size,
however, the left kidney was observed to be ruptured and was
therefore excised, while the right kidney was observed to be intact
and was successfully preserved. Following surgery, the patient was
subsequently administered an additional two cycles of chemotherapy,
in order to consolidate the efficacy of treatment. Currently, this
integrated treatment has proven to be effective.
In conclusion, IM metastasis to the kidneys is
rarely reported. This may be due to the lack of pathological
diagnosis performed on the majority of patients exhibiting GTN.
Nevertheless, the present case demonstrated that metastasis of IM
to the kidney is possible, and furthermore, that these metastatic
tumors may be fragile and possess the potential to cause
spontaneous kidney rupture.
Acknowledgements
The present study was supported by the Hunan
Provincial Natural Science Foundation of China (grant no. 14JJ3044)
and the Science Foundation of Health and Family Planning Commission
of Hunan Province (grant no. B2012-032)
References
|
1
|
Seckl MJ, Sebire NJ and Berkowitz RS:
Gestational trophoblastic disease. Lancet. 376:717–729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loukovaara M, Pukkala E, Lehtovirta P and
Leminen A: Epidemiology of hydatidiform mole in Finland, 1975 to
2001. Eur J Gynaecol Oncol. 26:207–208. 2005.PubMed/NCBI
|
|
3
|
Hancock BW, Nazir K and Everard JE:
Persistent gestational trophoblastic neoplasia after partial
hydatidiform mole incidence and outcome. J Reprod Med. 51:764–766.
2006.PubMed/NCBI
|
|
4
|
Shi YF, Li JQ, Zheng W, Chen XJ, Qiao YH,
Hao M, Zhou CW, Hu YL, Wan GM, Sha YC and Zheng X: Survey of
gestational trophoblastic disease incidence among 3.6 million
pregnancies in China. Zhonghua Fu Chan Ke Za Zhi. 40:76–78.
2005.(In Chinese). PubMed/NCBI
|
|
5
|
Sha YC: Investigation of Gestational
trophoblastic disease in Anhui Province during 1991-2000. Anhui
Medical Journal. 3:253–255. 2004.(In Chinese).
|
|
6
|
Song HZ, Yang XY and Xiang Y: Forty-five
year's experience of the treatment of choriocarcinoma and invasive
mole. Int J Gynaecol Obstet. 60(Suppl 1): S77–S83. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng FZ, Xiang Y, Shan Y, Wan XR and Oang
XY: Clinical analysis of patients with lung metastasis of invasive
mole before evacuation of hydatidiform mole. Zhonghua Fu Chan Ke Za
Zhi. 42:830–833. 2007.(In Chinese). PubMed/NCBI
|
|
8
|
Makangee A, Nadvi SS and Van Dellen JR:
Invasive mole presenting as a spinal extradural tumor: Case report.
Neurosurgery. 38:191–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malhotra B and Misra R: Metastatic
invasive mole in the urinary bladder. Indian J Cancer. 39:116–118.
2002.PubMed/NCBI
|
|
10
|
Ngan HY, Bender H, Benedet JL, Jones H,
Montruccoli GC and Pecorelli S: FIGO Committee on Gynecologic
Oncology: Gestational trophoblastic neoplasia, FIGO 2000 staging
and classification. Int J Gynaecol Obstet. 83(Suppl 1): S175–S177.
2003. View Article : Google Scholar
|
|
11
|
Lurain JR: Gestational trophoblastic
disease I: Epidemiology, pathology, clinical presentation and
diagnosis of gestational trophoblastic disease, and management of
hydatidiform mole. Am J Obstet Gynecol. 203:531–539. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chapman-Davis E, Hoekstra AV, Rademaker
AW, Schink JC and Lurain JR: Treatment of nonmetastatic and
metastatic low-risk gestational trophoblastic neoplasia: Factors
associated with resistance to single-agent methotrexate
chemotherapy. Gynecol Oncol. 125:572–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yarandi F, Eftekhar Z, Shojaei H, Kanani
S, Sharifi A and Hanjani P: Pulse methotrexate versus pulse
actinomycin D in the treatment of low-risk gestational
trophoblastic neoplasia. Int J Gynaecol Obstet. 103:33–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Escobar PF, Lurain JR, Singh DK, Bozorgi K
and Fishman DA: Treatment of high-risk gestational trophoblastic
neoplasia with etoposide, methotrexate, actinomycin D,
cyclophosphamide, and vincristine chemotherapy. Gynecol Oncol.
91:552–557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu WG, Ye F, Shen YM, Fu YF, Chen HZ, Wan
XY and Xie X: EMA-CO chemotherapy for high-risk gestational
trophoblastic neoplasia: A clinical analysis of 54 patients. Int J
Gynecol Cancer. 18:357–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cagayan MS: High-risk metastatic
gestational trophoblastic neoplasia. Primary management with EMA-CO
(etoposide, methotrexate, actinomycin D, cyclophosphamide and
vincristine) chemotherapy. J Reprod Med. 57:231–236.
2012.PubMed/NCBI
|
|
17
|
Neubauer NL, Latif N, Kalakota K, Marymont
M, Small W Jr, Schink JC and Lurain JR: Brain metastasis in
gestational trophoblastic neoplasia: An update. J Reprod Med.
57:288–292. 2012.PubMed/NCBI
|
|
18
|
Soper JT, Spillman M, Sampson JH,
Kirkpatrick JP, Wolf JK and Clarke-Pearson DL: High-risk
gestational trophoblastic neoplasia with brain metastases:
Individualized multidisciplinary therapy in the management of four
patients. Gynecol Oncol. 104:691–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Newman LB, Morgan TE, Bucy JG and Wise L:
Choriocarcinoma and bilateral renal metastases. Urology. 5:658–661.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang YE, Song HZ, Yang XY, Dong SY and Gan
N: Renal metastases of choriocarcinoma. A clinicopathological study
of 31 cases. Chin Med J (Engl). 104:716–720. 1991.PubMed/NCBI
|