Introduction
Cervical cancer is a common malignant tumor, and
according to the World Health Organization, 500,000 novel cases are
diagnosed and 274,000 mortalities occur due to this cancer every
year worldwide. In total, 83% of cases occur in developing
countries, and are associated with a decreasing age of onset
(1). Early stage cervical cancer may
be treated by surgery. However, resection of progressive tumors,
particularly recurrent or metastatic tumors, is considerably
limited. Patients with late-stage cervical cancer exhibit a poor
physical condition, which also limits the application of
radiotherapy, chemotherapy or the two therapies combined (2,3). The
pathogenesis of cervical cancer is not yet completely understood,
and there are currently no drugs available that may effectively
control the occurrence and development of this cancer. Previous
studies have indicated that the phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR)
signaling pathway regulates tumor cell proliferation, promotes cell
cycle progression, participates in neovascularization and induces
resistance to radiotherapy and chemotherapy in tumor cells
(4–6).
In addition, this signaling cascade is a major pathway in
suppressing tumor cell apoptosis (7,8). Cervical
cancer may be treated by triggering tumor cell apoptosis through
the combined application of radiotherapy and chemotherapy, which is
a focus in the molecular-targeted therapy of cervical cancer
(9).
Shikonin, a naphthoquinone derivative, is a
naturally occurring active chemical that is found in the root of
Lithospermum erythrorhizon. Shikonin has been reported to
demonstrate anti-cancer, anti-inflammation, anti-human
immunodeficiency virus and anti-fungal activity (10,11).
Shikonin demonstrates strong toxicity, but does not exhibit tumor
specificity, and therefore, shikonin has not been widely used in
the clinic (12). Previous studies of
the extraction or synthesis of high-performance non-toxic shikonin
derivatives has received considerable attention (13–15).
β-hydroxyisovaleryl-shikonin (β-HIVS) is a natural shikonin
derivative (16). The present study
investigated the inhibitory effect of β-HIVS on the proliferation
of human cervical cancer HeLa cells, and explored the molecular
mechanism of action of the PI3K/AKT/mTOR signaling pathway on
β-HIVS-induced apoptosis of HeLa cells.
Materials and methods
Reagents and equipment
HeLa human cervical cancer cells were purchased from
China Center for Type Culture Collection (Wuhan University, Wuhan,
Hubei, China). Fetal bovine serum was obtained from Hangzhou
Sijiqing Biological Engineering Materials Co. Ltd. (Hangzhou,
Zhejiang, China) and high-glucose Dulbecco's modified Eagle's
medium was purchased from Gibco Life Technologies (Carlsbad, CA,
USA). β-HIVS (>98% purity) was purchased from Wako Pure Chemical
Industries, Ltd. (Saitama, Japan). MTT was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and dimethyl sulfoxide was
purchased from Meilian Biological Technology Co., Ltd. (Shanghai,
China). Acridine orange/ethidium bromide double fluorescence kit
was obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing,
Jiangsu, China), Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide apoptosis kit was obtained from Roche
(Basel, Switzerland) and the flow cytometer used was obtained from
BD Biosciences (Franklin Lakes, NJ, USA). Rabbit anti-human PI3K,
AKT and 70-kDa ribosomal protein S6 kinase (P70S6K)
antibodies, and the mouse anti-human mTOR antibody were purchased
from Cell Signaling Technology (Danvers, MA, USA). The rabbit
anti-human β-actin polyclonal antibody was purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
The study protocol complied with all committee regulations approved
by the Ethics Committee for Experimentation of Yangzhou University
(Yangzhou, China).
Cell culture
Human cervical cancer HeLa cells were maintained in
high-glucose Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum, and were incubated in a 37°C incubator with
a 5% CO2 atmosphere, under saturated humidity (2406-2
CO2 incubator; Heraeus, Hanau, Germany). A high pressure
sterilization autoclave (ES 315; Tomy Company, Ltd., Tokyo, Japan
was used to prepare the phosphate-buffered saline (PBS) buffer
solution for cell passage cultivation. The cells were passaged with
0.25% trypsin (Nanjing KeyGen Biotech Co., Ltd.). The cells used in
the present study were in the logarithmic phase of growth at 24 h
subsequent to incubation. β-HIVS (2.3 mg) was dissolved in dimethyl
sulfoxide (592 µl) and the product (10 mM) was stored at −20°C
prior to use. A thermostatic bath (W201B; Senco Technology Co.,
Ltd., Shanghai, China) was used to rewarm the frozen cell
solutions.
Proliferation assay
The HeLa cells were seeded at a density of
1×105 cells/ml onto a 96-well plate, with 100 µl of cell
suspension being added to each well. The cells were then incubated
at 37°C for 24 h. The treatment groups consisted of a blank zero
group that contained no incubated cells, a control group that only
contained an equal volume of solvent and an experimental group.
Prior to the current experiment, cells were treated 0, 2.5, 5, 10,
20, 40, 80 and 160 µM β-HIVS to identify an optimum dose range.
Cells were obviously apoptotic with increasing concentrations of
β-HIVS, particularly at concentrations >40 µM. Therefore, the
experimental group was treated with final concentrations of 1.56,
3.13, 6.25, 12.50 and 25.00 µM β-HIVS in triplicate to determine
the effective and safe concentration of β-HIVS. Subsequent to
incubation at 37°C for 24, 48 or 72 h, 5 mg/ml MTT was added to
each well and the wells were incubated for 4 h. The supernatant was
discarded and 150 µl dimethyl sulfoxide was added to each well
prior to the wells being vortexed for 10 min. When the crystal had
completely dissolved, the absorbance values were measured at 490 nm
using a microplate reader (680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The toxicity exerted by β-HIVS on tumor cell
proliferation was determined by the inhibitory rate. The inhibitory
rate was calculated as follows: Inhibitory rate (%) = (absorbance
of the control group - absorbance of the experimental group) /
absorbance of the control group × 100. The experiment was
independently performed in triplicate.
Detection of apoptosis
Acridine orange/ethidium bromide double
fluorescence staining to detect morphological changes in apoptotic
cells
The HeLa cells were seeded at a density of
4×105 cells/ml in six-well plates, at a total volume of
1 ml/well, and the cells were then incubated for 24 h. The HeLa
cells were treated with β-HIVS at final concentrations of 0, 1, 5
and 10 µM for 24 or 48 h, and then treated with 0.25% trypsin,
washed twice with PBS and a 6×106 cell/ml suspension was
prepared. Subsequently, 25 µl of cell suspension was combined with
1 µl acridine orange/ethidium bromide dye and added to a clean
slide. Cell morphology was then observed and images were captured
under an inverted fluorescence microscope (TS-100; Nikon
Corporation, Tokyo, Japan) at a wavelength of 510 nm. The control
group acted as the normal cell culture group.
Annexin V-FITC/propidium iodide double staining
and flow cytometry to determine the apoptotic rate
The HeLa cells were seeded at a density of
4×105 cells/well onto six-well plates, incubated for 24
h, and then treated with 0, 1, 5 and 10 µM β-HIVS in triplicate. At
48 h, the cells were harvested and a single cell suspension was
prepared for each treatment group. The control group acted as the
normal cell culture group. The single cell suspension was washed
with 1 ml pre-cooled PBS at 4°C and centrifuged at 100 × g for 10
min (5810R; Eppendorf, Hamburg, Germany). The supernatant was
discarded and the procedure was repeated twice. Then, 100 µl of
binding buffer and 5 µl of Annexin V-FITC was added and the cells
were incubated in the dark for 30 min at room temperature. Next, 5
µl propidium iodide was added in the dark for 5 min. The samples
were quantitatively detected using a FACScan flow cytometer. Cells
without FITC-labeled Annexin V and propidium iodide were considered
as negative controls. The results were analyzed using Cell Quest
Software.
Flow cytometry analysis of cell cycle
distribution
The HeLa cells were seeded at a density of
4×105 cells/well, incubated for 24 h and then treated
with 0, 1, 5 and 10 µM β-HIVS in triplicate. Subsequent to 48 h,
the cells were harvested and made into single cell suspensions for
each treatment group. The control group acted as the normal cell
culture group. The cells were fixed in 70% cold ethanol at 4°C
overnight, and then the cell suspensions were centrifuged and the
fixative was removed. Following 2 washes with PBS, the samples were
treated with propidium iodide and RNase A for 30 min at 37°C at a
final concentration of 50 µM. The cell cycle distribution was
measured using a flow cytometer.
Reverse transcription-PCR
At 48 h subsequent to the treatment of HeLa cells
with 0, 1, 5 and 10 µM β-HIVS, total RNA was extracted using the
one-step TRIzol method (Invitrogen Life Technologies, Inc.,
Carlsbad, CA, USA). The concentration and purity of RNA was
determined using ultraviolet spectroscopy. The ratio of the
absorbance value at 260 nm to the absorbance value at 280 nm was
between 1.6 and 1.8. The extracted total RNA was stored at −80°C
prior to use or immediately reverse-transcribed into cDNA. Primers
for real-time quantitative PCR were synthesized by Takara Bio, Inc.
(Otsu, Shiga, Japan), according to the relevant accession number in
PubMed. The primer sequences were as follows: PI3K upstream,
5′-CTGTCAATCGGTGACTGTGTGG-3′ and downstream,
5′-AAACAGGTCAATGGCTGCATCATA-3′ (177 bp); AKT upstream,
5′-CTTGCTTTCAGGGCTGCTCA-3′ and downstream,
5′-CTTGCTTTCAGGGCTGCTCA-3′ (117 bp); MTOR upstream,
5′-AGAAACTGCACGTCAGCA CCA-3′ and downstream, 5′-CCATTCCAGCCAGTCATC
TTTG-3′ (83 bp); and P70S6K upstream, 5′-CTCAGTGAAAGT
GCCAATCAGGTC-3′ and downstream, 5′-GCTGCCAAT AAATCTTCGAGGTG-3′ (126
bp). Reverse transcription was performed using the PrimeScript RT
reagent kit (catalog no., RR037A; Takara Bio, Inc.) in a thermal
cycler (2720; Applied Biosystems, Inc., Foster City, CA, USA).
GAPDH acted as an internal reference. Quantitative PCR was
performed using SYBR Premix Ex Taq II (catalog no., RR820A; Takara
Bio, Inc.) and analyzed using a 7500 Real-Time PCR System (Life
Technologies, Grand Island, NY, USA). The results were compared
with the results of the GAPDH internal reference.
Western blotting
The HeLa cells were treated for 48 h with 0, 1, 5
and 10 µM β-HIVS and lysed. The protein concentration was measured
using a Coomassie Brilliant Blue assay (Beyotime Institute of
Biotechnology, Shanghai, China). Protein was isolated using 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(MiniPROTEAN® 3 Electrophoresis System; Bio-Rad Laboratories, Inc.)
and the bands were subseqeuntly transferred onto a polyvinylidene
difluoride membrane. The membrane was blocked with Tris-buffered
saline containing 50 mg/l skim milk for 1 h at room temperature,
and then incubated with primary antibody (dilution, 1:1,000) at 4°C
overnight. The membrane was then washed three times, incubated with
secondary horseradish peroxidase-labeled IgG antibody (dilution,
1:5,000) for 1 h at room temperature and washed an additional three
times. The blots were developed using the chemiluminescence LumiGLO
reagent (Cell Signaling Technology), photosensitized in a G:BOX gel
imaging system (Syngene, Cambridge, UK), visualized and fixed. Each
experiment was performed in triplicate. The bands were analyzed
using ImageJ analysis software (National Institutes of Health,
Bethesda, MA, USA), and the grayscale values were measured. β-actin
was used as an internal reference. The relative quantity of protein
was calculated as the ratio of the grayscale values of PI3K, AKT,
mTOR and P70S6K to β-actin.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Intergroup data were analyzed using an independent
samples t-test or Wilcoxon rank-sum test. The differences between
the dosing and time-point groups were compared using one-way
analysis of variance. Paired comparisons were performed between
each dosing group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of β-HIVS on HeLa cell
proliferation
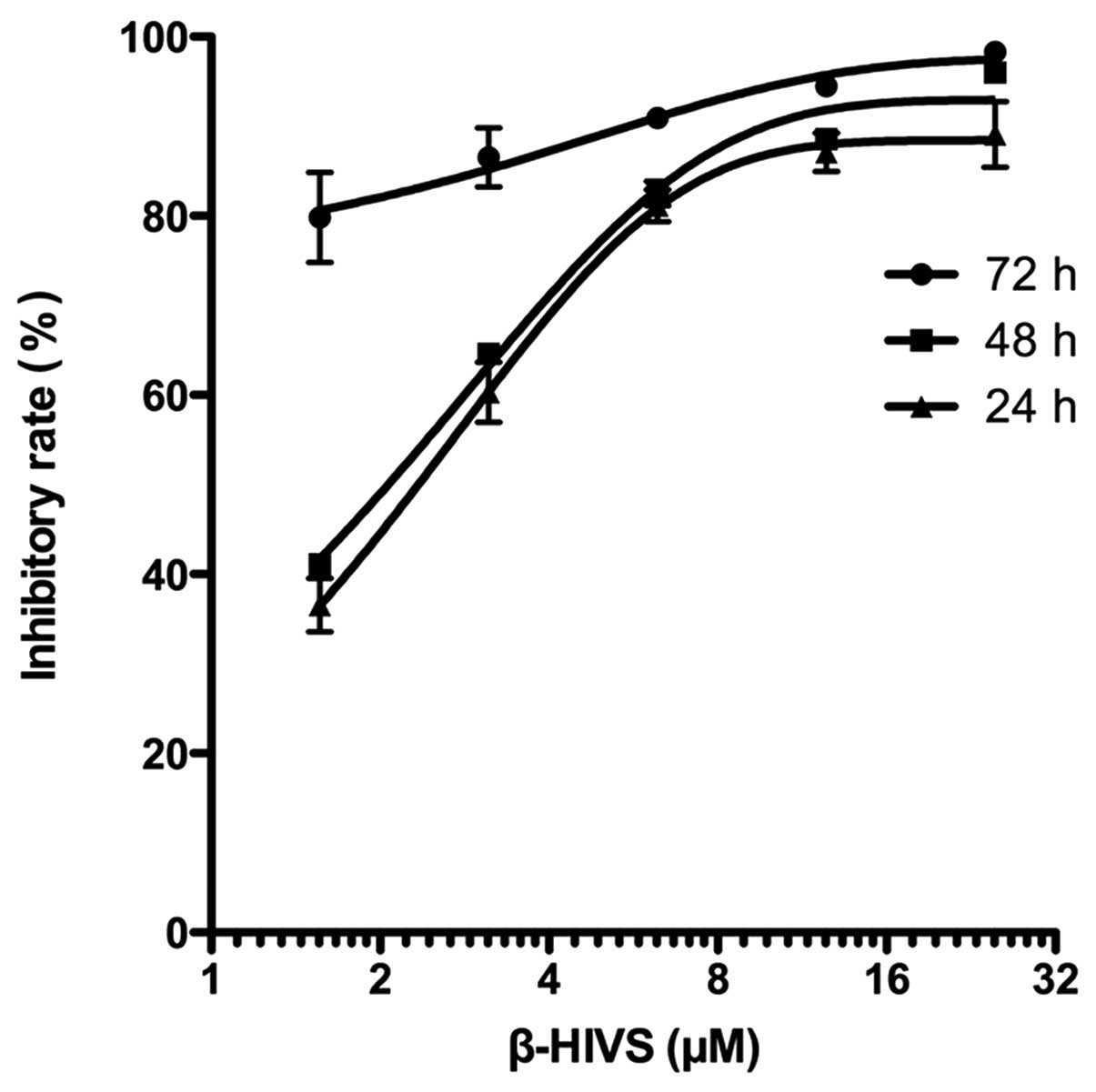
As shown in Table I
and Fig. 1, the results of the MTT
assay revealed that the inhibitory rate of HeLa cell proliferation
was gradually and significantly upregulated with the increased
concentration of β-HIVS at the same time point compared with the
control group (P<0.001). The rate of the inhibition of HeLa cell
proliferation gradually increased with time at the same β-HIVS
concentration, and a statistically significant difference was
detected between the 24-h group and the 72-h group (P<0.01).
However, no statistically significant difference was identified
between the 24 and 48 h treatment groups (P>0.05). These results
indicated that β-HIVS suppressed HeLa cell proliferation in a time-
and dose-dependent manner. At 24, 48 and 72 h subsequent to β-HIVS
treatment, the half maximal inhibitory concentrations
(IC50) of β-HIVS were 2.24, 2.04 and 0.23 µM,
respectively.
 | Table I.Effect of β-HIVS on HeLa cell
proliferation at various time points. |
Table I.
Effect of β-HIVS on HeLa cell
proliferation at various time points.
|
| Inhibitory rate,
% |
|---|
|
|
|
|---|
| Concentration of
β-HIVS, µM | 24 h | 48 h | 72 h |
|---|
| Control | 0.00 | 0.00 | 0.00 |
| 1.56 |
36.53±2.99a |
41.12±0.75a |
79.84±5.02a,b |
| 3.13 |
60.30±3.35a |
64.58±0.61a |
86.53±3.31a,b |
| 6.25 |
81.15±1.77a |
82.49±1.36a |
90.92±0.72a,b |
| 12.50 |
87.12±2.15a |
88.55±0.33a |
94.46±0.45a,b |
| 25.00 |
89.11±3.67a |
95.97±0.19a,b |
98.29±0.39a,b |
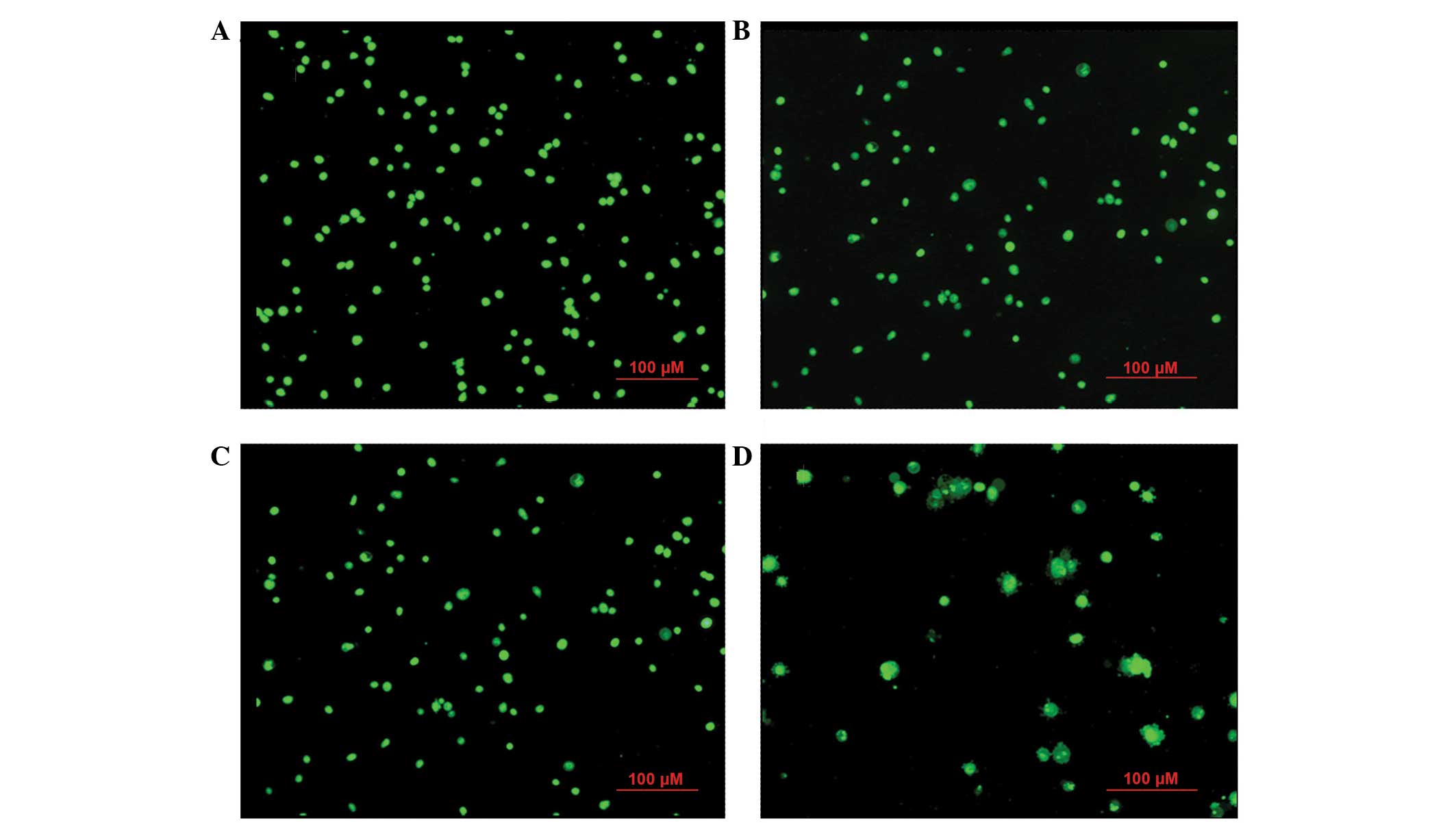
Morphological changes in apoptotic
HeLa cells induced by β-HIVS
At 24 h subsequent to β-HIVS treatment, cells in the
control group appeared rounded with green-stained nuclei of uniform
size. With increasing concentrations of β-HIVS, the number and
structure of apoptotic HeLa cells became evidently altered.
Subsequent to treatment with 1 µM β-HIVS, pyknosis appeared. At a
concentration of 5 µM β-HIVS, the cell shape was irregular and
certain cells exhibited early apoptotic changes, including
chromatin condensation, massive chromatin, pyknosis and
crescent-shaped cells. At a concentration of 10 µM β-HIVS, the cell
morphology was evidently altered and the proportion of apoptotic
cells had increased. Late apoptotic changes were visible, including
chromatin condensation, nuclear fragmentation, punctiform or
lobulated nuclei of various sizes, intact membrane and membrane
blebbing, with the presence of programmed cell death being
identified (Fig. 2).
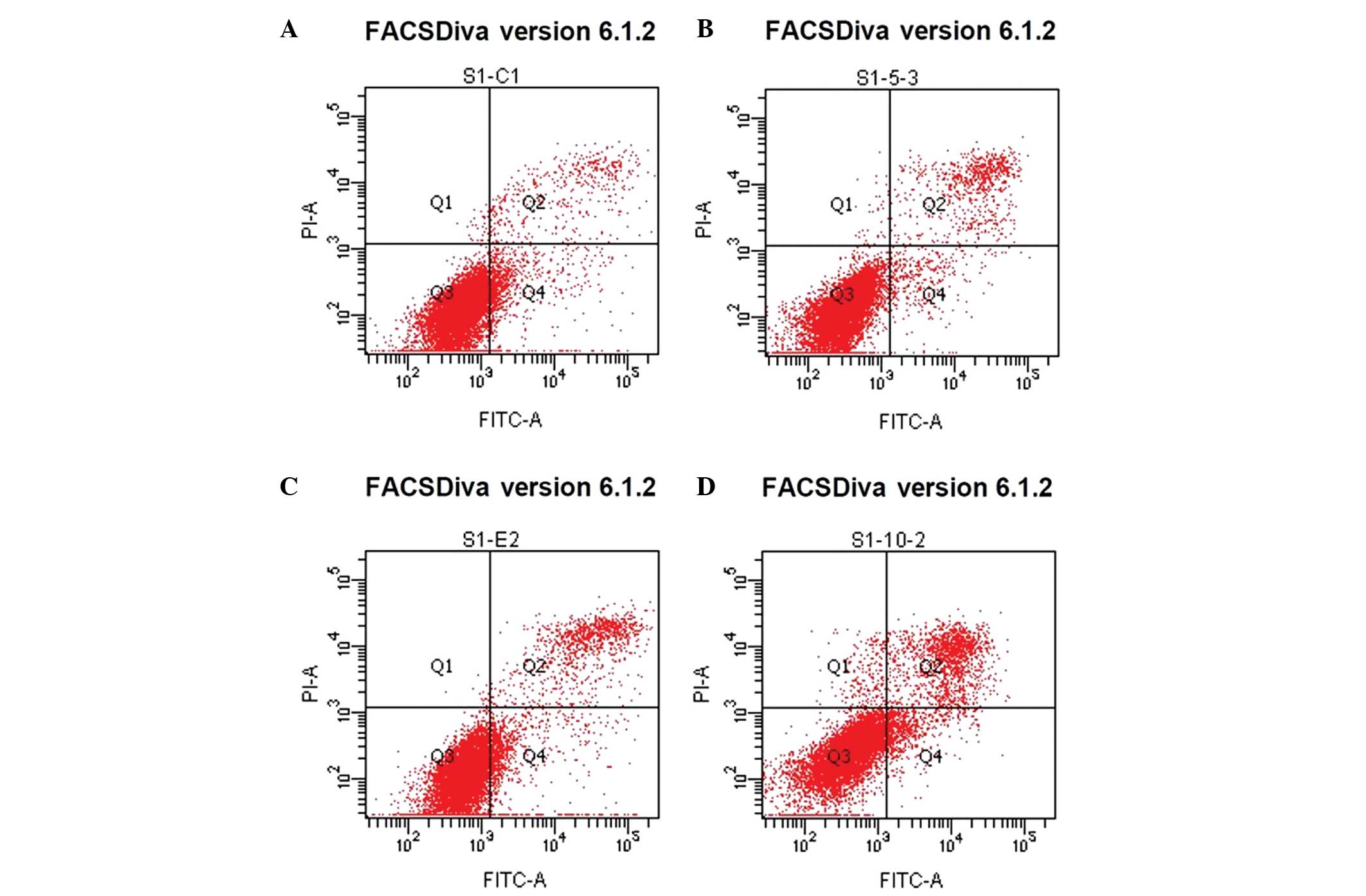
Effect of β-HIVS on the apoptotic rate
and cell cycle of HeLa cells
Treatment with β-HIVS promoted apoptosis in HeLa
cells. At 48 h subsequent to treatment with 1, 5 and 10 µM β-HIVS,
the apoptotic rates were 6.50, 8.93 and 15.77%, respectively.
Compared with the control group, which demonstrated an apoptotic
rate of 5.68%, the apoptotic rate was significantly higher in the
group treated with 10 µM β-HIVS (15.77%; P<0.001). However, no
statistically significant difference in the apoptotic rate was
determined between the cells treated with 1 and 5 µM β-HIVS
(P>0.05). β-HIVS-induced apoptosis appeared to be
dose-dependent, as the number of early apoptotic, late apoptotic
and necrotic cells gradually increased with increased
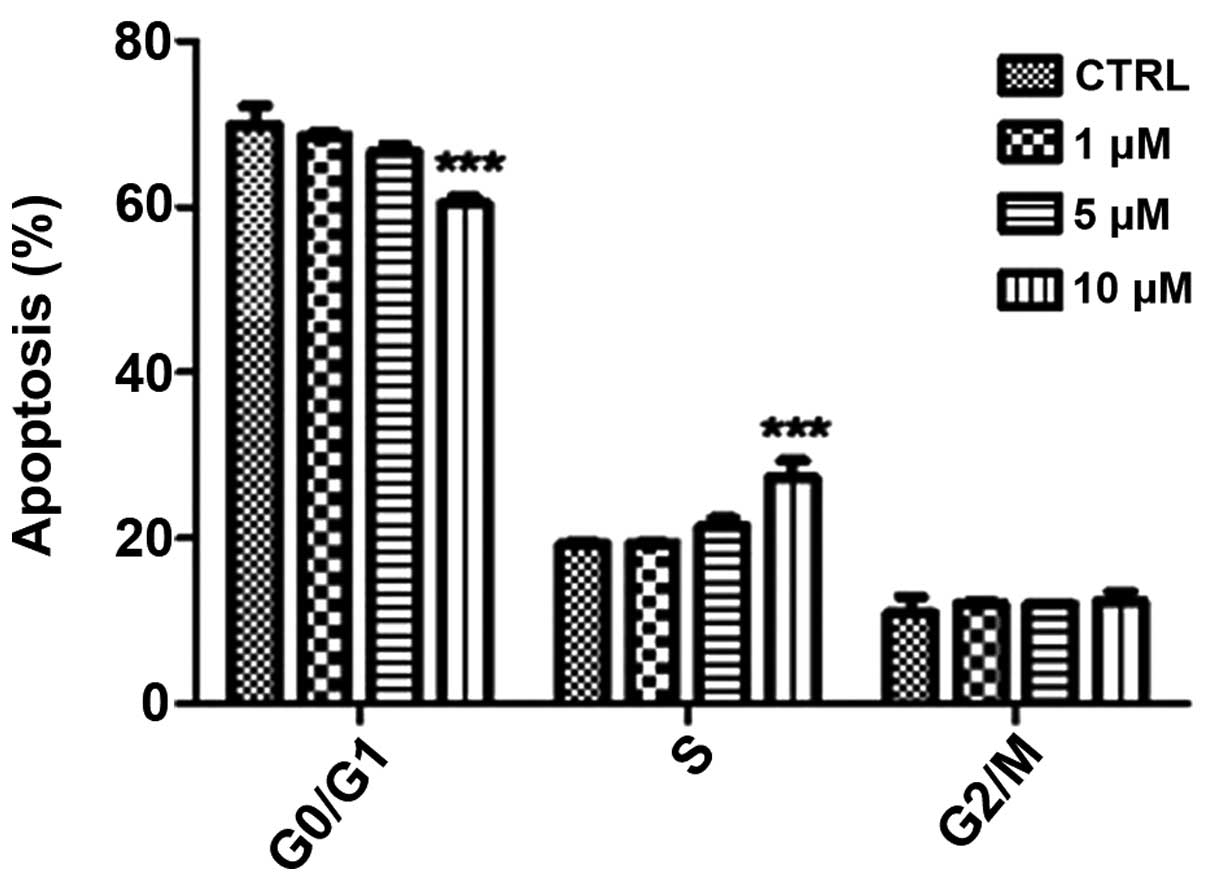
concentrations of β-HIVS. Cell cycle analysis revealed that
treatment with various concentrations of β-HIVS led to alterations
in HeLa cell cycle distribution. The number of cells in the
G0/G1 phase decreased and the number of cells
in the S phase increased, but the number of cells in the
G2/M phase did not evidently alter. Cell cycle blockade
occurred in the S phase. Subsequent to the treatment of HeLa cells
with 10 µM β-HIVS for 48 h, statistically significant differences
in the number of cells in the G0/G1, S and
G2/M phases were observed between the β-HIVS-treated
groups and the control group (P<0.001; Table II; Figs.
3 and 4).
 | Table II.Effect of β-HIVS on the apoptotic
rate and cell cycle distribution of HeLa cells. |
Table II.
Effect of β-HIVS on the apoptotic
rate and cell cycle distribution of HeLa cells.
|
|
| Cell cycle
distribution, % |
|---|
|
|
|
|
|---|
| Concentration of
β-HIVS, µM | Apoptotic rate,
% |
G0/G1 phase | S phase | G2/M
phase |
|---|
| Control |
5.68±0.55 | 69.89±2.33 | 19.24±0.33 | 10.88±2.00 |
| 1 |
6.50±0.32 | 68.55±0.45 | 19.35±0.17 | 12.10±0.28 |
| 5 |
8.93±1.66 | 66.66±0.84 | 21.34±1.04 | 11.99±0.20 |
| 10 |
15.77±0.37a |
60.36±0.86a |
27.30±2.05a |
12.34±1.19a |
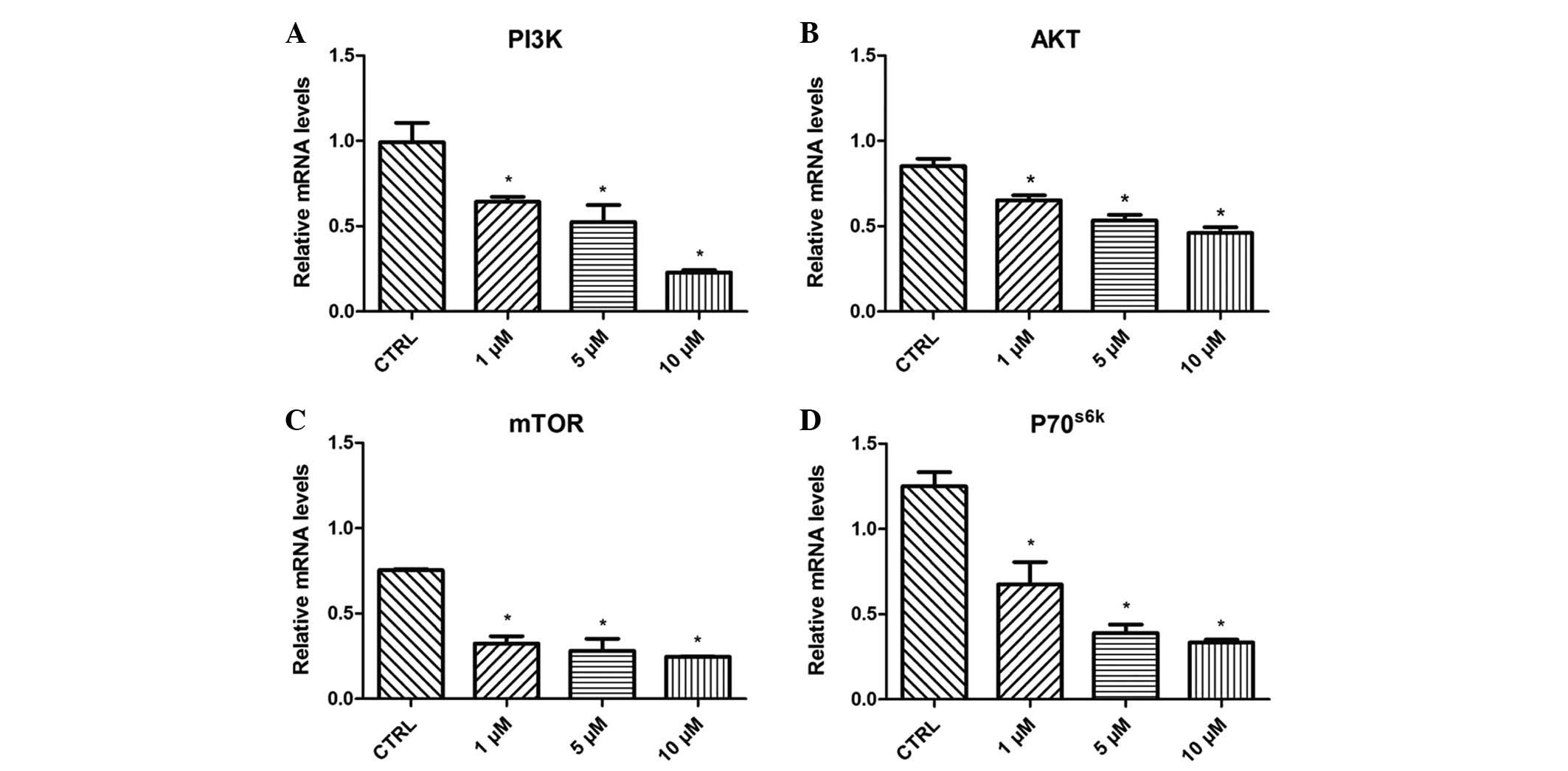
Effect of β-HIVS on PI3K, AKT, mTOR
and P70S6K mRNA expression
Treatment with 1, 5 and 10 µM β-HIVS resulted in
time- and concentration-dependent effects on the expression levels
of PI3K, AKT, mTOR and P70S6K mRNA in HeLa cells. At 48
h subsequent to treatment with β-HIVS, the expression of PI3K, AKT,
mTOR and P70S6K mRNA was significantly reduced. Compared
with the control group, the expression of PI3K in the 10 µM β-HIVS
group, AKT in the 1, 5 and 10 µM β-HIVS groups, mTOR in the 1, 5
and 10 µM β-HIVS groups and P70S6K in the 1, 5 and 10 µM
β-HIVS groups was significantly decreased (P<0.05; Fig. 5).
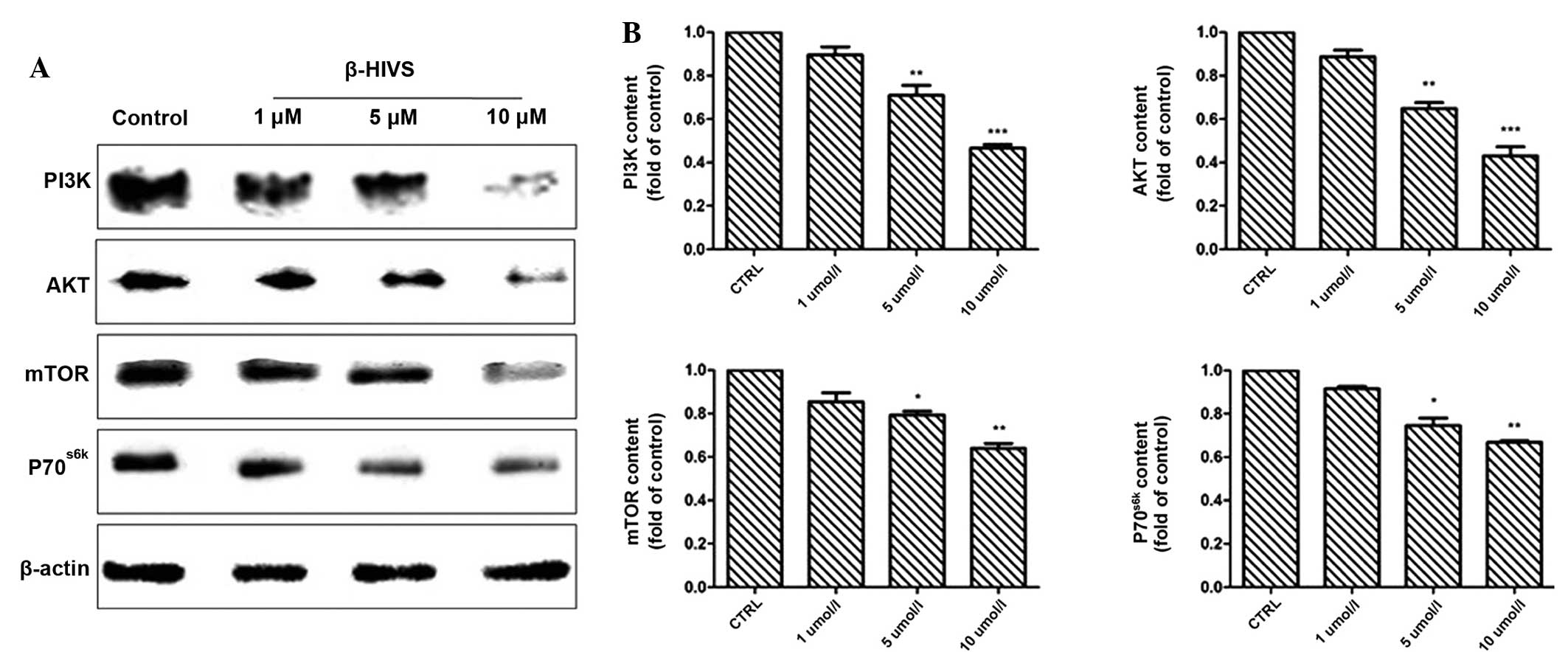
Effect of β-HIVS on PI3K, AKT, mTOR
and P70S6K protein expression
The results of western blot analysis revealed that
at 48 h subsequent to treatment with 1, 5 and 10 µM β-HIVS, the
expression of PI3K, AKT, mTOR and P70S6K was reduced. Compared with
the control group, the expression of PI3K in the 10 µM group, AKT
in the 1, 5 and 10 µM groups and mTOR in the 10 µM group was
significantly decreased (P<0.001), and the expression of mTOR in
the 5 µM group and P70S6K in the 5 and 10 µM groups was
also significantly decreased (P<0.05; Fig. 6).
Discussion
PI3K is a lipid kinase that regulates diverse
cellular processes, including the proliferation, adhesion,
survival, differentiation, apoptosis and motility of cells, and
exerts effects by activating AKT (17). Activated AKT contributes to cell
proliferation, the inhibition of apoptosis, cell cycle regulation,
the promotion of angiogenesis and chemotherapy resistance by
triggering downstream signaling, which results in the development
and progression of tumors (18,19).
mTOR is an atypical serine/threonine protein kinase,
with a molecular weight of 289 kDa (20–22), that
acts as a downstream substrate of AKT in the PI3K/AKT pathway
(20–22). AKT activates mTOR by phosphorylation
at serine 2,448 to improve the efficiency of mRNA translation,
increase the expression of a series of cell proliferation and
differentiation-associated proteins, and promote tumor development
and progression (23). Insulin-like
growth factor, nerve growth factor and platelet-derived growth
factor mediate and promote cell survival through various pathways,
and are important anti-apoptotic factors (18,24).
Welker et al (25) previously indicated that, through the
PI3K/AKT/mTOR signaling pathway, activated AKT activates mTOR and
regulates two downstream factors, consisting of eukaryotic
initiation factor 4E binding protein 1 and ribosomal S6 protein
kinase 1, in addition to controlling the translation of proteins
for cell proliferation and transformation. S6 protein kinase 1 and
4E binding protein 1 demonstrate positive and negative regulatory
effects on cell proliferation, respectively.
Gao et al (26)
verified that the activated PI3K/AKT/mTOR signaling pathway
upregulates the expression of cyclin and cyclin-dependent kinase 4
through P70S6K, promoting G1 progression, accelerating
the cell cycle and contributing to tumor progression. AKT activates
P70S6K, a downstream protein of mTOR, to promote actin
filament reconstruction and lead to tumor invasion and metastasis.
Therefore, the PI3K/AKT/mTOR cascade is a major signaling pathway
for protein synthesis, and the cascade is involved in cell
proliferation, differentiation and apoptosis, and tumor invasion
and metastasis (7,8). Phosphatase and tensin homolog is a tumor
suppressor that acts as a negative feedback regulator of
PI3K/AKT/mTOR signaling and antagonizes PI3K by converting
phosphatidylinositol-3,4,5-trisphosphate (PIP3) back to
phosphatidylinositol-4,5-bisphosphate by dephosphorylation of PIP3
at the 3′ position of the inositol ring. This negatively regulates
the activity of PI3K and downstream AKT/mTOR signaling, thus
inhibiting tumor growth (27).
It is important to identify the molecular targets of
natural antitumor drugs and to study the antitumor mechanism of
these agents. The root of Lithospermum erythrorhizon
(12), also termed arnebia, is a
perennial herb that contains complex chemical components. Diverse
natural shikonin-like compounds are derived from arnebia roots
using various methods. Natural shikonin-like compounds and the
derivatives of these compounds demonstrate cytotoxic actions and
antitumor effects. β-HIVS is a shikonin derivative that suppresses
tumor cell proliferation and induces tumor cell apoptosis (28,29).
Hashimoto et al (30)
confirmed that β-HIVS exerts cytotoxic effects on numerous types of
human carcinoma cell lines. When 10−6 M β-HIVS was used
to treat HL-60 cells for 3 h, typical apoptotic characteristics
were observed in the cells, including morphological changes,
nuclear fragmentation, DNA ladder formation and caspase-3
activation, which indicates that β-HIVS induced tumor cell
apoptosis through a caspase-3-dependent mechanism. Previous studies
(28,31) verified that β-HIVS exerted inhibitory
and proapoptotic effects on endometrial cancer, chorionic carcinoma
and ovarian cancer cells, with IC50 values ranging
between 10−6 and 10−8 M.
Furthermore, numerous studies have revealed that
β-HIVS is a selective inhibitor of topoisomerase I and an ATP
non-competitive inhibitor of protein tyrosine kinases (32–35).
β-HIVS regulates the cell cycle and apoptosis-associated protein
activity by inhibiting the activity of epidermal growth factor
receptor, decreasing dUTP nucleotidohydrolase activity and
suppressing vascular endothelial growth factor receptor activity
(36). β-HIVS has also been revealed
to inhibit tumor cell proliferation (35). Overall, there are various antitumor
targets of β-HIVS with complicated mechanisms of action, including
cell proliferation, apoptosis and signal transduction (16,34). Few
studies have assessed the effect of β-HIVS on cervical cancer or
the signal transduction pathway involved in mediating tumor cell
apoptosis.
The present study used MTT assays to detect the
effects of various concentrations of β-HIVS on human cervical
cancer HeLa cell proliferation at various time-points. The results
demonstrated that β-HIVS exerted significant inhibitory effects on
HeLa cell proliferation. In addition, with an increased β-HIVS
concentration and prolonged treatment time, the inhibitory effects
increased in a time- and dose-dependent manner. Tumor occurrence is
not only associated with abnormal cell proliferation, but is also
strongly correlated with abnormal apoptosis (37). Antitumor drugs used in the clinic
exert their effects mainly by inducing tumor cell necrosis and
apoptosis (38). In the present
study, an increased concentration of β-HIVS resulted in notable
alterations in the number and structure of apoptotic HeLa cells,
with a gradual increase in the apoptotic rate. Treatment with 10 µM
β-HIVS resulted in evident changes in cell morphology. The
proportion of apoptotic cells gradually increased, and late
apoptotic changes were observed, including chromatin condensation,
nuclear fragmentation, punctiform or lobulated nuclei, intact
membrane and membrane blebbing, with the presence of programmed
cell death. Flow cytometry revealed that the apoptotic rate of HeLa
cells evidently increased subsequent to treatment with β-HIVS,
indicating that β-HIVS suppressed HeLa cell proliferation by
inducing tumor cell apoptosis.
Therefore, the present study investigated the signal
transduction pathways that are activated by β-HIVS to result in the
apoptosis of HeLa cells. The PI3K/AKT/mTOR signaling pathway plays
an important role in tumor development and progression (9). Activation of this pathway is a key index
for the evaluation of the therapeutic effects of treatments and
patient prognosis (9). Thus, methods
of inhibiting this signaling pathway have been a focus in tumor
prevention and molecular-targeted therapy. The results from the
present study confirmed that β-HIVS exerts time- and
concentration-dependent effects on PI3K, AKT, mTOR and
P70S6K protein expression. The administration of a high
concentration of β-HIVS and a prolonged treatment time resulted in
an evident decrease in the expression of PI3K, AKT, mTOR and
P70S6K. Therefore, the present study hypothesized that
the molecular mechanism of the β-HIVS-mediated inhibition of HeLa
cell proliferation and promotion of apoptosis was as follows.
β-HIVS suppresses the activity of PI3K, downregulates PI3K protein
expression, regulates the AKT protein and the downstream target of
AKT mTOR, and also regulates P70S6K expression levels,
which results in the inhibition of HeLa cell proliferation. Thus,
this demonstrates the potential clinical value of β-HIVS.
In the present study, flow cytometry demonstrated
that β-HIVS elevated the apoptotic rate of HeLa cells, which was
strongly associated with the concentration of β-HIVS.
Simultaneously, β-HIVS altered the proliferation cycle of HeLa
cells, resulting in a reduced proportion of cells in the
G0/G1 phase, increased proportion of cells in
the S phase and no evident change in the proportion of
G2/M cells, in addition to cell cycle blockage in the S
phase. Overall, β-HIVS interfered with the cell cycle and then
suppressed tumor growth. Therefore, if β-HIVS is combined with
agents that target different phases of the cell cycle in the
clinic, chemotherapy resistance may be avoided.
The current study primarily explored the molecular
mechanism of β-HIVS on the induction of apoptosis in HeLa cells.
β-HIVS may inhibit tumor cell proliferation and increase the
apoptotic rate of tumor cells. In the current study, β-HIVS
promoted tumor cell apoptosis and exerted antitumor effects,
possibly by inhibiting the PI3K/AKT/mTOR signaling pathway and
affecting various downstream effector molecules in this cascade.
However, the involvement of other molecular pathways and the
association between these pathways remains poorly understood
(39). Numerous factors affect tumor
cell apoptosis, and regulation of apoptosis is complicated
(40). Thus, on the basis of these
in vitro findings regarding the PI3K/AKT/mTOR signaling
pathway, if the effects of β-HIVS on cervical cancer are studied
in vivo and the consistency of the in vivo and in
vitro experimental results is evaluated, a deeper understanding
of the occurrence, development, treatment and prognosis of cervical
cancer may be acquired. In addition, the present data revealed that
β-HIVS suppressed HeLa cell proliferation, induced tumor cell
apoptosis and interfered with the cell cycle of tumor cells,
highlighting the potential of β-HIVS as a novel high-performance
non-toxic antitumor agent. Blockade of the PI3K/AKT/mTOR signaling
pathway may effectively suppress tumor cell proliferation and
promote tumor cell apoptosis, which would be a novel strategy for
molecular-targeted therapy of cervical cancer.
Acknowledgements
This study was supported by the Jiangsu Provincial
Department of Science and Technology Social Development Project in
China (grant no. BS2006536) Jiangsu Provincial Higher Learning
Institute Postgraduate Science Research Innovation Project in China
(grant no. CXZZ13-0918).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roque DR, Wysham WZ and Soper JT: The
surgical management of cervical cancer: An overview and literature
review. Obstet Gynecol Surv. 69:426–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization: Diagnosis and
treatment of invasive cervical cancer. Comprehensive Cervical
Cancer Control: A Guide to Essential Practice (2nd). (Geneva).
World Health Organization. 153–175. 2014.
|
|
4
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eskander RN and Tewari KS: Exploiting the
therapeutic potential of the PI3K-AKT-mTOR pathway in enriched
populations of gynecologic malignancies. Expert Rev Clin Pharmacol.
7:847–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho DC: Targeting the PI3K/Akt/mTOR
pathway in malignancy: Rationale and clinical outlook. BioDrugs.
28:373–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han Z, Wu K, Shen H, Li C, Han S, Hong L,
Shi Y, Liu N, Guo C, Xue Y, et al: Akt1/protein kinase B alpha is
involved in gastric cancer progression and cell proliferation. Dig
Dis Sci. 53:1801–1810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J, Chen C and Zhao KN:
Phosphatidylinositol 3-kinase signaling as a therapeutic target for
cervical cancer. Curr Cancer Drug Targets. 13:143–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka S, Tajima M, Tsukada M and Tabata
M: A comparative study on anti-inflammatory activities of the
enantiomers, shikonin and alkannin. J Nat Prod. 49:466–469. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK-mediated process in
Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andújar I, Ríos JL, Giner RM and Recio MC:
Pharmacological properties of shikonin - a review of literature
since 2002. Planta Med. 79:1685–1697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andújar I, Recio MC, Giner RM and Ríos JL:
Traditional chinese medicine remedy to jury: The pharmacological
basis for the use of shikonin as an anticancer therapy. Curr Med
Chem. 20:2892–2898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu JJ, Bao JL, Wu GS, Xu WS, Huang MQ,
Chen XP and Wang YT: Quinones derived from plant secondary
metabolites as anti-cancer agents. Anticancer Agents Med Chem.
133:456–463. 2013. View Article : Google Scholar
|
|
15
|
Wang R, Yin R, Zhou W, Xu D and Li S:
Shikonin and its derivatives: A patent review. Expert Opin Ther
Pat. 22:977–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajasekar S, da Park J, Park C, Park S,
Park YH, Kim ST, Choi YH and Choi YW: In vitro and in vivo
anticancer effects of Lithospermum erythrorhizon extract on B16F10
murine melanoma. J Ethnopharmacol. 144:335–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martelli AM, Evangelisti C, Chiarini F and
McCubrey JA: The phosphatidylinositol 3-kinase/Akt/mTOR signaling
network as a therapeutic target in acute myelogenous leukemia
patients. Oncotarget. 1:89–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martelli AM, Evangelisti C, Chiarini F,
Grimaldi C, Cappellini A, Ognibene A and McCubrey JA: The emerging
role of the phosphatidylinositol 3-kinase/Akt/mammalian target of
rapamycin signaling network in normal myelopoiesis and
leukemogenesis. Biochim Biophys Acta. 1803:991–1002. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aimbetov R, Chen CH, Bulgakova O, Abetov
D, Bissenbaev AK, Bersimbaev RI and Sarbassov DD: Integrity of
mTORC2 is dependent on the rictor Gly-934 site. Oncogene.
31:2115–2120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang S and Houghton PJ: Targeting mTOR
signaling for cancer therapy. Curr Opin Pharmacol. 3:371–377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu G, Zhang W, Bertram P, Zheng XF and
McLeod H: Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR
pathway in common human tumors. Int J Oncol. 24:893–900.
2004.PubMed/NCBI
|
|
25
|
Welker ME and Kulik G: Recent syntheses of
PI3K/Akt/mTOR signaling pathway inhibitors. Bioorg Med Chem.
21:4063–4091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao N, Flynn DC, Zhang Z, Zhong XS, Walker
V, Liu KJ, Shi X and Jiang BH: G1 cell cycle progression and the
expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1
signaling in human ovarian cancer cells. Am J Physiol Cell Physiol.
287:C281–C291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Beta-hydroxyisovalerylshikonin has a profound
anti-growth activity in human endometrial and ovarian cancer cells.
Gynecol Oncol. 109:107–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rao Z, Liu X, Zhou W, Yi J and Li SS:
Synthesis and antitumour activity of β-hydroxyisovalerylshikonin
analogues. Eur J Med Chem. 46:3934–3941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hashimoto S, Xu M, Masuda Y, Aiuchi T,
Nakajo S, Cao J, Miyakoshi M, Ida Y, Nakaya K and Hashimoto S:
Beta-hydroxyisovalerylshikonin inhibits the cell growth of various
cancer cell lines and induces apoptosis in leukemia HL-60 cells
through a mechanism different from those of Fas and etoposide. J
Biochem. 125:17–23. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Anti-neoplastic effect of β-hydroxyisovalerylshikonin
on a human choriocarcinoma cell line. Mol Med Rep. 3:515–518. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakaya K and Miyasaka T: A shikonin
derivative beta-hydroxyisovalerylshikonin, is an
ATP-non-competitive inhibitor of protein tyrosine kinases.
Anticancer Drugs. 14:683–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashimoto S, Xu Y, Masuda Y, Aiuchi T,
Nakajo S, Uehara Y, Shibuya M, Yamori T and Nakaya K:
Beta-hydroxyisovalerylshikonin is a novel and potent inhibitor of
protein tyrosine kinases. Jpn J Cancer Res. 93:944–951. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kajimoto S, Horie M, Manabe H, Masuda Y,
Shibayama-Imazu T, Nakajo S, Gong XF, Obama T, Itabe H and Nakaya
K: A tyrosine kinase inhibitor, beta-hydroxyisovalerylshikonin,
induced apoptosis in human lung cancer DMS114 cells through
reduction of dUTP nucleotidohydrolase activity. Biochim Biophys
Acta. 1782:41–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Komi Y, Suzuki Y, Shimamura M, Kajimoto S,
Nakajo S, Masuda M, Shibuya M, Itabe H, Shimokado K, Oettgen P, et
al: Mechanism of inhibition of tumor angiogenesis by
beta-hydroxyisovalerylshikonin. Cancer Sci. 100:269–277. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishida M, Nasu K, Ueda T, Yuge A, Takai N
and Narahara H: Beta-hydroxyisovalerylshikonin induces apoptosis
and G0/G1 cell-cycle arrest of endometriotic stromal cells: A
preliminary in vitro study. Hum Reprod. 21:2850–2856. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boroughs LK and DeBerardinis RJ: Metabolic
pathways promoting cancer cell survival and growth. Nat Cell Biol.
17:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Castro J, Ribó M, Benito A and Vilanova M:
Mini-review: Nucleus-targeted ribonucleases as antitumor drugs.
Curr Med Chem. 20:1225–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin FP and Zhang M: Progress of
experimental researches on Chinese herbal compounds for inducing
tumor cell apoptosis. Chin J Integr Med. 16:565–571. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao X, Ai M, Guo Y, Zhou X, Wang L, Li X
and Yao C: Poly I: C-induced tumor cell apoptosis mediated by
pattern-recognition receptors. Cancer Biother Radiopharm.
27:530–534. 2012. View Article : Google Scholar : PubMed/NCBI
|