Introduction
Liver cancer is one of the most common neoplasms
worldwide, and hepatocellular carcinoma (HCC) is the most prevalent
type of liver cancer and accounts for 70–85% of all cases (1), ranking the sixth most common malignant
tumors worldwide (2). More than
600,000 people lose their lives due to HCC every year (3), and HCC is the third most frequent cause
of cancer-associated mortality (4).
Previous studies have demonstrated that the incidence rate of HCC
has been increasing. Surgical resection and liver-transplantation
are superior therapeutic methods for HCC compared with other
treatments, including radiofrequency ablation (RFA), transarterial
chemoembolization (TACE) and chemotherapy, the treatment outcomes
remain unsatisfactory owing to the high recurrence rate and high
fatality rate of HCC (2). If HCC was
detected and diagnosed earlier, surgical resection and
liver-transplantation would result in an improved curative effect,
therefore improving the disease-free and overall survival. The
serum levels of α-fetoprotein (AFP) and ultrasonography (US) are
widely used to screen for and assess HCC throughout the world.
However, the sensitivity and specificity of AFP for HCC diagnosis
and surveillance have certain limitations; US depends on the
operator's skill, and clinicians are not always able to distinguish
HCC clearly from other nodules (5).
Consequently, novel potential serum biomarkers are required for
prognosis and metastatic recurrence of HCC.
Hyaluronic acid (HA) is an extracellular matrix
(ECM) polymer and is frequently localized in the stroma of solid
tumors, which is synthesized by stromal fibroblasts in response to
paracrine factors produced by tumor cells (6). HA serves an important role in cellular
events, including cell migration, gene expression, signaling,
proliferation, motility, adhesion, metastasis and morphogenesis
(7,8).
The receptor for HA-mediated motility (RHAMM, also
known as CD168), which is an HA-binding protein, is located in
different parts of cell with different functions. For example, when
RHAMM combines with hyaluronan on the cell surface, it can activate
a signal transduction cascade to cause intracellular protein
tyrosine phosphorylation. In addition, it also locates in the
cytoplasm, centrosome, the cytoskeleton and nucleus (9). It can also interact with microtubules
and actin filaments, mitotic spindle assembly, which is important
to the organization of cytoskeletal network (10). RHAMM is not only a modulator of growth
factor receptor, but also has major roles in progression and
proliferation of various types of cancer (9,11). RHAMM
has been found in a variety of mammalian cells, including smooth
muscle cells, endothelial cells, nerve cells, macrophages,
spermatozoa (12,13), and certain types of tumor cell,
including prostate (14), breast
(15) and gastric (16) cancer, and B-cell chronic lymphocytic
leukemia (17). However, whether the
upregulation of RHAMM contributes to hepatocarcinogenesis of HCC
remains unclear. In the present study, it was demonstrated that
RHAMM was frequently upregulated in HCC and its upregulation was
closely associated with shorter disease-free and overall survival
of HCC patients.
Materials and methods
Patient specimens
A total of 187 HCC cancer patients who underwent
surgical resection at the Affiliated Hospital of Guilin Medical
University (Guilin, China) between November 2001 and April 2007
were enrolled in the present study. The patients were diagnosed by
the clinical symptoms, serological tests, US, computed tomography
(CT) scans, magnetic resonance imaging (MRI), and pathological
evaluations according to the ‘Primary Liver Cancer Clinical
Diagnosis and Staging Criteria’ (18). The clinicopathological characteristics
for these patients, including age, gender, family history,
hepatitis B surface antigen (HBsAg), AFP level, median size and
number, presence of combined liver cirrhosis, vascular invasion,
history of alcohol consumption, presence of distant metastasis,
tumor-node-metastasis (TNM), lymph node metastasis (LNM) and
incidence of postoperative recurrence, are presented in Table I. In addition, 10 specimens of normal
liver tissues adjacent to the hepatic hemangioma tissues were also
collected. Following the surgery, all the normal tissues were
examined by pathological examination. All of the samples were
immediately frozen in liquid nitrogen and then stored at −80°C
following the surgical resections. The present study was approved
by the Ethics Committee of the Affiliated Hospital of Guilin
Medical University. All patients provided written informed consent
to participate in this study according to the Declaration of
Helsinki.
 | Table I.Correlation between the
clinicopathologic variables and RHAMM mRNA expression in HCC. |
Table I.
Correlation between the
clinicopathologic variables and RHAMM mRNA expression in HCC.
|
|
|
| RHAMM mRNA |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical
characteristics | Variable | No. of patients | Low n (%) | High n (%) | χ2 | P-value |
|---|
| Age (years) | <55 | 129 | 41 (31.8) | 88
(68.2) | 1.101 | 0.294 |
|
| ≥55 | 58 | 23 (39.7) | 35
(60.3) |
|
|
| Gender | Male | 160 | 56 (35.0) | 104 (65.0) | 0.296 | 0.586 |
|
| Female | 27 | 8
(29.6) | 19
(70.4) |
|
|
| Family history | No | 158 | 53 (33.5) | 105 (66.5) | 0.209 | 0.647 |
|
| Yes | 29 | 11 (37.9) | 18
(62.1) |
|
|
| HBsAg | Negative | 31 | 10 (32.3) | 21
(67.7) | 0.064 | 0.801 |
|
| Positive | 156 | 54 (34.6) | 102 (65.4) |
|
|
| AFP (ng/ml) | <20 | 54 | 14 (25.9) | 40
(74.1) | 2.323 | 0.127 |
|
| ≥20 | 133 | 50 (37.6) | 83
(62.4) |
|
|
| Median size
(cm) | <5 | 69 | 25 (36.2) | 44
(63.8) | 0.196 | 0.658 |
|
| ≥5 | 118 | 39 (33.1) | 79
(66.9) |
|
|
| Cirrhosis | No | 15 | 4
(26.7) | 11
(73.3) | 0.414 | 0.520 |
|
| Yes | 172 | 60 (34.9) | 112 (65.1) |
|
|
| Tumor no. | Single | 128 | 42 (32.8) | 86
(67.2) | 0.359 | 0.549 |
|
| Multiple | 59 | 22 (37.3) | 37
(62.7) |
|
|
| Wine-drinking | No | 92 | 28 (30.4) | 64
(69.6) | 1.155 | 0.282 |
|
| Yes | 95 | 36 (37.9) | 59
(62.1) |
|
|
| TNM stage | I–II | 86 | 37 (43.0) | 49
(57.0) | 5.476 | 0.019 |
|
| III–IV | 101 | 27 (26.7) | 74
(73.3) |
|
|
| Vascular
invasion | No | 142 | 55 (38.7) | 87
(61.3) | 5.327 | 0.021 |
|
| Yes | 45 | 9
(20.0) | 36
(80.0) |
|
|
| Distant
metastasis | No | 169 | 57 (33.7) | 112 (66.3) | 0.192 | 0.661 |
|
| Yes | 18 | 7
(38.9) | 11
(61.1) |
|
|
| LNM | No | 173 | 60 (34.7) | 113 (65.3) | 0.215 | 0.643 |
|
| Yes | 14 | 4
(28.6) | 10
(71.4) |
|
|
| Recurrence | No | 124 | 49 (39.5) | 75
(60.5) | 4.578 | 0.032 |
|
| Yes | 63 | 15 (23.8) | 48
(76.2) |
|
|
The HCC patients were followed-up following surgery
by monitoring their serum AFP levels and performing US every 2
months and chest radiography every 6 months during the first 2
postoperative years and at 3–6 months intervals thereafter. A CT
scan or MRI was performed if recurrence was suspected based on a
rising of AFP level or abnormal US. Disease-free survival was
measured from the day of surgery to the date of recurrence,
metastasis, mortality or last follow-up. And overall survival was
measured from the day of surgery to the date of death or last
follow-up.
RNA extraction and cDNA synthesis
Total RNA was extracted from the frozen HCC tissue
samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To
reduce the risk of genomic DNA contamination, 1–2 µg RNA was
incubated with 2 µl DNase I (Invitrogen), 1 µl DNase buffer and 0.4
µl RNase out for 15 min at room temperature. The RNA concentration
was determined by spectrophotometry (170–2525, Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and total RNA integrity was
examined by visualization of the 28S and 18S ribosomal RNAs on a
1.2% agarose gel. First-strand cDNA was synthesized using the Prime
Script RT reagent kit (Takara Bio, Inc., Otsu, Japan) according to
the manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-PCR)
RT-qPCR analysis was performed using SYBR Premix Ex
Taq (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
instructions. The primer sequences for RHAMM (Invitrogen) were as
follows: F 5′-CAG CTG GAA GAT GAA GAA GGA-3′ and R 5′-GCA TGT AGT
TGT AGC TGA AAA GG-3′, and the length of the amplicon was 137 bp.
β-actin was used as the internal reference, with the primer
sequences (Invitrogen) designed as follows: F 5′-GAC AGG ATG CAG
AAG GAG ATT ACT-3′ and R 5′-TGA TCC ACA TCT GCT GGA AGGT-3′, and
the length of the amplicon was 142 bp. RT-qPCR amplification and
data analysis were performed using the ABI Prism 7500 Sequence
Detector system (Applied Biosystems, Foster City, CA, USA). Each
cDNA sample was mixed with 15 µl SYBR-Green PCR Master Mix (Applied
Biosystems). The thermal profile for the PCR reaction consisted of
an initial denaturation step at 95°C for 10 min, then 35 cycles of
denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec,
followed by extension at 72°C for 30 sec, and fluorescence
acquisition at 72°C. The mean Ct value for β-actin was subtracted
from the mean Ct value for RHAMM in each sample using the following
formula: RHAMM ΔCt = (mean RHAMM Ct - mean β-actin Ct). The
fold-change (2−RHAMMΔΔCt) of the RHAMM expression level
relative to the β-actin expression level was calculated for each
sample (19).
Immunohistochemistry (IHC) assay
The formalin-fixed, paraffin-embedded tissue blocks
were overlaid with the corresponding hematoxylin-eosin
(H&E)-stained slides for tissue microarray sampling. These
tissue array slides (HCC and ANLT) were reviewed by 2
histopathologists, and representative tumor areas that were free
form necrotic and hemorrhagic materials were pre-marked in the
paraffin blocks. These tissue microarray slides were de-waxed with
xylene (Sigma-Aldrich, St. Louis, MO, USA), rehydrated in graded
ethanol (Sigma-Aldrich), antigen-retrieved by pressure cooking for
3 min in ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.0;
Sigma-Aldrich), and washed in phosphate-buffered saline (PBS;
Sigma-Aldrich) 3 times (each wash was 3 min). The slides were then
immersed in 3% hydrogen peroxide (Sigma-Aldrich) for 10 min to
block endogenous peroxidase activity and pre-incubated with 10%
normal goat serum (Cayman Chemical Co., Ann Arbor, MI, USA) at room
temperature for 30 min to reduce nonspecific reaction.
Subsequently, the slides were incubated with a mouse polyclonal
anti-RHAMM antibody (catalog no. GR31505-2, 1:200 dilution; Abcam,
Cambridge, UK) at 4°C in a moist chamber overnight, and then washed
with PBS, incubated with a biotinylated goat anti-mouse antibody
(dilution, 1:2000; cat. no. ab97218, Abcam, Cambridge, UK) for 1 h,
and stained with 3,3′-diaminobenzidine tetrahydrochloride (DAB;
Sigma-Aldrich) at room temperature. The slides were counterstained
with Mayer's hematoxylin (Sigma-Aldrich), dehydrated and
mounted.
Statistical analysis
The statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Survival
curves were generated using the Kaplan-Meier method and checked for
statistical significance with the log-rank test. Qualitative
variables were compared with the Pearson's Chi-square
(χ2) test, and the quantitative variables were analyzed
by the independent Students t-test. Chi-square test was used for
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
RHAMM was upregulated in human
HCC
The levels of RHAMM mRNA expression in the 187 pairs
of human HCC and their ANLT were detected by RT-PCR. The results
demonstrated that RHAMM expression was upregulated in 123 (65.8%)
of the 187 HCC patients, while downregulated in 64 (34.2%) cases
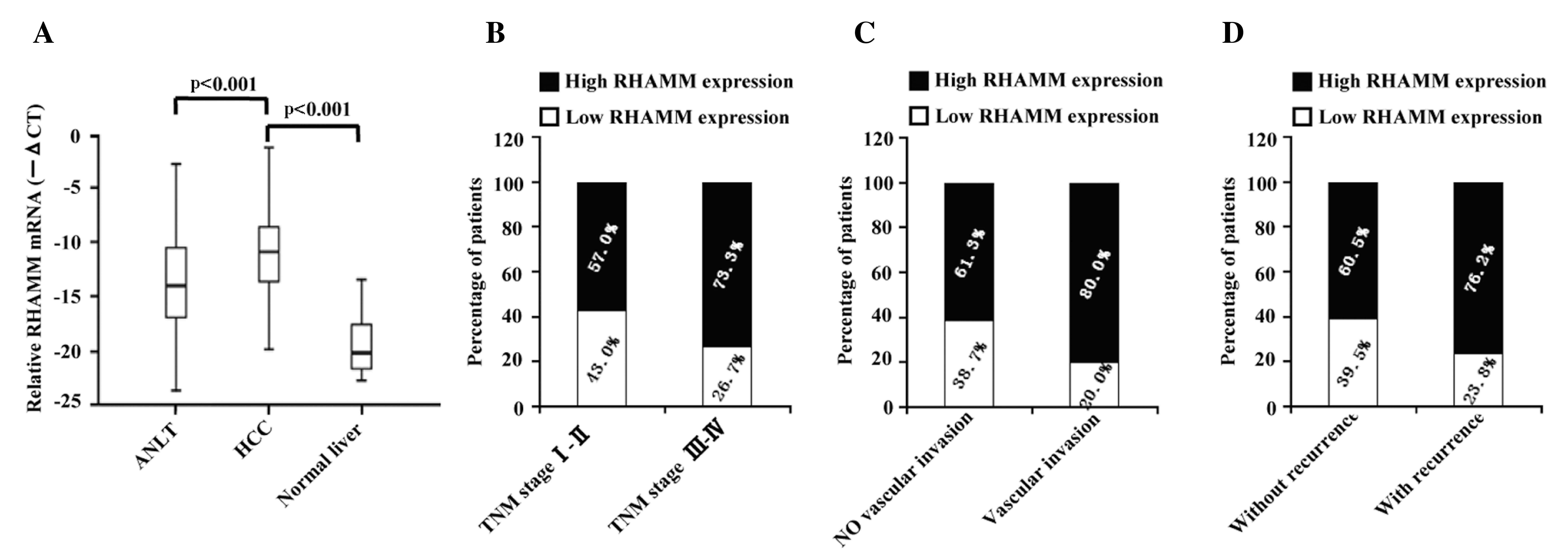
(P<0.001) (Fig. 1A), compared with
the corresponding ANLT. In addition, stratification analysis
demonstrated that the percentage of high RHAMM expression in the
patients whose TNM stage were III–IV was 73.3%, compared with 57%
in the patients with TNM stage I–II (P=0.019; Fig. 1B). The percentage of patients with
vascular invasion that expressed high levels of RHAMM was 80.0%,
compared with 61.3% patients without vascular invasion (P=0.021;
Fig. 1C). Furthermore, 76.2% of the
patients with recurrence expressed high levels of RHAMM, whereas
only 60.5% of patients without recurrence expressed high levels of
RHAMM (P=0.032 Fig. 1D).
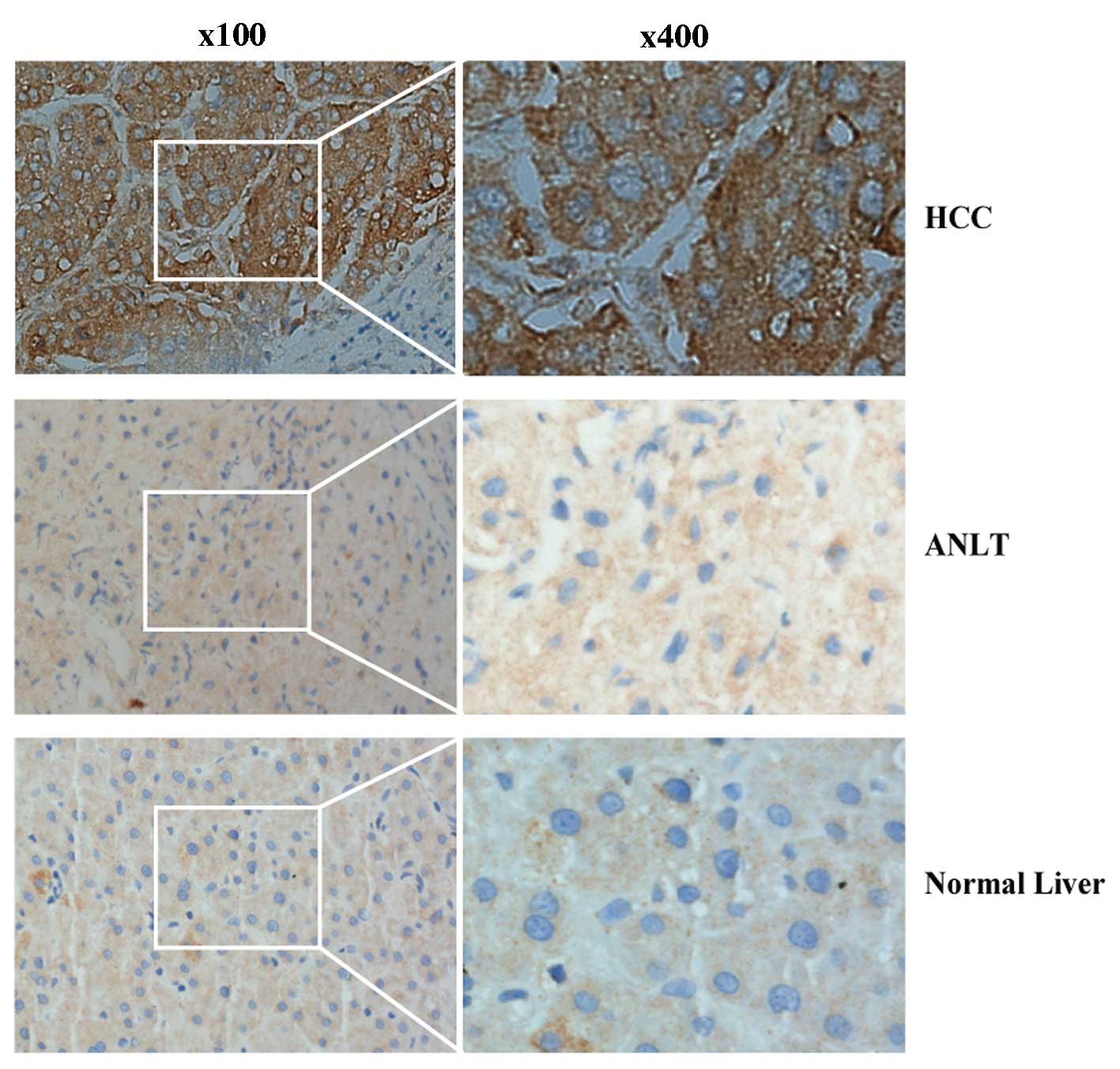
To further confirm the overexpression of RHAMM at
the protein level, IHC was performed on 25 pairs of HCC and
corresponding ANLT samples. The results demonstrated that RHAMM was
overexpressed in 18 (72%) of the 25 HCC tissues compared with their
ANLT (P=0.023), while RHAMM was overexpressed in only 10 (40%) of
the 25 ANLTs. In addition, the staining patterns indicated that
RHAMM was mostly anchored in the cytoplasm and ECM (Fig. 2). Taken together, these data indicate
that RHAMM expression levels were significantly upregulated in the
HCC samples compared with the ANLT.
Correlation between the expression of
RHAMM mRNA and clinicopathological data in HCC tissues
In order to further determine the correlation
between RHAMM expression and clinicopathological data in HCC, the
RHAMM expression and clinicopathological data were examined
(Table I). Notably, we found that
RHAMM expression was significantly higher in HCC patients with TNM
stage III–IV compared with those with TNM stage I–II
(χ2=5.476, P=0.019; Table
I). In addition, RHAMM expression was also much higher in HCC
patients with the presence of vascular invasion
(χ2=5.327, P=0.021) (Table
I) and recurrence (χ2=4.578, P=0.032) (Table I) compared with those without the
presence of vascular invasion and recurrence. No significant
association was observed between the RHAMM mRNA expression and age
(≥55 or <55 years), gender, family history, HBsAg, level of AFP
(≥20 ng/ml or <20 ng/ml), tumor median size (≥5 or <5cm) and
number, alcohol intake history, the presence of combined liver
cirrhosis, distant metastasis, and LNM (all P>0.05) (Table I). In conclusion, the upregulation of
RHAMM is associated with TNM stage, vascular invasion and
recurrence in HCC.
RHAMM overexpression, clinical
parameters were associated with disease-free and overall
survival
After analyzing all the data, it was observed that
high RHAMM mRNA expression levels, tumor size ≥5 cm, multiple tumor
number, TNM stage III–IV, and the presence of vascular invasion
(all P<0.01) were associated with shorter disease-free and
overall survival rates (Table II).
The presence of recurrence was also associated with shorter overall
survival rates.
 | Table II.Association between RHAMM expression,
clinical parameters and disease-free/overall survival. |
Table II.
Association between RHAMM expression,
clinical parameters and disease-free/overall survival.
|
|
|
| Disease-free
survival (months) | Overall survival
(months) |
|---|
|
|
|
|
|
|
|---|
| Clinical
characteristics | Category | No. of
patients | Mean | 95% CI | P-value | Mean | 95% CI | P-value |
|---|
| RHAMM
expression | Low | 64 | 49.62 | 40.80–58.43 | 0.005 | 52.95 | 44.70–61.21 | 0.008 |
|
| High | 123 | 32.67 | 26.92–38.42 |
| 39.30 | 33.63–44.97 |
|
| Age (years) | <55 | 129 | 38.86 | 32.66–45.06 | 0.560 | 43.71 | 37.91–49.51 | 0.630 |
|
| ≥55 | 58 | 41.75 | 32.85–50.64 |
| 45.76 | 37.37–54.16 |
|
| Gender | Female | 160 | 38.26 | 32.85–43.67 | 0.132 | 42.64 | 37.50–47.78 | 0.080 |
|
| Male | 27 | 45.79 | 33.92–57.66 |
| 50.98 | 40.31–61.64 |
|
| Family history | No | 158 | 39.23 | 33.69–44.76 | 0.611 | 43.62 | 38.43–48.82 | 0.459 |
|
| Yes | 29 | 43.42 | 30.42–56.42 |
| 48.52 | 36.23–60.81 |
|
| HBsAg | Negative | 31 | 44.69 | 31.72–57.66 | 0.501 | 48.73 | 36.96–60.50 | 0.492 |
|
| Positive | 156 | 39.05 | 33.52–44.57 |
| 43.81 | 38.58–49.04 |
|
| AFP (ng/ml) | <20 | 54 | 37.82 | 28.62–47.01 | 0.788 | 45.21 | 36.82–53.60 | 0.882 |
|
| ≥20 | 133 | 40.69 | 34.55–46.83 |
| 44.34 | 38.53–50.16 |
|
| Tumor size
(cm) | <5 | 69 | 58.02 | 50.23–65.80 |
<0.001 | 61.98 | 55.29–68.66 |
<0.001 |
|
| ≥5 | 118 | 29.01 | 23.11–34.91 |
| 33.76 | 28.06–39.45 |
|
| Cirrhosis | No | 15 | 38.91 | 20.49–57.33 | 0.942 | 45.11 | 28.78–61.43 | 0.777 |
|
| Yes | 172 | 39.68 | 34.35–45.00 |
| 44.28 | 39.27–49.28 |
|
| Tumor no. | Single | 128 | 45.65 | 39.44–51.86 |
<0.001 | 50.24 | 44.57–55.92 |
<0.001 |
|
| Multiple | 59 | 26.17 | 18.56–33.78 |
| 31.03 | 23.41–38.64 |
|
| Wine-drinking | No | 92 | 45.13 | 37.33–52.94 | 0.081 | 49.12 | 41.88–56.36 | 0.081 |
|
| Yes | 95 | 35.45 | 28.85–42.05 |
| 40.55 | 34.29–46.81 |
|
| TNM stage | I–II | 86 | 55.44 | 48.23–62.66 |
<0.001 | 59.72 | 53.34–66.10 |
<0.001 |
|
| III–IV | 101 | 25.82 | 19.99–31.66 |
| 31.44 | 25.51–37.38 |
|
| Vascular
invasion | No | 142 | 46.03 | 40.03–52.03 |
<0.001 | 50.66 | 45.17–56.15 |
<0.001 |
|
| Yes | 45 | 20.83 | 13.84–27.82 |
| 25.07 | 17.72–32.42 |
|
| Distant
metastasis | No | 169 | 40.90 | 35.49–46.30 | 0.193 | 45.50 | 40.43–50.56 | 0.191 |
|
| Yes | 18 | 27.07 | 15.04–39.09 |
| 33.36 | 20.50–46.23 |
|
| LNM | No | 173 | 40.88 | 35.52–46.24 | 0.114 | 45.42 | 40.41–50.42 | 0.137 |
|
| Yes | 14 | 26.19 | 12.85–39.54 |
| 31.91 | 17.63–46.18 |
|
| Recurrence | No | 124 |
|
|
| 37.54 | 31.60–43.49 |
<0.001 |
|
| Yes | 63 |
|
|
| 56.76 | 50.17–63.34 |
|
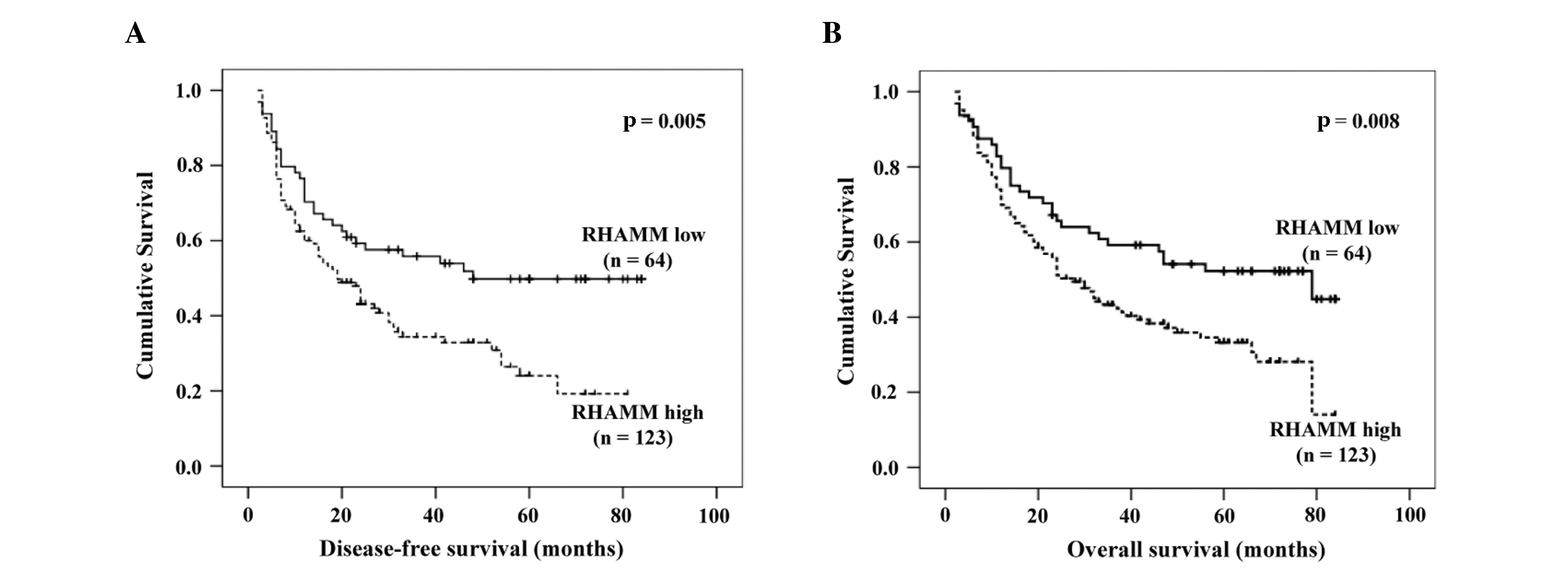
The median disease-free survival period for the 187
patients that expressed higher RHAMM levels was 32.67 months, with
a 95% confidence interval (CI) of 26.92–38.42 months; whereas, the
disease-free survival period for patients that expressed lower
RHAMM levels was 49.62 months, with a 95% CI of 40.80–58.43 months.
The results indicated that a higher RHAMM expression level was
associated with shorter disease-free survival period (P=0.005)
(Fig. 3A). The median overall
survival time for all patients with higher RHAMM expression levels
was 39.30 months, with a 95% CI of 33.63–44.97 months, whereas
those of patients with lower RHAMM expression levels was 52.95
months, with a 95% CI of 44.70–61.21 months. This result also
indicated that increased RHAMM expression levels were associated
with shorter overall survival rate (P=0.008) (Fig. 3B).
Overexpression of RHAMM was an
independent predictor for disease-free and overall survival
rates
In order to assess the prognostic significance of
RHAMM expression and clinical parameters, a Cox multivariate
proportional hazard model of independent predictors was established
for disease-free and overall survival (Table III). Tumor size ≥5 cm, multiple
tumor number, TNM stage III–IV, the presence of vascular invasion,
and overexpression of RHAMM were analyzed with Cox multivariate
proportional hazard model for disease-free and overall survival,
while recurrence was analyzed only used for overall survival.
 | Table III.Cox multivariate proportional hazard
model of independent predictors on disease-free and overall
survival. |
Table III.
Cox multivariate proportional hazard
model of independent predictors on disease-free and overall
survival.
| Variable | HR (95% CI) | P-value |
|---|
| Disease-free
survival |
|
|
| Tumor
size, cm (≥5 vs. <5) | 1.964
(1.188–3.247) |
0.008 |
| Tumor
no. (multiple vs. single) | 1.326
(0.877–2.005) |
0.181 |
| TNM
stage (III–IV vs. I–II) | 1.865
(1.157–3.007) |
0.011 |
|
Vascular invasion (yes vs.
no) | 1.375
(0.890–2.123) |
0.151 |
| RHAMM
expression (high vs. low) | 1.574
(1.025–2.419) |
0.038 |
| Overall
survival |
|
|
| Tumor
size, cm (≥5 vs. <5) | 1.738
(1.054–2.867) |
0.030 |
| Tumor
no. (multiple vs single) | 1.356
(0.922–2.106) |
0.125 |
| TNM
stage (III–IV vs. I–II) | 1.775
(1.109–2.843) |
0.017 |
|
Vascular invasion (yes vs.
no) | 1.599
(1.053–2.427) |
0.027 |
|
Recurrence (yes vs. no) | 2.194
(1.412–3.409) |
<0.001 |
| RHAMM
expression (high vs. low) | 1.739
(1.129–2.680) |
0.012 |
The median of hazard ratio (HR) of RHAMM expression
(high vs. low) was 1.574 with a 95% CI of 1.025–2.419 (P=0.038) for
disease-free survival, while the median was 1.739 with a 95% CI of
1.129–2.680 (P=0.012) for overall survival, which indicates that
high expression of RHAMM was an independent predictor for
disease-free and overall survival. Furthermore, other factors
including tumor size ≥5 cm (HR, 1.964; 95% CI, 1.188–3.247;
P=0.008), TNM stage III–IV (HR, 1.865; 95% CI, 1.157–3.007;
P=0.011) were also independent predictors for disease-free
survival. On the other hand, tumor size ≥5 cm (HR, 1.738; 95% CI,
1.054–2.867; P=0.030), III–IV of TNM stage (HR, 1.775; 95% CI,
1.109–2.843; P=0.017), the presence of vascular invasion (HR,
1.599; 95% CI, 1.053–2.427; P=0.027), and recurrence (HR, 1.739;
95% CI, 1.129–2.680; P<0.001) were independent predictors for
overall survival.
Discussion
Despite improvements in HCC treatment, the clinical
outcomes for HCC patients remain unsatisfactory, the reason for the
poor prognosis of HCC include the difficulty in early diagnosis and
the presence of tumor invasiveness, metastasis and recurrence
(20). Although serum AFP level has
been widely used for diagnosis of HCC, the sensitivity and
specificity of AFP for HCC are limited (21). Therefore, it is urgent to identify
novel potential biomarkers for early prognosis and monitoring of
tumor recurrence, which is helpful for clinicians to select
therapeutic strategies, so that the prognosis of HCC patients may
be improved. The present study was designed to investigate RHAMM
expression in HCC and its clinical significance.
The present study revealed that RHAMM mRNA
expression was significantly upregulated (65.78%, 123/187) in 187
pairs of primary HCC tissues compared with their paired ANLTs; this
result indicates that upregulation of RHAMM is a common incident in
HCC, which is in accordance with the a previous study that
demonstrated that RHAMM is upregulated in prostate cancer;
furthermore, RHAMM proteins responding to HA stimulation led to
activation of CD168/ROCK-signaling pathway, and the activation of
HA-mediated CD168 signaling were associated with the cancer stage,
Gleason's score, androgen-independent cell transformation and
metastatic status of high-grade prostate cancer (14). Notably, p53 has been proposed to be
able to repress expression of the RHAMM cell-surface and nuclear
matrix protein (22), thus the
expression of RHAMM, HA-mediated RHAMM signaling transduction and
metastasis may be repressed by p53. However, the underlying
mechanism of RHAMM upregulation in HCC remains unclear and future
research based on this molecule are strongly recommended.
Notably, the results of the present study indicated
that RHAMM upregulation is correlated with TNM stage, vascular
invasion and recurrence, which demonstrated that the upregulation
of RHAMM contributes to tumor node invasion, venous invasion and
recurrence in HCC. It has been demonstrated that RHAMM is
associated with tubulin and actin cytoskeletal elements (10,23),
indicating that RHAMM may be important to the movement and
reorganization of blastomeres during early embryonic development.
RHAMM has also been reported to be associated with cytoskeletal
interaction of mitochondria, thus, it may regulate mitochondrial
motility and positioning (24), which
is important for the physiological process of cytoplasmic
maturation and the further development of mammalian oocytes
(25). In addition, RHAMM regulates
Ras signaling and is involved in breast cancer progression
(26). The upregulation of RHAMM is
therefore closely involved in regulating tumorigenesis,
proliferation, tumor vascular invasion and node metastasis of HCC
cells.
In addition, the present study demonstrated that
upregulation of RHAMM is closely correlated with shorter
disease-free and overall survival rates in HCC patients as an
independent predictor. In HCC patients, disease-free survival is
closely associated with recurrence due to tumorigenesis,
proliferation, tumor cellular invasion, and metastasis of HCC
cells. Furthermore, IHC analysis demonstrated that RHAMM protein
level was higher in HCC tissues compared with their ANLTs, and the
RHAMM protein was mostly anchored in the cytoplasm and ECM, which
was in agreement with a study by Nedvetzki et al (27). RHAMM was the first discovered as
cell-associated hyaladherin with multiple forms on the cell surface
and in the cytoplasm. A previous study demonstrated that increased
expression of RHAMM has been reported to be associated with rapid
proliferation of tumor cells and reduced survival (9). RHAMM also forms complexes with CD44 (an
HA receptor), and the complexes modulate intracellular signaling to
promote invasion and metastasis of cancer cells (28). Furthermore, a study indicated that
high preoperative serum HA levels predict poor prognosis in
patients with HCC following hepatic resection (29). HA has been demonstrated to be involved
in different cellular processes, including cellular invasion,
tissue regeneration and angiogenesis (12,30,31). In
addition, HA suppresses HGF-induced cell differentiation through
RHAMM (32) and promotes cancer cell
motility through CD44-EGFR (33). HA
employs RHAMM to induce angiogenesis through recruitment of stromal
cells (34,35). In conclusion, upregulation of RHAMM
may result in the poor clinical prognosis observed in HCC
patients.
In conclusion, the present study provides evidence
that high expression of RHAMM was associated with TNM stage,
vascular invasion, and recurrence, demonstrating that it serves an
important role in tumor pathogenesis, vascular invasion and
recurrence of HCC. Furthermore, high RHAMM expression predicted
poor prognosis of HCC by analyzing the association between
expression of RHAMM and disease-free/overall survival of HCC
patients following surgical resections. Further evidenceis required
to determine the direct mechanisms of how RHAMM acts in regulating
tumorigenesis, proliferation, cellular invasion, and metastasis in
HCC cells. The results revealed an important role for RHAMM in HCC
progression and prognosis, and it may be regarded as a promising
therapeutic target for HCC.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (nos. 31370917,
30972797, 81430014, 81260328 and 81372163), the Natural Science
Foundation of Guangxi (nos. 2014GXNSFDA118019 and
2013GXNSFCA019012), the Science and Technology Planning Project of
Guangxi (no. 1140003B-79), the Lijiang Scholarship Foundation and
the Science and Technology Planning Project of Guilin (no.
20110119-1-8), the Project of Collaborative Innovation Center of
Universities in Guangxi, the Foundation of Distinguished Experts in
Guangxi, and the Science and Technology Planning Project of Guilin
(nos. 20140127-3 and 20100128-5).
References
|
1
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJF and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: World Gastroenterology Organisation Guidelines and
Publications Committee: World Gastroenterology Organisation
Guideline. Hepatocellular carcinoma (HCC): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
4
|
Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG and
Liang LJ: Prognostic significance of neutrophil-lymphocyte ratio in
hepatocellular carcinoma: A meta-analysis. BMC Cancer. 4:117–126.
2014. View Article : Google Scholar
|
|
5
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin SL, Chang D and Ying SY: Hyaluronan
stimulates transformation of androgen-independent prostate cancer.
Carcinogenesis. 28:310–320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stojkovic M, Krebs O, Kölle S, et al:
Developmental regulation of hyaluronan-binding protein
(RHAMM/IHABP) expression in early bovine embryos. Biol Reprod.
68:60–66. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park D, Kim Y, Kim H, Kim K, Lee YS, Choe
J, Hahn JH, Lee H, Jeon J, Choi C, et al: Hyaluronic acid promotes
angiogenesis by inducing RHAMM-TGFβ receptor interaction via
CD44-PKCδ. Mol Cells. 33:563–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shigeishi H, Higashikawa K and Takechi M:
Role of receptor for hyaluronan-mediated motility (RHAMM) in human
head and neck cancers. J Cancer Res Clin Oncol. 140:1629–1640.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Assmann V, Jenkinson D, Marshall JF and
Hart IR: The intracellular hyaluronan receptor RHAMM/IHABP
interacts with microtubules and actin filaments. J Cell Sci.
112:3943–3954. 1999.PubMed/NCBI
|
|
11
|
Koelzer VH, Huber B, Mele V, Iezzi G,
Trippel M, Karamitopoulou E, Zlobec I and Lugli A: Expression of
the hyaluronan-mediated motility receptor RHAMM in tumor budding
cells identifies aggressive colorectal cancers. Hum Pathol. Jul
29–2015.(Epub ahead of print). doi: 10.1016/j.humpath.2015.07.010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Savani RC, Cao G, Pooler PM, Zaman A, Zhou
Z and DeLisser HM: Differential involvement of the hyaluronan (HA)
receptors CD44 and receptor for HA-mediated motility in endothelial
cell function and angiogenesis. J Biol Chem. 276:36770–36778. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forteza R, Lieb T, Aoki T, Savani RC,
Conner GE and Salathe M: Hyaluronan serves a novel role in airway
mucosal host defense. FASEB J. 15:2179–2186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin SL, Chang D, Chiang A and Ying SY:
Androgen receptor regulates CD168 expression and signaling in
prostate cancer. Carcinogenesis. 29:282–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heldin P, Basu K, Olofsson B, Porsch H,
Kozlova I and Kahata K: Deregulation of hyaluronan synthesis,
degradation and binding promotes breast cancer. J Biochem.
154:395–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishigami S, Ueno S, Nishizono Y, Matsumoto
M, Kurahara H, Arigami T, Uchikado Y, Setoyama T, Arima H, Yoshiaki
K, et al: Prognostic impact of CD168 expression in gastric cancer.
BMC Cancer. 11:106–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannopoulos K, Li L, Bojarska-Junak A,
Rolinski J, Dmoszynska A, Hus I, Greiner J, Renner C, Döhner H and
Schmitt M: Expression of RHAMM/CD168 and other tumor-associated
antigens in patients with B-cell chronic lymphocytic leukemia. Int
J Oncol. 29:95–103. 2006.PubMed/NCBI
|
|
18
|
Verslype C, Rosmorduc O and Rougier P:
ESMO Guidelines Working Group: Hepatocellular carcinoma: ESMO-ESDO
Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23(Suppl 7): vii41–vii48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao W, Liu W, Yuan Q, Liu X, Ou Y, He S,
Yuan S, Qin L, Chen Q, Nong K, et al: Silencing of DLGAP5 by siRNA
significantly inhibits the proliferation and invasion of
hepatocellular carcinoma cells. PLOS One. 8:e80789–e80797. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YC, Xu Z, Zhang TF and Wang YL:
Circulating microRNAs as diagnostic and prognostic tools for
hepatocellular carcinoma. World J Gastroenterol. 21:9853–9862.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dong X, He H, Zhang W, Yu D, Wang X and
Chen Y: Combination of serum RASSF1A methylation and AFP is a
promising non-invasive biomarker for HCC patient with chronic HBV
infection. Diagn Pathol. 10:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Godar S and Weinberg RA: Filling the
mosaic of p53 actions: p53 represses RHAMM expression. Cell Cycle.
7:3479–3480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Assmann V, Marshall JF, Fieber C, Hofmann
M and Hart IR: The human hyaluronan receptor RHAMM is expressed as
an intracellular protein in breast cancer cells. J Cell Sci.
111:1685–1694. 1998.PubMed/NCBI
|
|
24
|
Lynn BD, Turley EA and Nagy JI:
Subcellular distribution, calmodulin interaction, and mitochondrial
association of the hyaluronan-binding protein RHAMM in rat brain. J
Neurosci Res. 65:6–16. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stojkovic M, Machado SA, Stojkovic P,
Zakhartchenko V, Hutzler P, Gonçalves PB and Wolf E: Mitochondrial
distribution and adenosine triphosphate content of bovine oocytes
before and after in vitro maturation: Correlation with
morphological criteria and developmental capacity after in vitro
fertilization and culture. Biol Reprod. 64:904–909. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Thor AD, Moore DH II, Zhao Y,
Kerschmann R, Stern R, Watson PH and Turley EA: The overexpression
of RHAMM, a hyaluronan-binding protein that regulates ras
signaling, correlates with overexpression of mitogen-activated
protein kinase and is a significant parameter in breast cancer
progression. Clin Cancer Res. 4:567–576. 1998.PubMed/NCBI
|
|
27
|
Nedvetzki S, Gonen E, Assayag N, Reich R,
Williams RO, Thurmond RL, Huang JF, Neudecker BA, Wang FS, Turley
EA, et al: RHAMM, a receptor for hyaluronan-mediated motility,
compensates for CD44 in inflamed CD44-knockout mice: A different
interpretation of redundancy. Proc Natl Acad Sci USA.
101:18081–18086. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Guo L, Li JW, Liu N, Qi R and Liu J:
Expression of hyaluronan receptors CD44 and RHAMM in stomach
cancers: relevance with tumor progression. Int J Oncol. 17:927–932.
2000.PubMed/NCBI
|
|
29
|
Mima K, Beppu T, Ishiko T, Chikamoto A,
Nakagawa S, Hayashi H, Watanabe M, Sakamaki K and Baba H:
Preoperative serum hyaluronic acid level as a prognostic factor in
patients undergoing hepatic resection for hepatocellular carcinoma.
Br J Surg. 101:269–276. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bourguignon LY, Gilad E, Peyrollier K,
Brightman A and Swanson RA: Hyaluronan-CD44 interaction stimulates
Rac1 signaling and PKN gamma kinase activation leading to
cytoskeleton function and cell migration in astrocytes. J
Neurochem. 101:1002–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Contreras EG, Gaete M, Sánchez N, Carrasco
H and Larraín J: Early requirement of Hyaluronan for tail
regeneration in Xenopus tadpoles. Development. 136:2987–2996. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Washio A, Kitamura C, Jimi E, Terashita M
and Nishihara T: Mechanisms involved in suppression of NGF-induced
neuronal differentiation of PC12 cells by hyaluronic acid. Exp Cell
Res. 315:3036–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim Y, Lee YS, Choe J, Lee H, Kim YM and
Jeoung D: CD44-epidermal growth factor receptor interaction
mediates hyaluronic acid-promoted cell motility by activating
protein kinase C signaling involving Akt, Rac1, Phox, reactive
oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem.
283:22513–22528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koyama H, Hibi T, Isogai Z, Yoneda M,
Fujimori M, Amano J, Kawakubo M, Kannagi R, Kimata K, Taniguchi S,
et al: Hyperproduction of hyaluronan in neu-induced mammary tumor
accelerates angiogenesis through stromal cell recruitment: Possible
involvement of versican/PG-M. Am J Pathol. 170:1086–1099. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Golshani R, Lopez L, Estrella V, Kramer M,
Iida N and Lokeshwar VB: Hyaluronic acid synthase-1 expression
regulates bladder cancer growth, invasion, and angiogenesis through
CD44. Cancer Res. 68:483–491. 2008. View Article : Google Scholar : PubMed/NCBI
|