Introduction
As an important fragment with biological functions,
mature microRNA-126 (miRNA/miR-126) is associated with the
angiogenesis, proliferation and differentiation of stem cells, as
well as the development of various tumors (1–4).
Osteosarcoma, the most common primary malignant tumor, has
exhibited a high prevalence in the past 20 years, therefore, the
establishment of novel chemotherapy drugs for osteosarcoma is
urgently required (5–7). Approximately 900 novel cases are
diagnosed each year in the USA (8,9). It is
estimated that osteosarcoma accounts for ~20% of bone cancers, the
incidence rate of osteosarcoma is 4.6–5.6% for the range 0–19 years
per year per million persons worldwide (10). Previous studies have demonstrated that
DDP plus gemcitabine was associated with a significant survival
advantage without the addition of substantial toxicity and MTX was
proved to improve disease-specific outcomes and reduce collateral
damage in patients with rheumatoid arthritis (11,12).
Cisplatin (DDP) and methotrexate (MTX) are commonly used as
clinical chemotherapy drugs, although their roles and effects in
the current treatment of osteosarcoma have not been fully
elucidated (13,14). Aberrant activation of the
PI3K/Akt/mTOR pathway is observed in numerous types of cancer and
is considered to serve a major role in breast cancer cell
proliferation and anti-cancer drug resistance (15), Moreover, Akt promotes protein
synthesis and cell growth by activating mTOR through effects on the
intermediary tuberous sclerosis 1/2 complex (16). In the present study, lentiviral
vectors overexpressing and silencing miR-126 were constructed to
infect a variety of osteosarcoma cells, so as to observe the
sensitivity of miR-126 to the clinical use of DDP and MTX in the
treatment of osteosarcoma cells. The study aimed to obtain results
supporting the treatment of osteosarcoma by DDP and MTX, thereby
enabling the use of personalized treatment programs and further
reducing the drug resistance to chemotherapy drugs.
Materials and methods
Cell culture and dosage
The MG63 and U-2 OS cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich,
St Louis, MO, USA) and placed in a 5% CO2 incubator.
When 80% of cell fusion was completed, the cells were digested with
0.25% trypsin and subcultured in accordance with requirements in
different Petri dishes until the cells grew to 70% confluence. In
this experiment, rapamycin (RAPA; 100 nm), DDP (20 µM) and MTX (100
µM) (Sigma-Aldrich) were selected, and the cells were treated with
the drugs for 48 h. Subsequent experiments could then be performed.
The study was approved by the ethics committee of the Second
Xiangya Hospital (Central South University, Changsha, China).
Construction of lentiviral vectors
overexpressing and silencing miR-126
A target gene, chemically synthesized and linearly
ligated into the AgeI lentiviral vector, was transformed
into competent bacterial cells. Once the first colony was
identified by polymerase chain reaction (PCR), the positive clones
were sequenced and comparatively analyzed. The total volume of the
reaction was 20 µl: 5 µl of sense oligonucleotide (200 µmol/l), 5
µl of antisense oligonucleotide (200 µmol/l), 2 µl of 10X annealing
buffer and 8 µl of ddH2O. A proper comparison was made
to successfully clone a target plasmid, while purified viruses were
measured by ELISA (KHB, Shanghai, China).
Establishing stably-infected
osteosarcoma cell lines
The level of miR-126 expression in the two
osteosarcoma cell lines, MG-63 and U-2 OS, was detected by
quantitative PCR following miR-126-overexpressing and -silencing
lentiviral vector infection. Total RNA was isolated from using
MirVana TM miRNA Isolation Kit (ABI, Roche, Branchburg, NJ, USA).
Then the total RNA was transcribed into cDNA at 16°C for 30 min,
42°C for 30 min, 85°C for 30 min, and stored at 4°C. The
reverse-transcription for GAPDH were conducted at 45°C for 1 h,
then 70°C for 10 min, and saved at 4°C. The detection of miR-126
and GAPDH were conducted using the fluorescent dye SYBR Green. The
sequences of the primers used for amplification were as follows:
miR-126, F 5′-ACA CTC CAG CTG GGT CGT ACC GTG AGT AAT-3′ and R
5′-TGG TGT CGT GGA GGA GTC-3′; GAPDH, F 5′-GAA GGT CGG AGT CAA CGG
ATT-3′ and R 5′-ATG GGT GGA ATC ATA TTG GAA-3′. The PCR reaction
for miR-126 were 95°C for 5 min, 95°C for 15 sec, 60°C for 15 sec
and a total 40 cycles were performed. GAPDH were conducted under
95°C for 5 min, 95°C for 15 sec, 60°C for 1 min and total of 50
cycles were performed. Fluorescence signals were collected at 85°C
and GAPDH was used as an internal reference. After PCR was
performed, the mRNA level was calculated by a comparative threshold
cycle (Ct) method using the formula 2−Δ∆Ct. miR-126
expression in the stably-transfected osteosarcoma cell lines was
tested for using immunofluorescence analysis.
MTT analysis of the effects of three
different chemotherapy drugs upon transfected cell
proliferation
Culture medium containing 10% calf serum was used to
prepare a single cell suspension, and ~10,000 cells per well were
seeded in 96-well plates. The cells were cultured overnight until
the cell density increased to 70%. The medium was replaced by 100
µl serum-free medium, cultured for 24 h and then synchronized. In
the experiment, the cells were divided into different treatment
groups, forming the control, RAPA (100 nM), DDP (20 µM) and MTX
(100 µM) groups. A total of 10 µl MTT (5 mg/ml) was added to each
well at different times (12, 24 and 48 h). The cells were incubated
for 4 h and then the culture was terminated. With the culture
supernatant in the net absorption hole, 150 µl dimethyl sulfoxide
was added to each well. Optical density values at a 570-nm
wavelength were measured with an automatic microplate reader
(Dynatech MR4000; Dynatec Laboratories, Inc., El Paso, TX,
USA).
Flow cytometry to detect the effects
of chemotherapeutic drugs on the apoptosis of transfected
cells
A total of 2 ml (1×106 cells/ml) of cells
were seeded in 6-well plates, then treated with 100 nM of RAPA, 20
µM of DDP and 100 µM of MTX and the culture was stopped after 48 h.
Following centrifugation for 5 min at 800 × g, the cells were
collected through sedimentation, while the supernatant was
discarded and washed twice with pre-cooled phosphate-buffered
saline (PBS). Cold 75% ethanol was added overnight at a fixed
temperature of 4°C. Following centrifugation at 1,500 × g for 5
min, the supernatant was discarded and the cells were washed with 3
ml of PBS once. In total, 400 µl ethidium bromide (50 µg/ml) and
100 µl RNase A (100 µg/ml) (Sigma-Aldrich) were added at 4°C in the
dark for 30 min. A total of 20,000 cells were counted according to
standard procedures through flow cytometry (Becton Dickinson,
Franklin Lakes, NJ, USA) and the treatment results were analyzed
with ModFit (Verity Software House, Chula Vista, CA, USA), the DNA
analysis software.
Flow cytometry for the detection of
the cell cycle in transfected cells
The cells in the logarithmic growth phase were
digested and counted. The cell concentration was adjusted to
1×105/ml and seeded in 6-well plates. Each well was
provided with 2 ml cell solution and the cells were cultured in a
5% CO2 incubator at 37°C. For the treatment groups, RAPA
(100 nm), DDP (20 µM) or MTX (100 µM) were then added in the
logarithmic growth phase. Meanwhile, a solvent control group was
set, with two wells at the same concentration. Once the cell
culture plate was cultured in the 5% CO2 incubator for
48 h, the fluid culture was collected in a centrifuge tube where
the cells were digested and collected at 4°C. The cells were
centrifuged at a rate of 1,000 × g for 5 min. The supernatant was
discarded and the cells were washed once with ice cold PBS. The
cells were collected in a centrifuge tube at 4°C and centrifuged at
1,000 × g for 5 min. The supernatant was discarded, while the ice
cold PBS cells were re-suspended, transferred to the Eppendorf tube
at 4°C and centrifuged at 1,000 × g for 5 min. The supernatant was
discarded again and 1 ml PBS was added. The re-suspended cells were
pre-cooled and fixed with 70% ethanol at 4°C. The cells were
centrifuged at 1,000 × g for 5 min and the ethanol solution was
discarded. Next, the cells were washed twice with PBS and filtered
through a 400-mesh sieve, prior to propidium iodide dye being added
at 4°C in darkness for staining for 30 min. The proportion of cells
in the G0/G1 phase, S phase and G2
phase was then detected by flow cytometry.
Statistical analysis
SPSS software, version 19.0 (SPSS, Inc., Chicago,
IL, USA) were conducted for data analysis. Continuous data were
presented with mean ± standard deviation and examined with test of
normality. Comparisons between two groups were conducted using
Student's t-test and comparisons among multi groups were performed
using homogeneity test of variance and one-way analysis of
variance. Pairwise comparisons on mean values were determined using
LSD t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-126 overexpression is associated
with the inhibitory effects of DDP and MTX upon the proliferation
of osteosarcoma cells
The titer of miR-126-overexpressing and -silencing
recombinant lentivirus was 1.07×109 U/ml. Reverse transcription-PCR
analysis showed that the miR-126 mRNA expression was increased in
the stably-transected osteosarcoma cells overexpressing miR-126,
while miR-126 mRNA expression was lowered in the osteosarcoma cells
transfected with miR-126-silencing lentiviral vectors (data not
shown).
The MTT assay showed that DDP and MTX exhibited
inhibitory effects on the proliferation of the two osteosarcoma
cell lines, MG63 and U-2 OS (both P<0.05), while RAPA, an
inhibitor of the mammalian target of rapamycin (mTOR) signaling
pathway, did not significantly inhibit cell proliferation compared
with the control (P>0.05). During miR-126 overexpression, cell
proliferation was observed to be weaker, whereas cell proliferation
became stronger as the expression of miR-126 was lowered in the
DDP-treated cells at the same concentration (Fig. 1A and B).
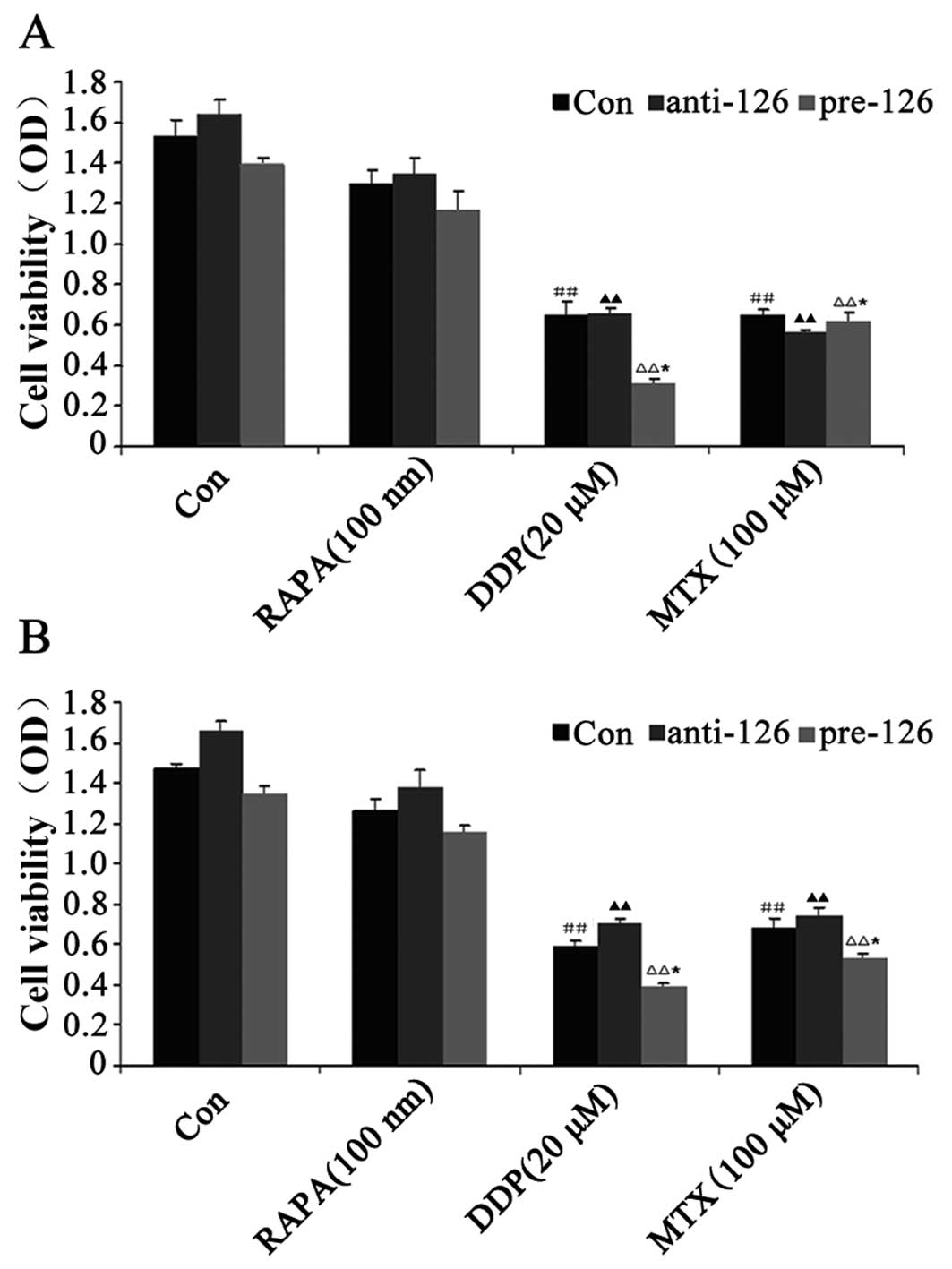 | Figure 1.Effect of DDP, MTX and RAPA on
osteosarcoma cell proliferation. (A) MG63 osteosarcoma cells were
infected with miR-126-overexpressing or -silencing lentiviral
vectors. The effect of DDP, MTX and RAPA on osteosarcoma cell
proliferation was then observed by MTT. (B) U-2 OS osteosarcoma
cells were infected with miR-126-overexpressing or -silencing
lentiviral vectors. The effect of DDP, MTX and RAPA on bone tumor
cell proliferation was then observed by MTT.
##,▲▲P<0.01 vs. control group; *P<0.05 vs.
anti-miR-126 group. DDP, cisplatin; MTX, methotrexate; RAPA,
rapamycin; pre-miR-126, osteosarcoma cells infected with
miR-126-overexpressing lentiviral vectors; anti-miR-126,
osteosarcoma cells infected with miR-126-silencing lentiviral
vectors; OD, optical density. |
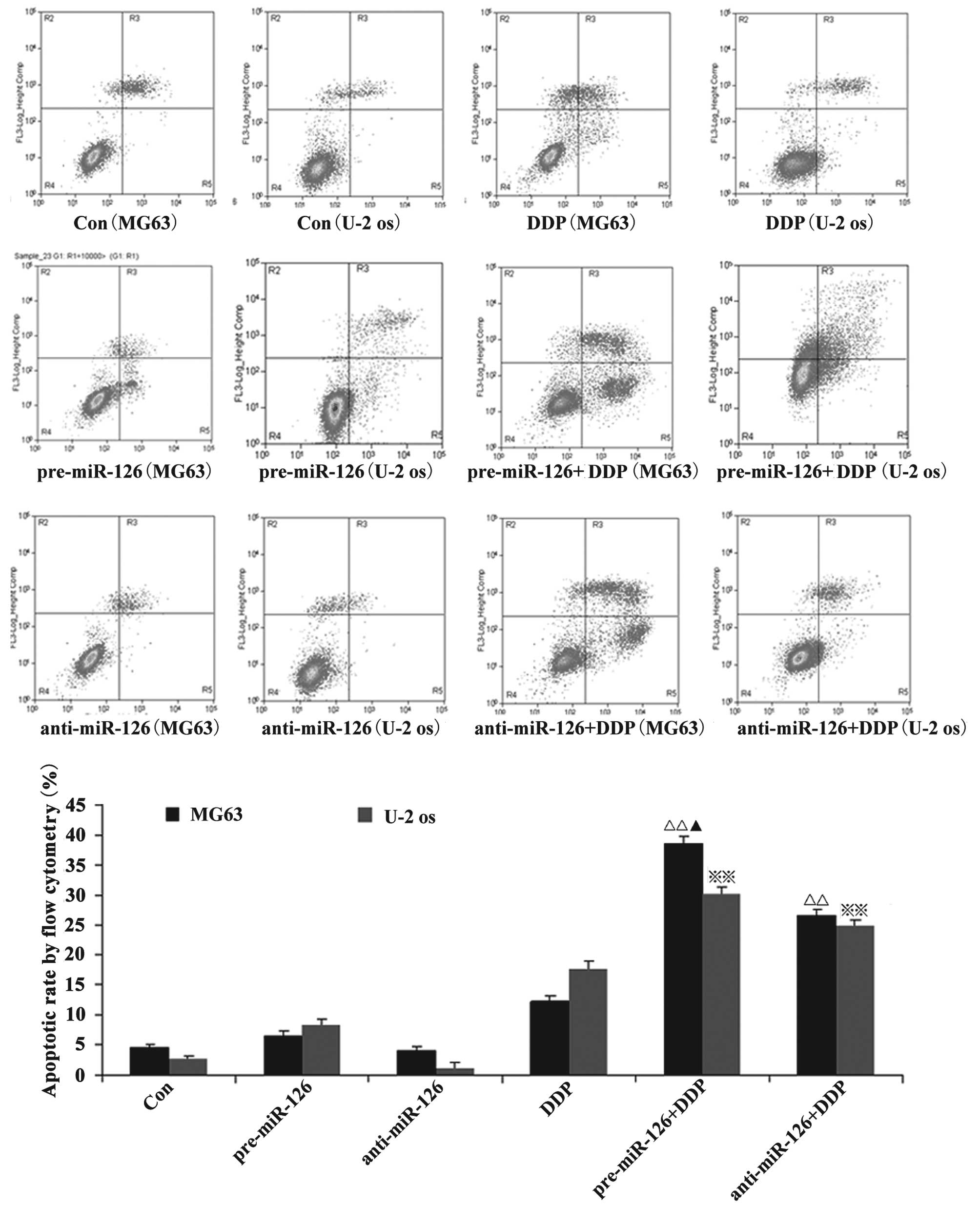
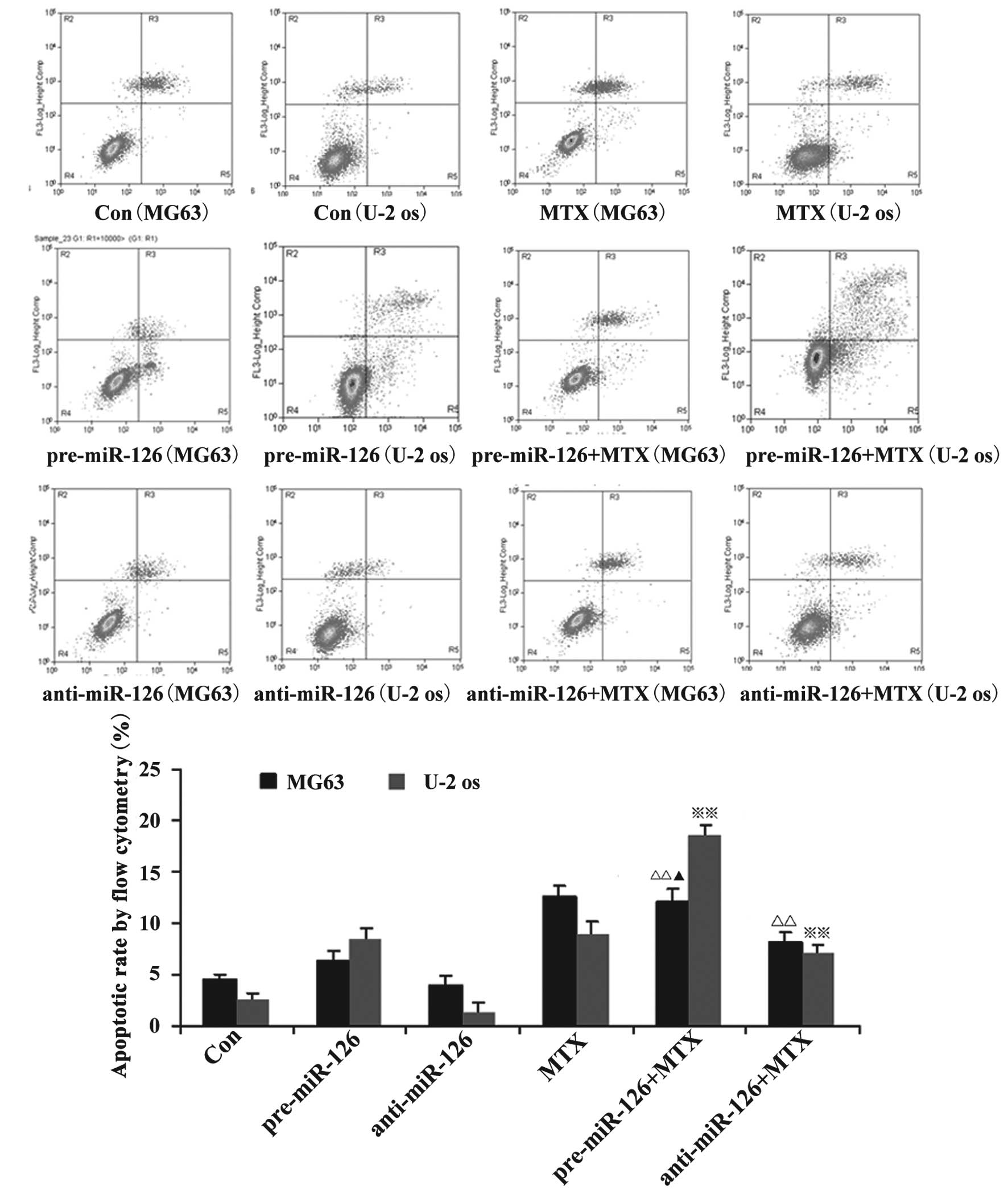
miR-126 overexpression is associated
with the effects of DDP and MTX in the promotion of apoptosis in
osteosarcoma cells
Flow cytometry showed that the apoptotic rate of the
osteosarcoma cells increased with the overexpression of miR-126,
while the silencing of miR-126 expression interfered with the
apoptotic rate. When DDP or MTX were applied, the apoptotic rate of
the osteosarcoma cells was significantly promoted by miR-126
overexpression (Figs. 2 and 3; both P<0.05).
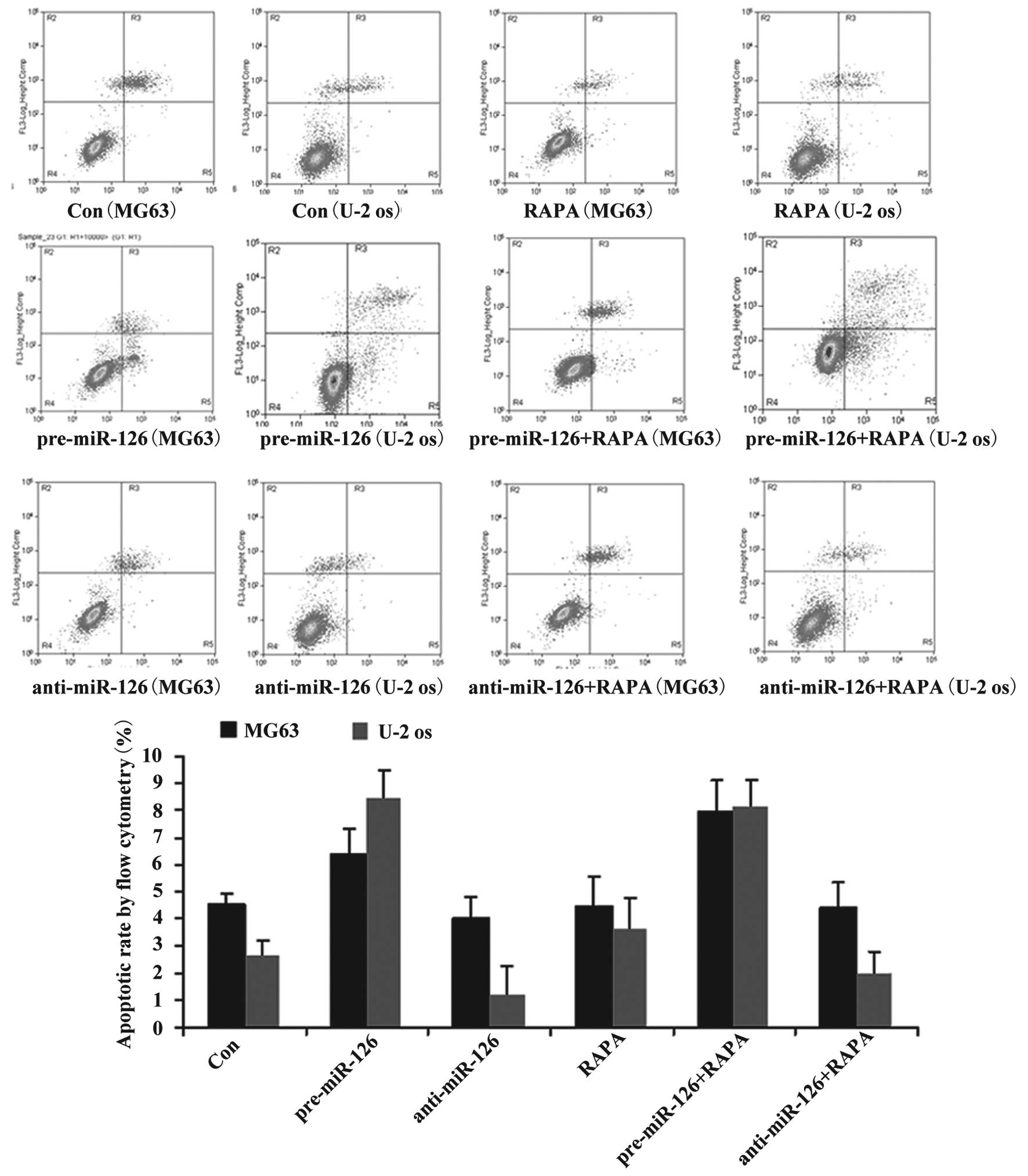
Although DDP or MTX have significant effects on the
apoptotic rate in the miR-126-silenced osteosarcoma cells (both
P<0.05), RAPA did not significantly promote the apoptosis of the
osteosarcoma cells (Fig. 4;
P>0.05).
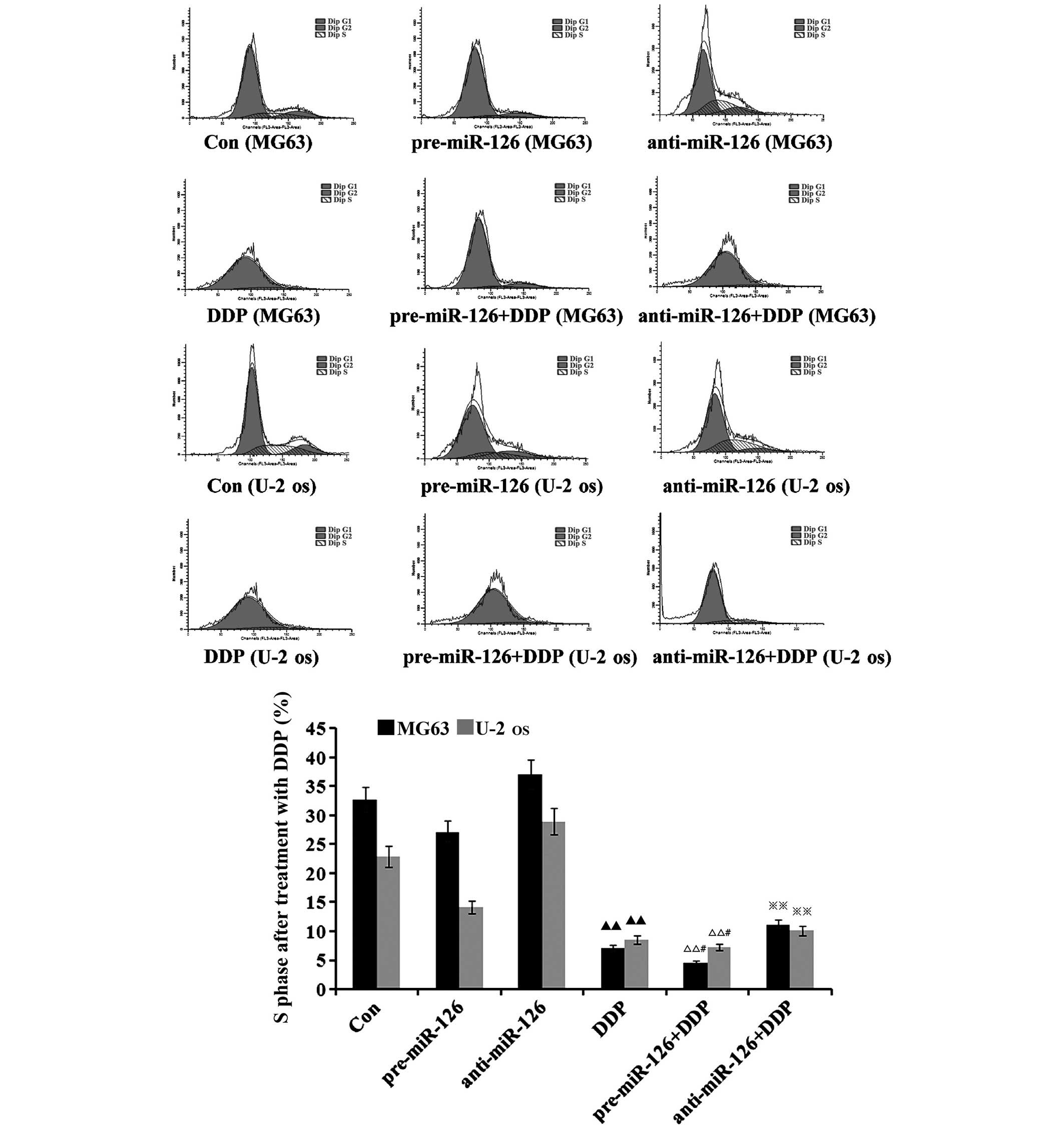
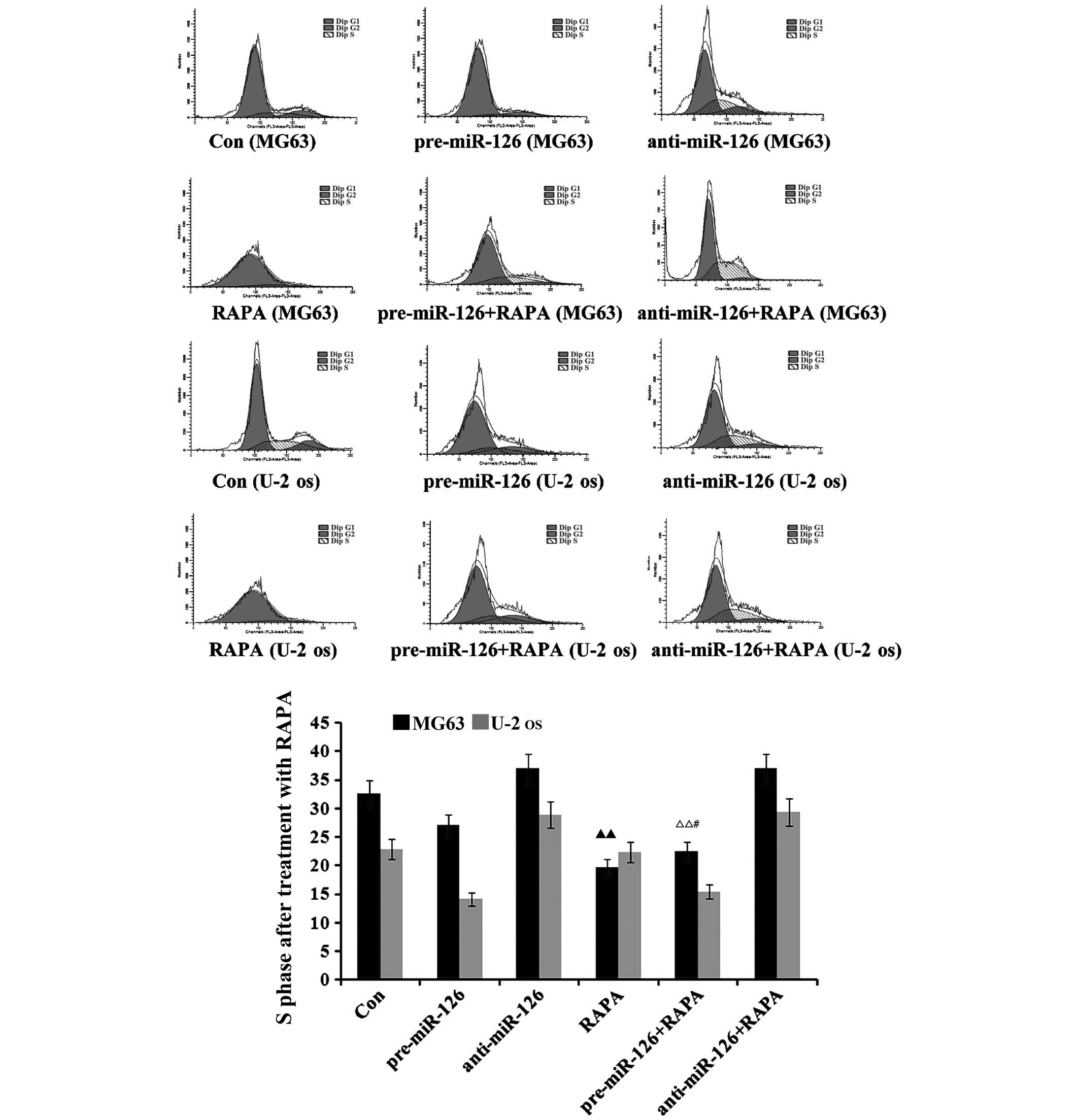
miR-126 overexpression is associated
with the inductive effects of DDP upon cycle of osteosarcoma
cells
The flow cytometry suggested that there would be a
decrease in the proportion of osteosarcoma cells in the S phase as
DDP, MTX and RAPA were added to the osteosarcoma cells (Figs. 5–7). DDP
had the best inhibitory effect (both P<0.05), which was
connected with changes in the miR-126 expression in the two
osteosarcoma cell lines; the proportion of osteosarcoma cells in
the S phase decreased with the increase of miR-126, while it
increased as miR-126 was silenced (Fig.
5; both P<0.01).
Discussion
As regulators of gene expression, miRNAs are
involved in regulating multiple biological processes, including
cell growth, apoptosis, tissue growth, tissue morphogenesis and the
regulation of tumor growth (17–19).
Highly expressed in human endothelial cells, miR-126 is closely
associated with several types of cancer and is possibly one of the
future means for cancer treatment (4,20). Each
tumor has its specific miRNA expression profiles, so miRNAs may be
utilized for the early diagnosis of tumors and for predicting
prognosis (21,22). The changes to the expression of miRNAs
are closely connected with the formation and progression of human
cancer. Current literature suggests that ~50% of miRNAs are located
within the genomic region associated with cancer, so miRNAs can be
used as oncogenes or tumor suppressor genes.
Osteosarcoma is a progressive and fatal malignant
bone tumor with a nearly unchanged case fatality rate (23). At present, the majority of studies
advocate the comprehensive treatment of osteosarcoma, including the
use of surgery, radiotherapy, chemotherapy and immune therapy
(24–26). Chemotherapy was first used for the
palliative treatment of advanced cases and achieved effects to a
certain degree, thus promoting its application in the adjuvant
treatment of early-stage patients to prevent metastasis. A previous
study found that osteosarcoma cells exhibited low level miR-126
expression and showed that the roles of this miRNA in osteosarcoma
are not fully clear yet.
MTX has been primarily used for treating leukemia,
choriocarcinoma, lung cancer, breast cancer and malignant lymphoma
in the early stages (27,28). Due to problems with drug resistance,
its efficacy is somewhat limited when used at a conventional dose.
Commonly used in the clinic as an anticancer drug, the relevant
literature at home and abroad has reported that DDP has significant
anti-tumor effects, is able to treat a variety of advanced cancers
(including ovarian, breast and lung cancer) and can achieve ideal
results in osteosarcoma cases when it is used together with MTX as
a combination chemotherapy. In the present study,
miR-126-overexpressing and -silencing lentiviral vectors were
created to observe the impact of DDP and MTX on osteosarcoma cell
proliferation, apoptosis and the cell cycle in two different
osteosarcoma cell lines, MG63 and U-2 OS.
The experimental results showed that DDP and MTX
inhibited the proliferation of the two osteosarcoma cell lines with
miR-126 overexpression. As a tumor suppressor gene, miR-126 may
directly affect the sensitivity of cells to DDP through its
expression. During the overexpression of miR-126, the proliferative
ability of the osteosarcoma cells became weak, while the inhibitory
effect upon cell proliferation when the miR-126 expression was
lowered and the osteosarcoma cells were treated with DDP at the
same concentration. This indicates that the level of miR-126
expression may not be associated with the inhibitory effect of DDP
on the proliferation of osteosarcoma cells. In this study, it was
observed that the apoptosis rate increased after miR-126
overexpression, but decreased after miR-126 expression was
silenced. With the application of DDP and MTX, the apoptosis rate
of the cells with miR-126 overexpression was significantly
increased. It is therefore clear that miR-126 is associated with
the apoptosis of osteosarcoma cells. Furthermore, DDP and MTX, as
chemotherapy drugs, are involved in the regulation of this
apoptosis of osteosarcoma cells through miR-126. Moreover, Zhang
et al (29) demonstrated that
cisplatin treatment could induce the knockdown of Akt2 expression
to enhance the efficacy of chemotherapy in patients with
osteosarcoma. Consistent with the results of the present study, a
previous study showed that miR-126, as a tumor suppressor in
osteosarcoma, whose ectopic expression inhibited invasion and
induced apoptosis in osteosarcoma cells (30).
Flow cytometry suggested that the proportion of
osteosarcoma cells in the S phase was reduced following the
addition of DDP and MTX to the osteosarcoma cells. Furthermore, DDP
exhibited the best inhibitory effects upon the cell cycle and was
associated with the changes to the miR-126 expression level in the
two osteosarcoma cell lines. This indicates that miR-126 is
associated with the cell cycle of osteosarcoma cells and that DDP
affects this cycle through miR-126, while the effects of
chemotherapy may be enhanced when MTX is used together with DDP. To
observe the signaling pathways through which miR-126 inhibits the
proliferation and apoptosis of osteosarcoma cells, RAPA, an
inhibitor of the mTOR pathway, was selected in this study. The
results suggested that miR-126 may fail to give its roles into play
in osteosarcoma cells via mTOR signals and its specific regulatory
mechanisms remain to be further studied.
In summary, in the present study, DDP and MTX
inhibited the proliferation of two osteosarcoma cell lines and
promoted their apoptosis. Meanwhile, the effects of the drugs were
dependent upon the high expression of miR-126 in the osteosarcoma
cells. This further indicates that miR-126 may strengthen the
sensitivity of osteosarcoma cells to DDP and MTX. This means that
it is necessary to first detect the expression of miR-126 in
patients when DDP and MTX are clinically used for treating
osteosarcoma, so as to aid in increasing their sensitivity to the
drugs and to improve the therapeutic effects.
Acknowledgements
This study was supported by the independent
exploration and innovation project of Central South University
Doctoral Students (grant no. 2013zzts096).
Glossary
Abbreviations
Abbreviations:
|
DDP
|
cisplatin
|
|
RAPA
|
rapamycin
|
|
miR
|
microRNA
|
|
MTX
|
methotrexate
|
References
|
1
|
Chang SH and Hla T: Post-transcriptional
gene regulation by HuR and microRNAs in angiogenesis. Curr Opin
Hematol. 21:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kane NM, Howard L, Descamps B, Meloni M,
McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, et
al: Role of microRNAs 99b, 181a and 181b in the differentiation of
human embryonic stem cells to vascular endothelial cells. Stem
Cells. 30:643–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mishra RR, Kneitz S and Schartl M:
Comparative analysis of melanoma deregulated miRNAs in the medaka
and Xiphophorus pigment cell cancer models. Comp Biochem Physiol C
Toxicol Pharmacol. 163:64–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu JQ, Liu P, Si MJ and Ding XY:
MicroRNA-126 inhibits osteosarcoma cells proliferation by targeting
Sirt1. Tumour Biol. 34:3871–3877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bago-Horvath Z, Schmid K, Rössler F,
Nagy-Bojarszky K, Funovics P and Sulzbacher I: Impact of RANK
signalling on survival and chemotherapy response in osteosarcoma.
Pathology. 46:411–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guma SR, Lee DA, Ling Y, Gordon N and
Kleinerman ES: Aerosol interleukin-2 induces natural killer cell
proliferation in the lung and combination therapy improves the
survival of mice with osteosarcoma lung metastasis. Pediatr Blood
Cancer. 61:1362–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohba T, Cole HA, Cates JM, Slosky DA, Haro
H, Ando T, Schwartz HS and Schoenecker JG: Bisphosphonates inhibit
osteosarcoma-mediated osteolysis via attenuation of tumor
expression of MCP-1 and RANKL. J Bone Miner Res. 29:1431–1445.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rabinowicz R, Barchana M, Liphshiz I,
Futerman B, Linn S and Weyl-Ben-Arush M: Cancer incidence and
survival among children and adolescents in Israel during the years
1998 to 2007. J Pediatr Hematol Oncol. 34:421–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salas SI, Jiguet-Jiglaire C, Campion L,
Bartoli C, Frassineti F, Deville JL, De Maues Paula A, Forest F,
Jézéquel P, Gentet JC and Bouvier C: Correlation between ERK1 and
STAT3 expression and chemoresistance in patients with conventional
osteosarcoma. BMC Cancer. 14:6062014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al: ABC-02 Trial Investigators: Cisplatin plus gemcitabine
versus gemcitabine for biliary tract cancer. N Engl J Med.
362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westlake SLI, Colebatch AN, Baird J, Kiely
P, Quinn M, Choy E, Ostor AJ and Edwards CJ: The effect of
methotrexate on cardiovascular disease in patients with rheumatoid
arthritis: a systematic literature review. Rheumatology (Oxford).
49:295–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin F, Wang Q, Yu W, Tang L, Zheng S, Sun
Y, Shen Z, Yao Y and Dong Y: Clinical analysis of Chinese limb
osteosarcoma patients treated by two combinations of methotrexate,
cisplatin, doxorubicin and ifosfamide. Asia Pac J Clin Oncol.
7:270–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang TM, Qi SN, Zhao N, Yang YJ, Yuan HQ,
Zhang B and Jin S: Induction of apoptosis through
caspase-independent or caspase-9-dependent pathway in mouse and
human osteosarcoma cells by a new nitroxyl spin-labeled derivative
of podophyllotoxin. Apoptosis. 18:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghayad SE and Cohen PA: Inhibitors of the
PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent
Pat Anticancer Drug Discov. 5:29–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markman B, Dienstmann R and Tabernero J:
Targeting the PI3K/Akt/mTOR pathway - beyond rapalogs. Oncotarget.
1:530–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gits CM, van Kuijk PF, Jonkers MB, Boersma
AW, Smid M, van Ijcken WF, Coindre JM, Chibon F, Verhoef C,
Mathijssen RH, et al: MicroRNA expression profiles distinguish
liposarcoma subtypes and implicate miR-145 and miR-451 as tumor
suppressors. Int J Cancer. 135:348–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guled M, Pazzaglia L, Borze I, Mosakhani
N, Novello C, Benassi MS and Knuutila S: Differentiating soft
tissue leiomyosarcoma and undifferentiated pleomorphic sarcoma: A
miRNA analysis. Genes Chromosomes Cancer. 53:693–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tafra R, Brakus SM, Vukojevic K, Kablar B,
Colovic Z and Saraga-Babic M: Interplay of proliferation and
proapoptotic and antiapoptotic factors is revealed in the early
human inner ear development. Otol Neurotol. 35:695–703. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Zhou Y, Feng X, Yang P, Yang J, An
P, Wang H, Ye S, Yu C, He Y and Luo H: Low expression of
microRNA-126 is associated with poor prognosis in colorectal
cancer. Genes Chromosomes Cancer. 53:358–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rabinowits G, Gercel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
23
|
Guma SR, Lee DA, Yu L, Gordon N, Hughes D,
Stewart J, Wang WL and Kleinerman ES: Natural killer cell therapy
and aerosol interleukin-2 for the treatment of osteosarcoma lung
metastasis. Pediatr Blood Cancer. 61:618–626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rainusso N, Brawley VS, Ghazi A,
Gottschalk S, Rosen JM and Ahmed N: Immunotherapy targeting HER2
with genetically modified T-cells eliminates tumor-initiating cells
in osteosarcoma. Cancer Gene Ther. 19:212–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luckasson R and Schalock RL: What's at
stake in the lives of people with intellectual disability? Part II:
Recommendations for naming, defining, diagnosing, classifying and
planning supports. Intellect Dev Disabil. 51:94–101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moritake H, Kamimura S, Kojima H,
Shimonodan H, Harada M, Sugimoto T, Nao-I N and Nunoi H:
Cytomegalovirus retinitis as an adverse immunological effect of
pulses of vincristine and dexamethasone in maintenance therapy for
childhood acute lymphoblastic leukemia. Pediatr Blood Cancer.
60:329–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang G, Li M, Zhu X and Yang C: Knockdown
of Akt sensitizes osteosarcoma cells to apoptosis induced by
cisplatin treatment. Int J Mol Sci. 12:2994–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|