Introduction
Basal cell carcinoma (BCC) is the most prevalent
cutaneous tumor, and accounts for ~70% of all malignant skin
diseases (1). BCCs display a large
degree of morphological variability, and a number of
histopathological subtypes have been defined (1). BCC is an epithelial tumor, which arises
from progenitor cells of the interfollicular epidermis and upper
infundibulum (2). Three histological
subtypes of BCC exist: Superficial, nodular and infiltrative
(1). An additional type of epithelial
tumor, trichoepithelioma (TE), originates from the outer root
sheath of the hair follicle (3). In
contrast to BCC, TE is a benign tumor with clear follicular
differentiation. Three major variants of trichoepithelioma have
been identified, namely the solitary, multiple and desmoplastic
subtypes. The histological features of the solitary and multiple
subtypes are identical (3). In
certain cases, it may be difficult to clinically or microscopically
distinguish TE and BCC. It is important to accurately differentiate
the two neoplasms, as they exhibit distinct biological behaviors,
for example malignancy. Furthermore, each condition has distinct
potential treatment strategies, thus an accurate diagnosis is
significant for prognosis.
Human sebaceous glands and hair follicles are skin
structures targeted by androgen action. These structures contain
steroid enzymes capable of transforming weak androgens into the
tissue-active androgens testosterone and dihydrotestosterone, which
bind to the androgen receptor (AR) and regulate the transcription
of target genes (4). The detection of
AR (using antibodies) is a promising tool for use in the
differention between BCC and TE (5).
In normal skin, AR is expressed in the sebaceous glands,
interfollicular epidermal keratinocytes, pilosebaceous duct
keratinocytes, dermal fibroblasts and secretory coil cells of the
eccrine sweat glands (6–8). AR expression has been suggested to occur
in a number of cutaneous neoplasms, including BCC (6). By contrast, AR appears not to be
expressed in mature hair follicles, the epidermis or benign hair
follicle tumors, for example TE (8).
Cluster of differentiation 10 (CD10) is a 100 kDa
transmembrane glycoprotein, which was initially identified as
common acute lymphoblastic leukemia antigen (9). CD10 expression is associated with
cellular growth rates, and is elevated in malignant tumors and
regenerating tissues, although its expression is not
lineage-specific (10). Furthermore,
CD10 expression has been detected in peritumoral fibroblast-like
stromal cells within the invasive areas of certain types of cancer,
including prostate, breast, colorectal and lung carcinomas
(11). In healthy adult skin, CD10
immunopositivity is observed in the inner sheath of the hair
follicles, hair matrix and perifollicular fibrous sheath (12). Additionally, CD10 is expressed in a
number of types of skin cancer, including dermatofibroma,
dermatofibrosarcoma protuberans and melanoma (13).
The aim of the present study was to compare the CD10
and AR expression patterns in BCC and TE cases, and to determine
whether they may serve as useful diagnostic markers.
Materials and methods
Case selection
Tumor samples were obtained by total excisional
resection, and incisional biopsy specimens were excluded. The data
were collected over 2 years, between 2010 and 2012. Ethical
approval for this study was obtained from the Ankara Training and
Research Hospital's institutional review board. A total of 39 BCC
and 15 TE cases were retrieved from the pathology department
archives at the Ankara Training and Research Hospital (Ankara,
Turkey) for analysis of immunohistochemical staining patterns.
Hematoxylin and eosin (H&E)-stained sections were reviewed by
two pathologists with experience in dermatopathology (Dr Hesna M.
Astarci and Professor Huseyin Ustun), and a diagnosis of BCC or TE
was confirmed. The 39 BCC samples were classified into 4 groups:
Superficial (6 cases), nodular (30 cases), infiltrative (1 case)
and mixed (2 cases) (1). Biopsies
were obtained from the face and back.
Immunohistochemistry
Immunohistochemical analysis was performed on all of
the specimens (54 cases). The present study compared TE with BCC.
Formalin (10% solution; pH 7.0–7.6)-fixed, paraffin-embedded tumor
tissues were prepared for immunohistochemical evaluation. In each
case, a pair of 4-µm sections was placed on poly-L-lysine-coated
slides (Sigma-Aldrich, St. Louis, MO, USA). The tissue sections
were dried for 12 h in a 37°C oven, deparaffinized with xylene and
rehydrated using graded alcohol (Merck Millipore, Darmstadt,
Germany). Antigen retrieval was performed by heating the slides
under pressure with EDTA (ScyTek Laboratories, Inc., Logan, UT,
USA) for 9 min. The sections were placed in the aforementioned
solutions for 20 min at room temperature and then placed in
phosphate-buffered saline (PBS) solution (ScyTek Laboratories,
Inc.). Endogenous peroxidase activity was inhibited by incubation
in 1% H2O2 for 15 min. Following PBS washes,
the samples were incubated with Ultra V Block (ScyTek Laboratories,
Inc.) to inhibit non-specific binding. Each pair of tissue sections
was incubated for 2 h at room temperature with mouse monoclonal
primary antibody against human CD10/CALLA (neutral endopeptidase)
(clone 56C6; #MS-728-R7; Thermo Fisher Scientific, Fremont, CA,
USA). The sections were then washed four times with PBS and
incubated for 20 minutes at room temperature with a biotinylated
goat anti-polyvalent secondary antibody (UltraTek HRP
Anti-Polyvalent Lab Pack; #UHP125; ScyTek Laboratories, Inc.). The
slides were then washed with PBS and incubated in a
diaminobenzidine chromogen-substrate (ScyTek Laboratories, Inc.)
for 5 min. The sections were subsequently counterstained with
Mayer's hematoxylin solution (Sigma-Aldrich), then dehydrated with
alcohol and cleared with xylene. Finally, entellan (Merck
Millipore) was added to the slides, and the samples were mounted
with coverslips. In each case, human small intestine tissue
sections were stained for CD10 for use as a positive control (i6000
Automatic Staining System, Biogenex, Fremont, CA, USA). Anti-CD10
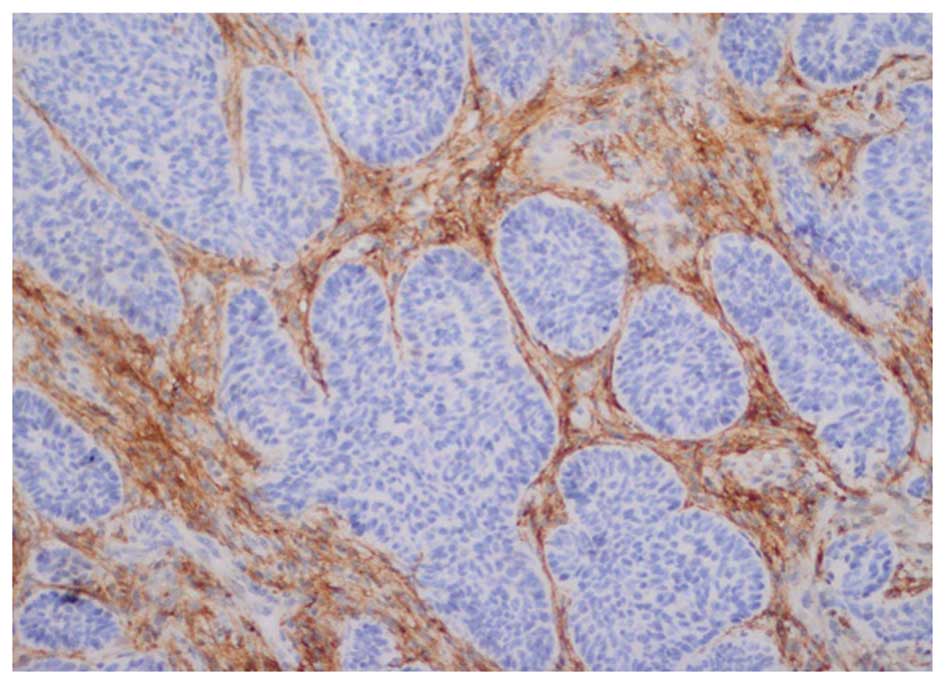
localization to the stroma and/or tumor cells was determined
according to the following four immunoreactivity groups: Stromal
staining, peripheral staining, central staining and no staining
(Fig. 1). Paraffin-embedded blocks
were also stained for AR expression using a standard
immunoperoxidase technique (using the i6000 Automatic Staining
System) (14). In brief, the sections
were deparaffinized, and antigen retrieval was performed by heat
treatment at pH 9.5 for 3 min in Borg Decloaker solution (Biocare
Medical, Inc., Concord, CA, USA). Sections were incubated overnight
at room temperature with a monoclonal mouse anti-human AR antibody
(diluted 1:75; clone AR441; #M3562; Dako North America, Inc.,
Carpinteria, CA, USA). The sections were subsequently incubated
with a goat anti-mouse IgG Biotinylated Universal Link secondary
antibody (#GU600; Biocare Medical, Inc.) for 20 min and
streptavidin-enzyme conjugate (Streptavidin-horseradish peroxidase;
Biocare Medical, Inc.) for 20 min at room temperature. The sections
were finally stained with a 3,3′-diaminobenzidine chromogen kit and
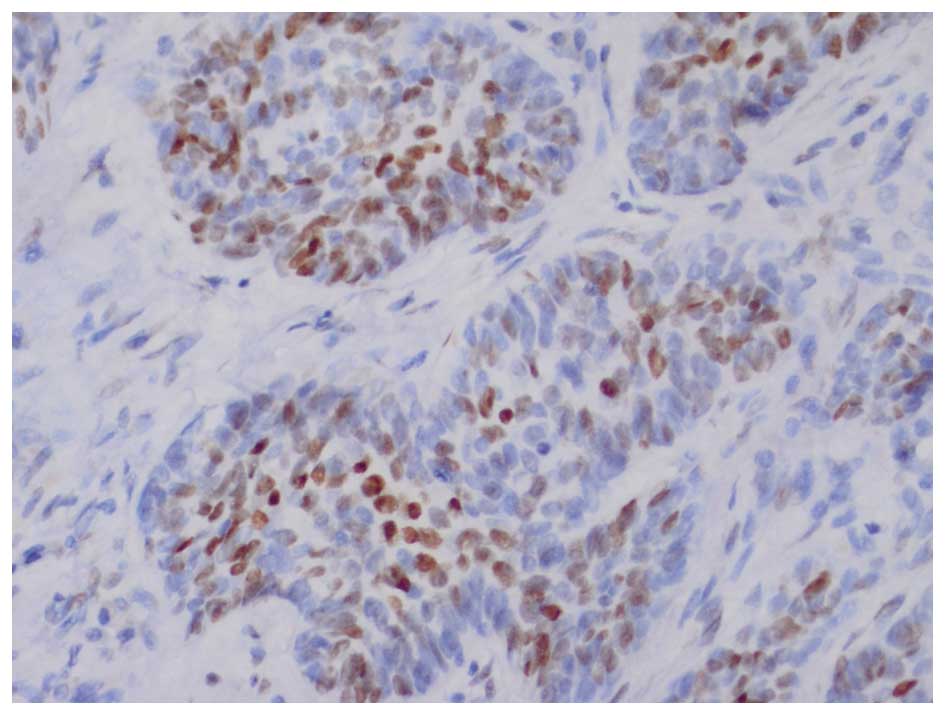
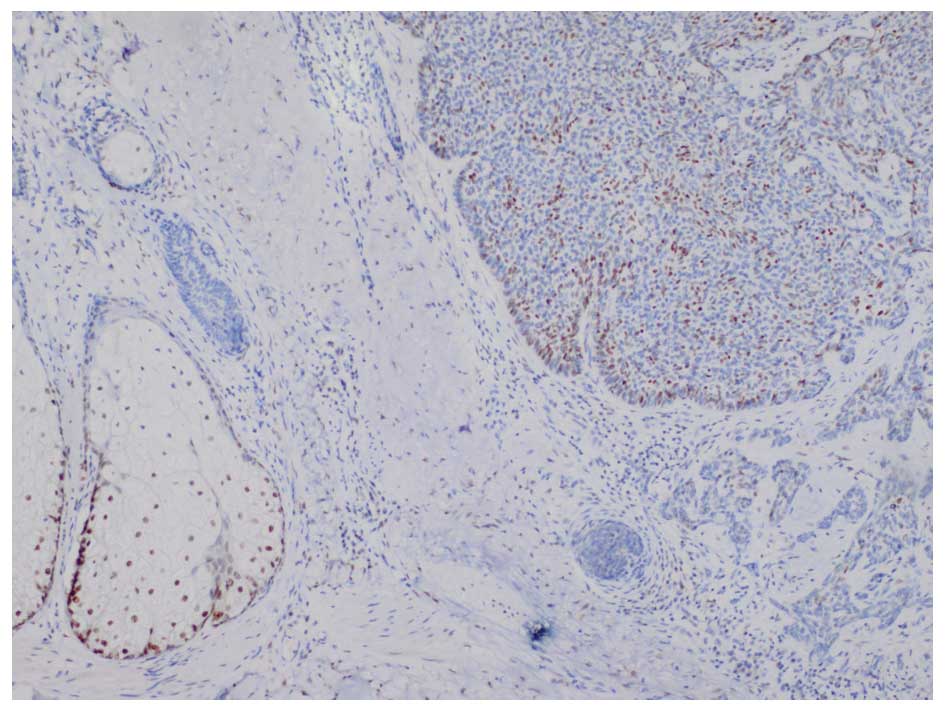
counterstained with Mayer's hematoxylin. In BCCs, AR expression is
frequently distributed focally, therefore, any tumor displaying
focal nuclear AR staining was classified as AR-positive (14). The AR-stained specimens required
individual interpretation and comparison with an internal positive
control (sebaceous glands) on the same slide (Figs. 2 and 3).
In BCC, AR expression frequently exhibits a focal and nuclear
distribution, and AR-positive immunostaining generally appears as
scattered clusters and as individual cells. AR immunoreactivity was
classified into the following five groups: Negative, (<1% AR
positive cells); 1+, focal positive (1–5%); 2+, focal positive
(5–25%); 3+, focal positive (25–49%); and 4+, diffuse positive
(>50%).
Statistical analysis
Data were collected and statistical analysis was
performed using SPSS version 20.0 (IBM SPSS, Armonk, NY, USA).
Fisher's exact and χ2 tests were used for comparisons
between the nominal variables. P<0.05 was considered to indicate
a statistically significant difference.
Results
CD10 expression in BCC and TE
patients
The present study involved 11 (28.2%) female and 28
(71.8%) male patients with BCC. The age of the patients ranged from
34–89 years, with a mean age of 65.74±12.388 years. The patients
with TE comprised 7 (46.7%) females and 8 (53.3%) males. The
patients' ages ranged from 24–74 years, with a mean age of
55.40±21.33 years. The majority of the BCC cases and all of the TE
cases were localized in the head region. The BCC cases were
classified into four groups. Furthermore, CD10 expression was
classified into four groups and was compared between the BCC and TE
groups (Table I). One tumor displayed
multiple CD10 staining patterns. A total of 9 TE (60%) and 11 BCC
cases (28.2%) presented stromal CD10 staining. Stromal CD10
staining was significantly more common in TE cases than in BCC
cases (P=0.030). In addition, 23 BCC (59%) and 4 TE cases (26.7%)
displayed peripheral CD10 staining. Peripheral CD10 staining was
significantly more common in BCC cases than in TE cases
(P=0.033).
 | Table I.CD10 expression patterns in BCC (n=39)
and TE (n=15) groups. |
Table I.
CD10 expression patterns in BCC (n=39)
and TE (n=15) groups.
| CD10 staining | BCC, n (%) | TE, n (%) | P-value |
|---|
| Stromal | 11 (28.2) | 9 (60.0) | 0.030 |
| Peripheral | 23 (59.0) | 4 (26.7) | 0.033 |
| Central | 10 (25.6) | 3 (20.0) | 0.480 |
| None | 7
(17.9) | 3 (20.0) | 0.571 |
AR expression in BCC and TE
patients
None of the TE cases were positive for AR staining,
and none of the BCC cases displayed a diffuse nuclear staining
pattern (staining, >50%). In total, 16 BCC cases (41%) were
negative for AR staining (AR staining, <1%), while 23 (59%) BCC
cases were positive for AR staining (Table II). Of the AR (+) cases, 5 (21.7%)
were female, and 18 (78.3%) were male (Table III). A total of 17 BCC cases (43.6%)
displayed ulceration, whereas none of the TE cases displayed
ulceration. In addition, 26 BCC (66.7%) and 5 TE cases (33.3%)
displayed inflammatory infiltrates. Inflammatory infiltrates were
more common in BCC cases than in TE cases, however, no
statistically significant difference was identified (P=0.27). Cysts
were observed in 21 BCC (53.8%) and 3 (20%) TE cases. Cysts were
statistically significantly more common in BCC cases than in TE
cases (P=0.025). Clefts were observed in 1 TE (6.7%) and 28 BCC
cases (71.8%) and were thus significantly more common in BCC cases
than in TE cases (P=0.0001; Table
IV). Of the AR (+) BCC cases, 21.7% displayed stromal CD10
staining, 60.9% displayed peripheral CD10 tumor cell staining and
30.4% displayed central CD10 tumor cell staining. By contrast,
17.9% of AR (+) BCC cases were negative for CD10 staining. The
results suggest the sensitivities and specificities for the
diagnosis of TE, when the differential is BCC. The sensitivity and
specificity of CD10 and AR are presented in Table V.
 | Table II.AR staining in BCC cases (n=39). |
Table II.
AR staining in BCC cases (n=39).
| AR staining, % | BCC cases, n (%) |
|---|
| <1 | 16 (41.0) |
| 1–5 | 12 (30.8) |
| 6–25 | 9
(23.1) |
| 26–50 | 2
(5.1) |
 | Table III.Gender ratio of AR (+) basal cell
carcinoma cases (n=23). |
Table III.
Gender ratio of AR (+) basal cell
carcinoma cases (n=23).
| Gender | AR (+) cases, n
(%) |
|---|
| Female | 5
(21.7) |
| Male | 18 (78.3) |
 | Table IV.Histopathological features of BCC
(n=39) and TE (n=15) cases. |
Table IV.
Histopathological features of BCC
(n=39) and TE (n=15) cases.
| Histopathological
feature | BCC, n (%) | TE, n (%) | P-value |
|---|
| Pigmentation | 15 (38.5) | 2
(13.3) |
0.069 |
| Inflammation | 26 (66.7) | 5
(33.3) |
0.027 |
| Cyst | 21 (53.8) | 3
(20.0) |
0.025 |
| Cleft | 28 (71.8) | 1 (6.7) | <0.001 |
| Calcification | 5
(12.8) | 1 (6.7) |
0.461 |
 | Table V.Sensitivity and specificity of CD10
staining, histopathological features and AR (+) in TE and BCC
cases. |
Table V.
Sensitivity and specificity of CD10
staining, histopathological features and AR (+) in TE and BCC
cases.
|
| TE | BCC |
|---|
|
|
|
|
|---|
| Feature | Sens. | Spec. | Sens. | Spec. |
|---|
| CD10 staining |
|
|
|
|
|
Stromal | 0.400 | 0.282 | 0.718 | 0.400 |
|
Peripheral | 0.733 | 0.590 | 0.410 | 0.733 |
|
Central | 0.800 | 0.256 | 0.744 | 0.800 |
| Histopathology |
|
|
|
|
|
Pigmentation |
0.867 |
0.385 | 0.615 |
0.867 |
|
Inflammation |
0.667 |
0.667 | 0.333 |
0.667 |
|
Cyst |
0.800 |
0.538 | 0.462 |
0.800 |
|
Cleft |
0.933 |
0.718 | 0.282 |
0.933 |
|
Calcification |
0.933 |
0.128 | 0.282 |
0.933 |
| AR (+) | <0.001 | <0.001 | 0.590 | <0.001 |
Discussion
In the present study, AR (+) staining in the BCC
group was enriched in the 1–5% staining group (30.8% of samples).
Furthermore, AR (+) sensitivity was calculated as 59% and
specificity was 0%. The tumor cells in the BCC group displayed a
patchy, focal staining pattern. Tumor cells were typically
scattered in tumor nodules. A number of tumor islands within the
same lesion exhibited no staining, thus, the BCC staining pattern
suggested that incisional punch biopsy specimens may exhibit
negative staining. Therefore, incisional punch biopsy specimens
have the potential to present false-negative staining results, if
the total tumor excision is insufficient. In the current study, all
cases consisted of excisional biopsy specimens, in order to
demonstrate the staining sensitivity and pattern of the tumor cells
and stroma in whole lesions. AR staining <1% was considered to
be negative, thus, 59.9% of the cases were AR (+). Izikson et
al (14) and Katona et al
(15) reported AR staining in 78% and
65% of their cases, respectively. In addition, Asadi-Amoli et
al (16) reported that 33% of
cases were AR (+), whereas Costasche et al (17) reported AR staining in 100% of cases.
In the present study, none of the BCC cases displayed a diffuse
nuclear pattern (staining, >50%), a result that was consistent
with those of the previous study by Asadi-Amoli et al
(16).
Costache et al (17) demonstrated that cytokeratin 20 (CK20)
and AR expression aided the differentiation between BCC and TE;
however, interpretation was difficult in certain cases. The study
also reported that no differences in B cell lymphoma-2 (Bcl-2) and
CD34 staining were observed between BCC and TE. By contrast,
Kirchmann et al (18) and
Illueca et al (19) reported
the utility of CD34 as a marker, which is not detected in the
stroma of BCC but is present in TE (18,19). A
study by Katona et al (15)
supported the utility of CK20 and AR expression in the
differentiation of BCC and TE. An AR (−), CK20 (+) immunophenotype
was sensitive (87%) and specific (100%) for TE, but was specific
(100%) and moderately sensitive (61%) for BCC (15). Furthermore, Choi et al
(20) reported that elastic fiber
staining and CK15 expression patterns may aid in the
differentiation of TE from BCC. Carvalho et al (21) investigated CD23 expression in
desmoplastic TE and morpheaform BCC, but observed no statistically
significant difference in CD23 expression in these tumors.
Furthermore, additional immunohistochemical markers, including
Bcl-2, transforming growth factor-β and Ber-EP4, may be potentially
useful in the differential diagnosis of TE and BCC (22–27).
Sengul et al (28) reported that stromal CD10
immunopositivity in benign cutaneous appendage tumors originating
from the hair follicle (trichoepithelioma, trichoblastoma,
trichofolliculoma or trichoadenoma) is increased compared with that
of BCC (P=0.003). However, peripheral, regionally positive CD10
expression was stronger for BCC than for benign tumours of
cutaneous appendages originating from the hair follicle (P=0.03),
which is similar to the results of the present study.
Izikson et al (14) observed positive nuclear AR
immunostaining in ~78% of BCCs, whereas positive nuclear AR
immunostaining was demonstrated in ~59% of BCCs. In the present
study, <1% AR staining was considered to be negative, which may
explain the differences in AR percentages reported in these
studies. In the same lesions, AR and CD10 staining exhibited
distinct staining intensities, and the varied distribution was
marked. Incisional punch biopsy specimens may potentially lead to
false-negative results. Therefore, AR and CD10 staining are more
accurate markers when complete excision of the lesion is performed,
particularly in cases where a histopathological differential
diagnosis of BCC and TE is difficult.
In the present study, AR positive staining was
detected in 59% of BCC cases, while TE cases exhibited no
significant AR staining. In the majority of BCC cases, the
expression pattern of AR was focal and nuclear, and the staining
typically appeared as scattered clusters and individual tumor
cells. None of the TE cases were positive for AR staining. In
total, 23 (59%) of the BCC cases exhibited positive AR staining,
and of these, 21.7% exhibited stromal CD10 staining, 60.9%
exhibited peripheral CD10 tumor cell staining and 30.4% exhibited
central tumor cell staining. Furthermore, 17.9% of the AR (+) BCC
cases were negative for CD10 staining. Additionally, the current
study demonstrated that the BCC cases had increased inflammatory
infiltrates, cysts, ulceration and clefts compared with those of TE
cases. AR positivity of BCC cases was calculated as 59% sensitive
and 0% specific.
AR and CD10 expression demonstrated differing
staining intensities and distributions within specific lesions.
This observation is significant since incisional punch biopsy
specimens may therefore detect negative CD10 and AR staining. On
the basis of these results, CD10 and AR staining in incisional
punch biopsy specimens may not correctly reflect the diagnosis,
which may be accurately determined using a total tumor biopsy. For
example, it is possible that specimens obtained by incisional punch
biopsy exhibiting negative AR staining may demonstrate AR
positivity when evaluated following total excision biopsy.
In conclusion, a differential diagnosis of BCC and
TE using the CD10 and AR markers requires the use of total excision
tumor specimens.
References
|
1
|
Patterson JW: Chapter 31 - Tumors of the
epidermis. Weedon's Skin Pathology (4th). Elsevier. 783–835.
2015.
|
|
2
|
Youssef KK, Van Keymeulen A, Lapouge G,
Beck B, Michaux C, Achouri Y, Sotiropoulou PA and Blanpain C:
Identification of the cell lineage at the origin of basal cell
carcinoma. Nat Cell Biol. 12:299–305. 2010.PubMed/NCBI
|
|
3
|
Patterson JW: Chapter 33 - Tumors of
cutaneous appendages. Weedon's Skin Pathology (4th). Elsevier.
903–965. 2015.
|
|
4
|
Sawaya ME: Purification of androgen
receptors in human sebocytes and hair. J Invest Dermatol.
98(Suppl): S92–S96. 1992. View Article : Google Scholar
|
|
5
|
Arits AH, Parren LJ, van Marion AM, Sommer
A, Frank J and Kelleners-Smeets NW: Basal cell carcinoma and
trichoepithelioma: A possible matter of confusion. Int J Dermatol.
47(Suppl 1): S13–S17. 2008. View Article : Google Scholar
|
|
6
|
Bayer-Garner IB, Givens V and Smoller B:
Immunohistochemical staining for androgen receptors: A sensitive
marker of sebaceous differentiation. Am J Dermatopathol.
21:426–431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choudhry R, Hodgins MB, Van der Kwast TH,
Brinkmann AO and Boersma WJ: Localization of androgen receptors in
human skin by immunohistochemistry: Implications for the hormonal
regulation of hair growth, sebaceous glands and sweat glands. J
Endocrinol. 133:467–475. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shikata N, Kurokawa I, Andachi H and
Tsubura A: Expression of androgen receptors in skin appendage
tumors: An immunohistochemical study. J Cutan Pathol. 22:149–153.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong HY, Gorczyca W, Liu Z, Tsang P, Wu
CD, Cohen P and Weisberger J: B-cell lymphomas with coexpression of
CD5 and CD10. Am J Clin Pathol. 119:218–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wagoner J, Keehn C and Morgan MB: CD-10
immunostaining differentiates superficial basal cell carcinoma from
cutaneous squamous cell carcinoma. Am J Dermatopathol. 29:555–558.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahara M, Chen S, Kido M, Takeuchi S,
Uchi H, Tu Y, Moroi Y and Furue M: Stromal CD10 expression, as well
as increased dermal macrophages and decreased Langerhans cells, are
associated with malignant transformation of keratinocytes. J Cutan
Pathol. 36:668–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yada K, Kashima K, Daa T, Kitano S,
Fujiwara S and Yokoyama S: Expression of CD10 in basal cell
carcinoma. Am J Dermatopathol. 26:463–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pham TT, Selim MA, Burchette JL Jr, Madden
J, Turner J and Herman C: CD10 expression in trichoepithelioma and
basal cell carcinoma. J Cutan Pathol. 33:123–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Izikson L, Bhan A and Zembowicz A:
Androgen receptor expression helps to differentiate basal cell
carcinoma from benign trichoblastic tumors. Am J Dermatopathol.
27:91–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katona TM, Perkins SM and Billings SD:
Does the panel of cytokeratin 20 and androgen receptor antibodies
differentiate desmoplastic trichoepithelioma from
morpheaform/infiltrative basal cell carcinoma? J Cutan Pathol.
35:174–179. 2008.PubMed/NCBI
|
|
16
|
Asadi-Amoli F, Khoshnevis F, Haeri H,
Jahanzad I, Pazira R and Shahsiah R: Comparative examination of
androgen receptor reactivity for differential diagnosis of
sebaceous carcinoma from squamous cell and basal cell carcinoma. Am
J Clin Pathol. 134:22–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costache M, Bresch M and Böer A:
Desmoplastic trichoepithelioma versus morphoeic basal cell
carcinoma: A critical reappraisal of histomorphological and
immunohistochemical criteria for differentiation. Histopathology.
52:865–876. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kirchmann TT, Prieto VG and Smoller BR:
CD34 staining pattern distinguishes basal cell carcinoma from
trichoepithelioma. Arch Dermatol. 130:589–592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Illueca C, Monteagudo C, Revert A and
Llombart-Bosch A: Diagnostic value of CD34 immunostaining in
desmoplastic trichilemmoma. J Cutan Pathol. 25:435–439. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi CW, Park HS, Kim YK, Lee SH and Cho
KH: Elastic fiber staining and cytokeratin 15 expression pattern in
trichoepithelioma and basal cell carcinoma. J Dermatol. 35:499–502.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carvalho J, Fullen D, Lowe L, Su L and Ma
L: The expression of CD23 in cutaneous non-lymphoid neoplasms. J
Cutan Pathol. 34:693–698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verhaegh ME, Arends JW, Majoie IM,
Hoekzema R and Neumann HA: Transforming growth factor-beta and
bcl-2 distribution patterns distinguish trichoepithelioma from
basal cell carcinoma. Dermatol Surg. 23:695–700. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smoller BR, Van de Rijn M, Lebrun D and
Warnke RA: Bcl-2 expression reliably distinguishes
trichoepitheliomas from basal cell carcinomas. Br J Dermatol.
131:28–31. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Basarab T, Orchard G and Russell-Jones R:
The use of immunostaining for bcl-2 and CD34 and the lectin peanut
agglutinin in differentiating between basal cell carcinomas and
trichoepitheliomas. Am J Dermatopathol. 20:448–452. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poniecka AW and Alexis JB: An
immunohistochemical study of basal cell carcinoma and
trichoepithelioma. Am J Dermatopathol. 21:332–336. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abdelsayed RA, Guijarro-Rojas M, Ibrahim
NA and Sangueza OP: Immunohistochemical evaluation of basal cell
carcinoma and trichepithelioma using Bcl-2, Ki67, PCNA and P53. J
Cutan Pathol. 27:169–175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Swanson PE, Fitzpatrick MM, Ritter JH,
Glusac EJ and Wick MR: Immunohistologic differential diagnosis of
basal cell carcinoma, squamous cell carcinoma, and
trichoepithelioma in small cutaneous biopsy specimens. J Cutan
Pathol. 25:153–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sengul D, Sengul I, Astarci MH, Ustun H
and Mocan G: CD10 for the distinct differential diagnosis of basal
cell carcinoma and benign tumours of cutaneous appendages
originating from hair follicle. Pol J Pathol. 61:140–146.
2010.PubMed/NCBI
|