Introduction
Resistance to chemotherapy is a major problem facing
current cancer therapy (1).
Scientists continue to search for the mechanisms underlying drug
resistance; meanwhile, identifying improved predictive biomarkers
for responsiveness to chemotherapy remains important, since the
traditional tumor markers such as MUC1 antigen (CA15.3) do not
accurately reflect the actual effect of cancer treatment (2). It is necessary to define specific
characteristics that provide the possibility of optimizing
individual treatment (3), and in this
era of high-throughput methods, a deluge of novel biomarkers have
been reported for prognostic and predictive purposes. However, of
these, only a few have been incorporated into clinical practice
(3).
Intercellular communication is a hallmark of
multicellular organisms and may be mediated through direct
cell-cell contact or the transfer of secreted molecules (4). In the last 2 decades, a third mechanism
of intercellular communication has emerged that involves the
transfer of extracellular vesicles (EVs) shed from the cell plasma
membrane (4). EVs are generally
referred to as microvesicles, exosomes, shedding vesicles, or
microparticles, among others (5–8).
Numerous diverse biological functions have been
attributed to EVs (9), and it is now
commonly accepted that exosomes and microvesicles are important
vehicles of intercellular communication between cells locally and
at a distance (4). Tumor cells, in
addition to other cells in the tumor microenvironment, also release
EVs, and there is evidence that they contribute to tumor
progression by promoting angiogenesis and metastasis (4). Our previous study demonstrated that
circulating EVs containing the Ca2+-permeable channel
TrpC5 transfer chemoresistance to previously non-chemoresistant
recipient cells (10). This study led
to the hypothesis that EVs may serve an essential role in clinical
chemoresistance, therefore the present study aimed to investigate
the association between EVs and drug resistance.
ABCG2/breast cancer resistance protein (BCRP) is a
member of the adenosine triphosphate-binding cassette (ABC)
transporter protein family. It is referred to as a ‘half-type’ ABC
transporter, functions as a homodimer, and transports anticancer
agents such as irinotecan out of cells (11). It has been demonstrated that elevated
BCRP levels in vitro result in resistance to anticancer
drugs, including topotecan and irinotecan, however the underlying
mechanism(s) require further study (12), particularly whether BCRP acts
via circulating EVs. If so, BCRP-containing EVs may have
clinical applications. To investigate these possibilities, the
present study investigated the association between BCRP and
circulating EVs, carefully comparing patients demonstrating a poor
response to chemotherapy with those without chemotherapy. The
results indicated that there is a role of BCRP-containing EVs in
clinical prognosis.
Materials and methods
Patient tumor specimens and peripheral
blood samples
Tumor specimens for assessing the response to
chemotherapy prior to surgery: Patients were recruited at the
Affiliated Hospital of Jiangnan University (Wuxi, China) between
2010 and 2012. The anthracycline-taxane-based neoadjuvant
chemotherapy regimen TEC (docetaxel, epirubicin, and
cyclophosphamide) was used with large (>3 cm) and
locally-advanced breast cancers (T3, T4, or N2) in order to reduce
the size of the primary tumor and increase the likelihood of breast
conservation, and to eliminate occult systemic metastases in order
to improve survival. Tumor samples were acquired following 2–4
cycles of TEC therapy (n=3) and from patients who did not receive
chemotherapy prior to surgery (n=6). The resected primary tumor
tissues were kept in liquid nitrogen following surgery. The
response of primary tumors to TEC treatment was quantified
according to the RECIST criteria (Response Evaluation Criteria in
Solid Tumors).
Blood samples for assessing the response to
chemotherapy following surgery: Peripheral blood samples were
collected into polypropylene tubes containing EDTA (Vacutainer
System, BD Biosciences, San Jose, CA, USA). The samples consisted
of 2 groups: i) Patients who received 3–6 cycles of TEC therapy
following surgery, extracted before the final cycle (n=34); ii)
Samples extracted when the pathological diagnosis of the patients
was made at surgery (n=21). The patient demographics and clinical
pathological characteristics are listed in the Table I. Tumor assessment was performed by
MRI and/or ultrasound depending on the method used at baseline, and
the response to chemotherapy was assessed by the RECIST criteria
(13). Patients achieving a complete
(CR) or partial (PR) response were considered to be responders;
those with stable disease (SD) or progressive disease (PD) were
considered to be non-responders. The use of clinical samples in
this study was approved by the Review Board at the Fourth
Affiliated Hospital of Soochow University.
 | Table I.Clinical and pathological
characteristics of 34 patients who received chemotherapy and 21
patients who did not receive chemotherapy after surgery (blood
drawn at the time of primary diagnosis of breast cancer). |
Table I.
Clinical and pathological
characteristics of 34 patients who received chemotherapy and 21
patients who did not receive chemotherapy after surgery (blood
drawn at the time of primary diagnosis of breast cancer).
| Characteristics | All patients
(n=55) | +Chemotherapy
(n=34) | −Chemotherapy
(n=21) |
|---|
| Age, years |
|
|
|
|
<50 | 15 | 10 | 5 |
| ≥50 | 40 | 24 | 16 |
| Gender |
|
|
|
| Male | 0 | 0 | 0 |
|
Female | 55 | 34 | 21 |
| Histology |
|
|
|
|
Ductal | 53 | 32 | 21 |
| Paget
disease | 2 | 2 | 0 |
| Tumor size |
|
|
|
| pTx | 3 | 3 | 0 |
| pT1 | 11 | 7 | 4 |
| pT2 | 26 | 12 | 14 |
| pT3 | 9 | 7 | 2 |
| pT4 | 6 | 5 | 1 |
| Lymph node
status |
|
|
|
| N0 | 21 | 8 | 13 |
| N1 | 15 | 10 | 5 |
| N2 | 9 | 8 | 1 |
| N3 | 10 | 8 | 2 |
| AJCC Substage |
|
|
|
| I | 6 | 4 | 2 |
| II | 28 | 13 | 15 |
| III | 13 | 9 | 4 |
| IV | 8 | 8 | 0 |
| Adjuvant
Chemotherapy |
|
|
|
|
Anthracycline | 10 | 10 | 0 |
|
Taxane | 9 | 9 | 0 |
|
Anthracycline/taxane | 15 | 15 | 0 |
| ER Status |
|
|
|
|
Positive | 38 | 22 | 16 |
|
Negative | 15 | 10 | 5 |
|
Missing | 2 | 2 | 0 |
| PR Status |
|
|
|
|
Positive | 37 | 21 | 16 |
|
Negative | 15 | 10 | 5 |
|
Missing | 3 | 3 | 0 |
| Her-2 Status |
|
|
|
|
Positive | 31 | 18 | 13 |
|
Negative | 16 | 8 | 8 |
|
Missing | 8 | 8 | 0 |
Isolation of microvesicles
The methods for collecting isolated EVs from plasma
were as previously described. The plasma supernatant was obtained
by centrifugation at 850 × g for 3 min. The EVs were isolated after
1:2 dilution in PBS by sequential centrifugation (10 min at 500 × g
to remove cell debris; 30 min at 2,000 × g to remove platelets; and
70 min at 100,000 × g to obtain precipitate). After washing in PBS,
EVs were re-suspended in PBS for analysis.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Isolated EVs were lysed with 200 µl TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and processed
according to the manufacturer's instructions to obtain total RNA.
Total RNA was reverse-transcribed using a Reverse Transcriptase
M-MLV (RNase H-) kit (Takara Bio Inc., Otsu, Japan) according to
the manufacturer's instructions. The single-stranded cDNA was
amplified by PCR in a Thermal Cycler C1000 Tech (Bio-Rad) using
DreamTaq Green PCR Master Mix (2×) (Thermo-Scientific). GAPDH was
used as an endogenous control. The PCR was performed under the
following conditions: 3 min at 95°C; 30 sec at 94°C, 30 sec at
58°C, and 45 sec at 72°C for 35 cycles; and 72°C for 10 min. The
primer sequences were as follows: BCRP, F 5′-CAG CCG TGG AAC TCT
TTG TGG TAG AGA AG-3′ and R 5′-CTG TTG CAT TGA GTC CTG GGC AGA
AG-3′; flotillin-2, F 5′-AGA TCC GGC AGG AAG AGA TT-3′ and R 5′-GCT
TCT GCC TTG AGC TTC AT-3′; MUC1, F 5′-CGA CTA CTA CCA AGA GCT GCA
GAG AGA CAT-3′ and R 5′-TGT AAG AGA GGC TGC TGC CAC CAT TAC CTG-3′;
GAPDH, F 5′-CTC CTG CAC CAC CAA CTG CTT AGC-3′ and R 5′-CGC CTG CTT
CAC CAC CTT CTT GAT-3′. Equal amounts of RT-PCR products were
loaded onto 1.5% agarose gels. The data were analyzed with ImageJ
software (v.149; NIH, Bethesda, MD, USA). All reactions were
performed in triplicate.
Immunofluorescence (IF) analysis
IF staining was performed as described previously
(10). Isolated EVs were resuspended
in PBS, and then passed through a 0.8-µm filter (Stericup, Merck
Millipore, Billerica, MA, USA), allowing EVs of diameters <0.8
µm to remain in the filtrate. The filtrate was then passed through
a 0.1-µm filter (Stericup), so purified EVs with diameters from 0.1
to 0.8 µm were retained on the filter surface. The EVs on the
filter underwent IF staining. The purified EVs and surgical
specimens were fixed with 4% paraformaldehyde (Sigma-Aldrich, St.
Louis, MO, USA) for 15 min, then blocked with 10% BSA/PBS with 0.1%
Triton X-100 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in PBS
for 30 min at room temperature. Slides were incubated with primary
antibodies overnight at 4°C followed by the appropriate secondary
fluorescence-labeled antibody for 1 h at room temperature. DAPI
(1:1,000; Beyotime Institute of Biotechnology, Haimen, China) was
used to stained nuclei. Images were captured with a confocal
microscope (Leica TCS SP8, Leica Microsystems GmbH, Wetzlar,
Germany). The primary antibody mouse anti-BCRP/ABCG2 monoclonal
antibody (Abcam, Cambridge, UK; ab3380, diluted 1:10) was used for
IF staining in frozen sections of surgical specimens; mouse
anti-BCRP/ABCG2 monoclonal antibody (Abcam, ab3380, diluted 1:10)
and goat anti-MUC1 monoclonal antibody (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; sc-6825, diluted 1:20) were used for IF
staining of purified EVs. The secondary antibodies were Alexa Fluor
488-labeled donkey anti-mouse IgG (H+L) antibody and Alexa Fluor
546-labeled donkey anti-goat IgG (H+L) antibody (Invitrogen Life
Technologies; diluted 1:100).
Fluorescent in situ hybridization
(FISH) analysis
FISH was performed using the locked nucleic acid
(LNA)-modified oligonucleotide probe (Redlandbio Technology Co.,
Ltd., Guangzhou, China). The sequences of the hBCRP/ABCG2 probes
were as follows: hBCRP probe 1, 5′-ATG CTG CAA AGC CGT AAA TCC ATA
TCG TG-3′; hBCRP probe 2, 5′-TAA GAT GAC ACT CTG TAG TAT CCG CTG
ATG-3′; and hBCRP probe 3, 5′-CTC TAC TCT ACC CAC AGT TCC AAA CCC
TCA-3′. Surgical specimens were washed with 0.1 mM PBS,
permeabilized with 0.4% Triton X-100, and treated with 1 µg/ml
proteinase K (Beyotime Institute of Biotechnology). The specimens
were washed with 4% PFA for 5 min to inhibit the effects of the
proteinase. To reduce non-specific signals, slides were washed once
with 0.25% acetic anhydride (Sigma-Aldrich). Hybridization with the
probe (1 µM) was performed at 40°C for 16 h after incubation in 50%
formamide (Sangon Biotech Co., Ltd., Shanghai, China) that had been
deionized for 30 min at 37°C. The slides were washed once with 4X
saline-sodium citrate (SSC) for 15 min at 37°C, and once with NTE
buffer (500 mM NaCl, 10 mM Tris, 1 mM EDTA, 20 µg/ml RNase A) for
30 min at 37°C to digest the single-stranded RNA, then rinsed once
with 1X SSC and 0.5X SSC (15 min each) at 37°C, and finally washed
twice with Buffer 1 (100 mM Tris, 150 mM NaCl, pH 7.5) for 10 min.
All FISH images of BCRP were captured by a confocal laser scanning
microscope (Leica TCS SP8, Leica Microsystems GmbH).
Flow cytometry
Analysis of purified EVs. Purified EVs [10 µg as
measured by Bradford assay (Sangon Biotech Co., Ltd.)] were
incubated with 10 µl latex beads 3.1 µm in diameter (Life
Technologies, Gaithersberg, MD, USA) in a 1.5-ml microcentrifuge
tube for 15 min at room temperature. PBS was added to a final
volume of 1 ml and incubated on a test-tube rotator overnight at
4°C. Glycine was added (110 µl of 1 M; 100 mM final concentration),
mixed gently, incubated at room temperature for 30 min, and then
centrifuged for 3 min at 1,500 × g at room temperature. The
supernatant was removed and discarded. The bead pellet was
re-suspended in 1 ml PBS/0.5% BSA and centrifuged for 3 min at
1,500 × g at room temperature. The supernatant was removed and
discarded, and the pellet washed twice. EV-coated beads were
incubated for 1 h at room temperature in 50 µl PSB/5% BSA
containing the primary antibody, either mouse anti-BCRP/ABCG2
monoclonal antibody (Abcam; ab3380, diluted 1:10), mouse anti-MUC1
monoclonal antibody (Abcam; ab70475, diluted 1:50), mouse
anti-flotillin-2 monoclonal antibody (Santa Cruz Biotechnology;
sc-28320, diluted 1:50), or mouse isotype-matched control IgG1
(Abcam: ab18443, diluted 1:100). The pellet was resuspended in 1 ml
PBS/0.5% BSA and centrifuged for 3 min at 1,500 × g at room
temperature. The secondary antibody Alexa Fluor 488-labeled donkey
anti-mouse IgG (H+L) (Invitrogen Life Technologies, diluted 1:100)
was incubated for 45 min at room temperature, followed by washing
and resuspension with PBS. The antibody-stained EV-coated latex
beads were analyzed on a FACSCalibur flow cytometer (BD
Biosciences, Mountain View, CA, USA). Data were analyzed using
Flowjo software, version 7.6 (Flowjo, LLC, Ashland, OR, USA).
Statistical analysis
Results are presented as the mean ± standard error
of the mean. All experiments were performed in triplicate.
Statistical differences were determined by the paired Student's
t-test, using Graphpad Prism software, version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). All statistical tests were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
BCRP expression is upregulated at the
mRNA level in tumor specimens from patients who received
neoadjuvant chemotherapy prior to surgery
A total of 3 tumor specimens were collected from
each patient who received TEC therapy and was assessed as
non-responsive (PD/SD according to the RECIST criteria) and those
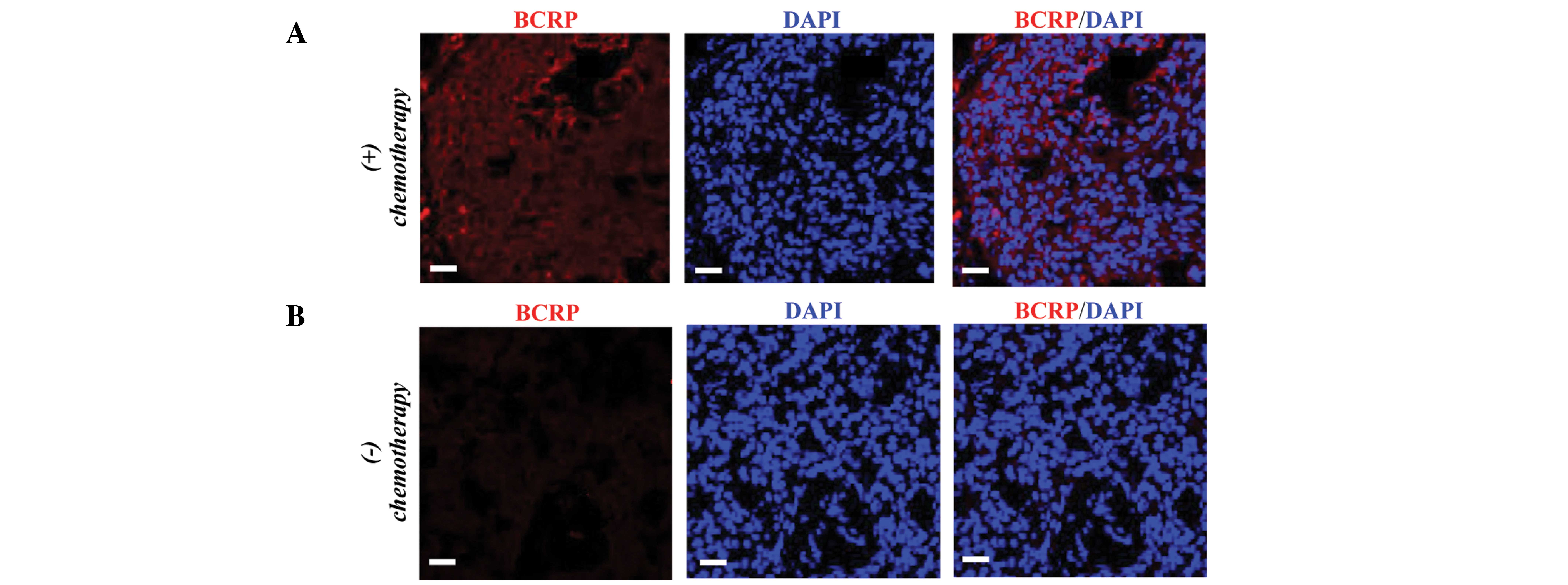
who received no chemotherapy. FISH analysis demonstrated that the
tumor samples from non-responsive patients had higher mRNA
expression levels of BCRP compared with the group with no
chemotherapy (Fig. 1), indicating a
potential role of BCRP in drug resistance.
BCRP and flotillin-2 expression in
tumor specimens is enhanced following neoadjuvant chemotherapy
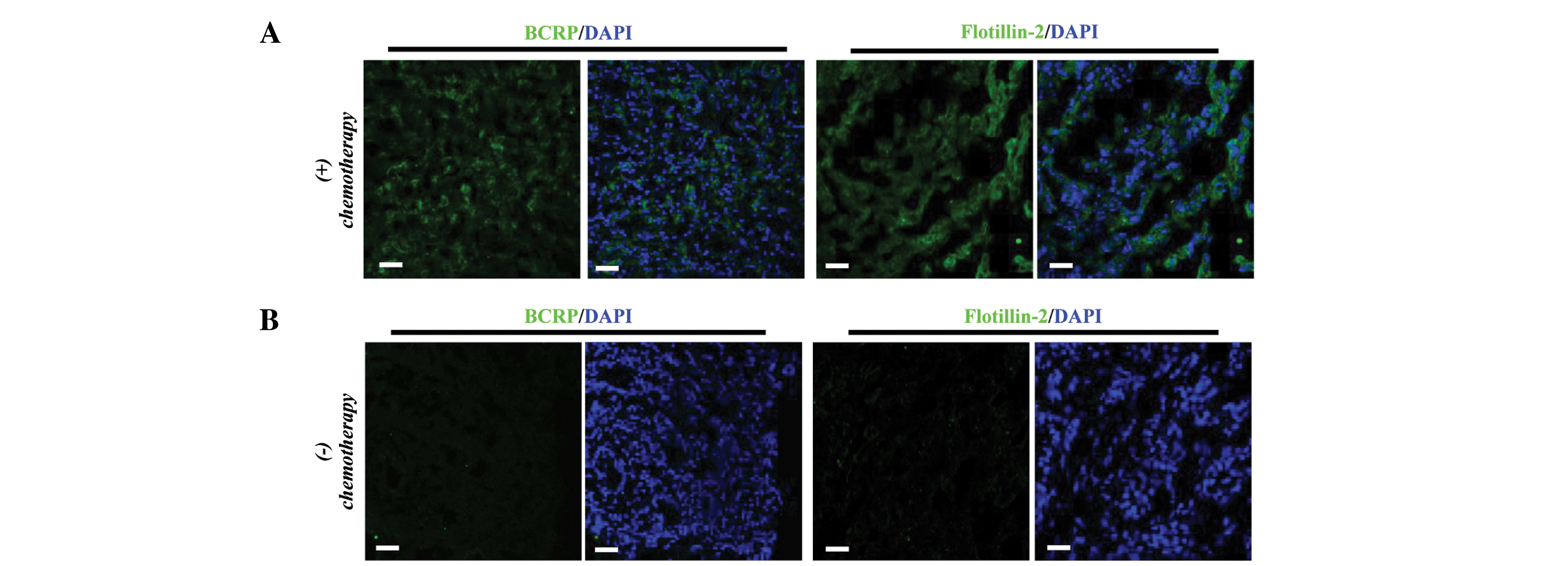
IF was used to assess the expression of BCRP and the
EV marker flotillin-2 in specimens from non-responsive patients and
those without chemotherapy by IF. The results demonstrated that
both BCRP and flotillin-2 were expressed at higher levels in
samples from non-responsive patients compared with controls
(Fig. 2A and B). These results
indicated that BCRP and flotillin-2 may be potentially used as
indicators of the response to neoadjuvant chemotherapy.
Patients with PD/SD (non-responders)
demonstrate higher BCRP expression levels in circulating EVs
As noted above, both BCRP and flotillin-2 were
upregulated in tumor specimens from non-responsive patients. Since
flotillin-2 is an EV marker, to further study the association
between BCRP and EVs, the BCRP levels were assessed in circulating
EVs isolated from patients at the mRNA and protein levels using
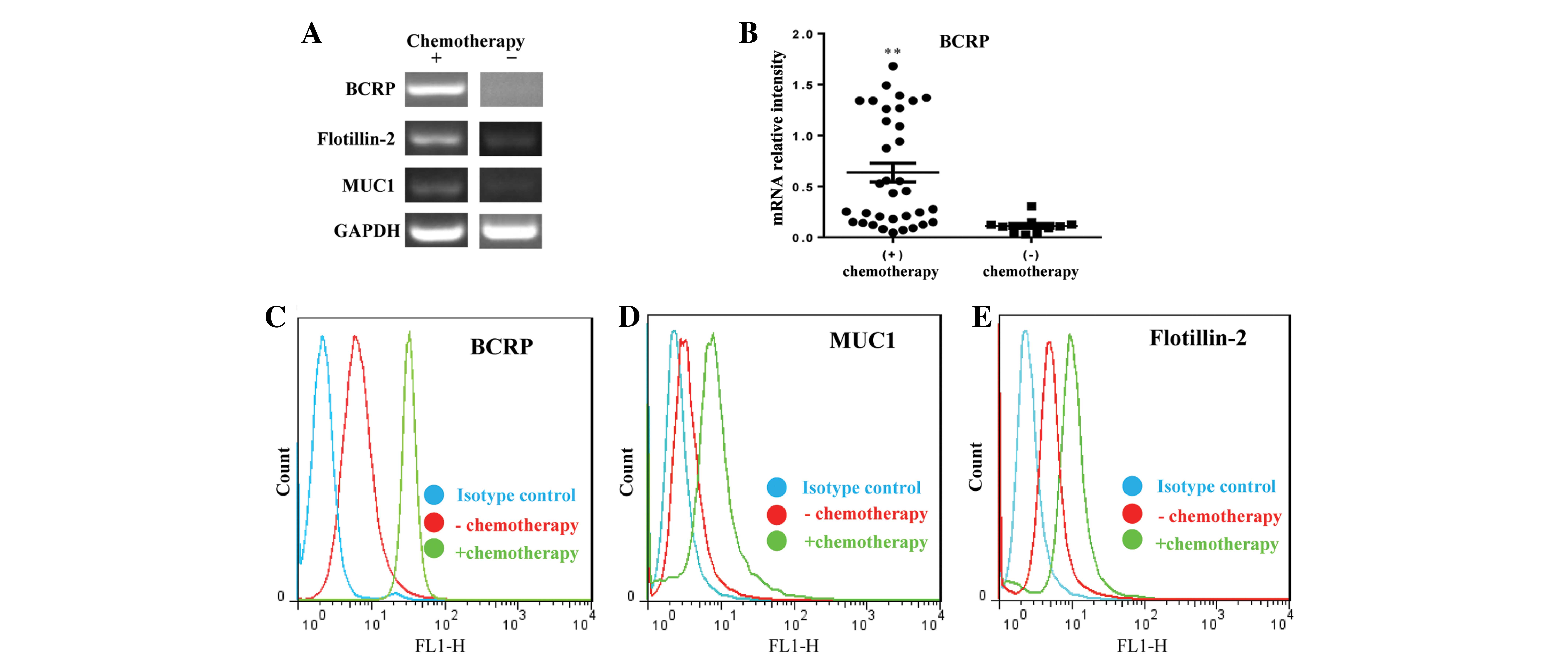
RT-PCR and FACS, respectively. It is known that MUC1 is frequently
expressed in breast cancer, is sorted into rafts by a
flotillin-2-dependent mechanism, and is exported via EVs (14–16).
Therefore, RT-PCR was performed to simultaneously identify the
transcript expression of BCRP, flotillin2, and MUC1. As
hypothesized, these transcripts were expressed at significantly
higher levels in blood samples from non-responsive patients
compared with those without chemotherapy after surgery (Fig. 3A). Samples were obtained from 34
patients who received 4–6 cycles of TEC therapy and 21 patients
without chemotherapy (Table I).
Analysis of BCRP transcription levels (Fig. 3B) demonstrated that BCRP transcription
was only detectable in the circulating EVs of 10/21 (47.6%) of
patients without chemotherapy; in 11/21 patients without
chemotherapy it was too low to be detected. The FACS results were
in accordance with the RT-PCR by demonstrating increased expression
levels of BCRP, flotillin-2, and MUC1 in EVs from non-responsive
patients (Fig. 3C-E). Taken together,
circulating EVs containing BCRP of tumor origin may be potentially
used as a prognostic biomarker for the response to clinical
chemotherapy.
Tumor-derived circulating EVs
expressing BCRP demonstrate notable potential for predicting
chemotherapeutic outcome
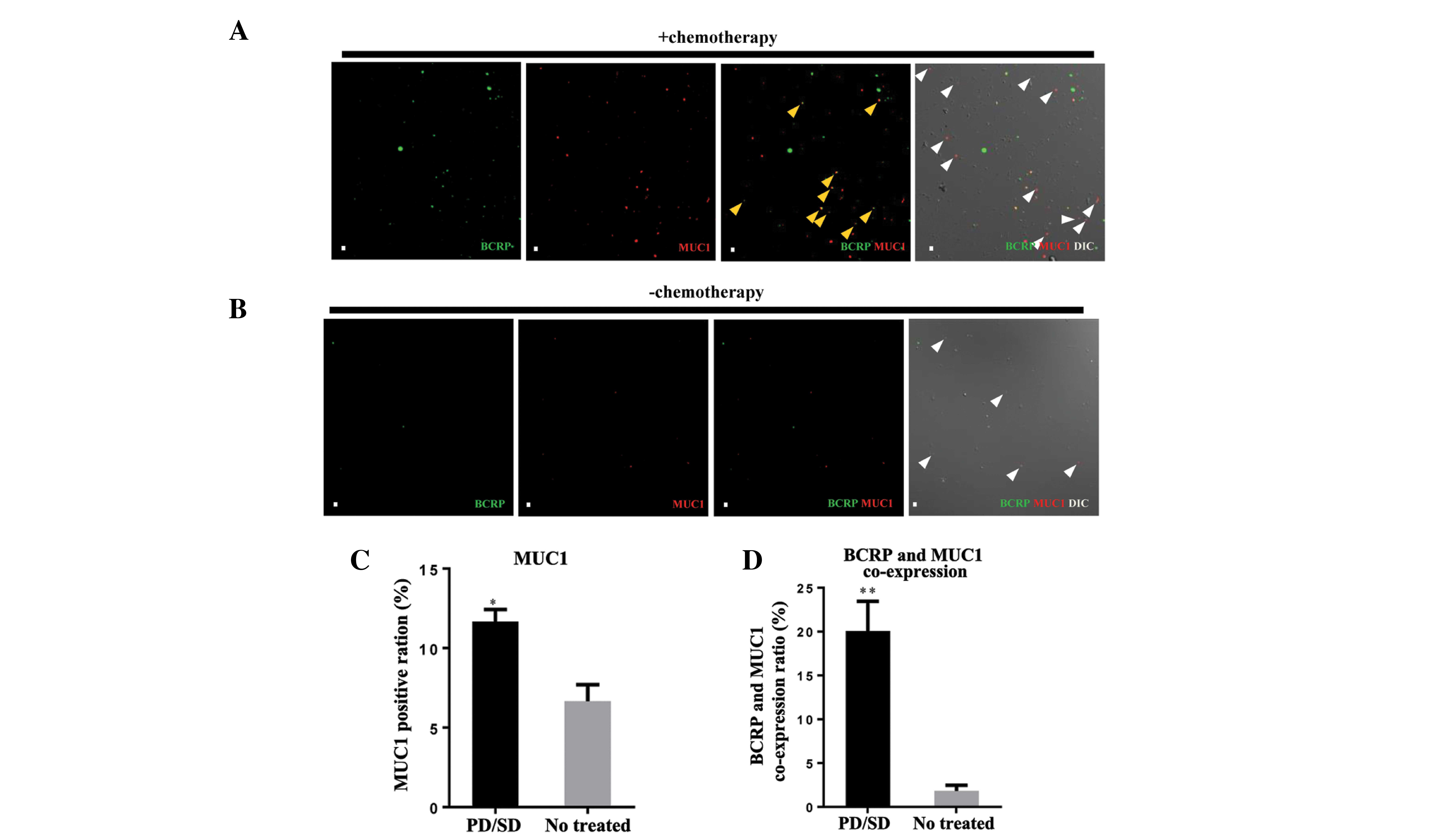
To present the association between BCRP and MUC1
visually, immunostaining was used with specific anti-BCRP/ABCG2 and
anti-MUC1 monoclonal antibodies to visualize their expression in
EVs from the peripheral blood of patients. The results demonstrated
that the EVs from non-responsive patients had higher MUC1
expression (Fig. 4A–C), indicating
their higher release of EVs into the circulation. Notably, BCRP was
co-expressed with MUC1 in samples from non-responsive patients,
however this was almost absent from the control group (Fig. 4 A, B and D), which indicates that the
development of chemoresistance may be due to a progressive
enrichment of BCRP in EVs and their release. All these findings
support the idea that tumor-derived circulating EVs that carry BCRP
could be used as a prognostic biomarker to predict the
chemotherapeutic outcome in breast cancer.
Discussion
BCRP can confer a multidrug-resistant phenotype on
cancer cells and affects drug absorption, distribution, metabolism,
and excretion in normal tissues (11), however, the underlying mechanism
remains unknown. To the best of our knowledge, this is the first
study to investigate the association between BCRP and circulating
EVs, elucidating the mechanism of clinical drug resistance.
Previous studies have demonstrated that recipient
cells can acquire drug resistance by the transmission of
P-glycoprotein or Ca2+-permeable channels via EVs
(10). The association between
BCRP-containing EVs and poor outcomes of clinical chemotherapy were
further verified. In the present study, higher BCRP expression at
both the mRNA and proteins levels was observed in tumor specimens
and EVs in blood samples from patients with a poor chemotherapeutic
outcome (non-responders) compared with those with no chemotherapy.
It has previously been reported that flotillin-2 overexpression is
associated with a poor prognosis and reduced survival in patients
with both early- and late-stage breast cancer (17). Moreover, Ma et al (10) demonstrated that flotillin-2 expression
is significantly upregulated following chemotherapy and the present
study confirmed this. Based on these findings, BCRP and flotillin-2
may be indicators of the potential response to neoadjuvant
chemotherapy. Moreover, the co-expression level of MUC1 and BCRP
also demonstrated a close associationwith the outcome of
chemotherapy.
It has been reported that, apart from BCRP, the
expression levels of MDR2, LRP, and MRP1 all have predictive value
for the clinical outcome of adjuvant chemotherapy (18). The present study indicated that a high
level of BCRP co-expression with MUC1 was induced by TEC therapy
but the underlying mechanism needs to be further defined. To assess
the clinical response to chemotherapy, blood tumor markers such as
CA15-3, carcinoembryonic antigen, and erythrocyte sedimentation
rate are usually used as treatment guidelines (19). However, they do not reflect accurately
the status of patients in response to chemotherapy. Therefore, it
is important to identify more appropriate biomarkers to predict the
chemotherapeutic response. In the present study, significantly
different levels of BCRP-containing EVs were observed between
patients with or without chemoresistance, and the novel hypothesis
that the level of tumor-derived circulating EVs in which BCRP is
expressed can predict the chemotherapeutic response was proposed,
which provides a convenient means of determining an individual
therapeutic strategy when facing the challenge of drug
resistance.
Nevertheless, questions remain, such as: i) The
exact level at which BCRP-containing EVs determine
non-responsiveness. ii) Whether the underlying mechanism of BCRP
enrichment in circulating EVs is intrinsic or acquired. iii)
Whether specific chemotherapy drugs have specific relationships
with EVs. iv) Whether the TNM stage can be determined by measuring
the level of BCRP-containing circulating EVs. v) Whether estrogen
receptor/progesterone receptor/human epidermal growth factor
receptor 2 status or overall/progression-free survival can be
determined by EV measurement. Therefore, further exploration of
these problems may be worthwhile for tapping the potential of
BCRP-containing EVs as a predictor of chemotherapeutic outcome.
Acknowledgements
The authors would like to thank Prof. Iain C. Bruce
for critical reading of the manuscript. The present study was
supported by the Major Research plan of the National Natural
Science Foundation of China (grant no. 91439131 to Dr Xin Ma); the
Natural Science Foundation for Distinguished Young Scholars of
Jiangsu Province (grant no. BK20140004 to Dr Xin Ma); the Program
for New Century Excellent Talents in University of The Ministry of
Education of China (Grant no. NCET-12-0880 to Dr Xin Ma);
Fundamental Research Funds for the Central Universities (grant
no's. JUSRP51311A and JUSRP51516 to Dr Xin Ma); China National
Natural Science Foundation grants (grant no. 81100185 to Dr Xin Ma,
81273437 and 31200126 to Dr Jian Jin); and an NSFC-RGC joint grant
(grant no. 81361168001 to Dr Jian Jin). The Clinical Science and
Technology Projects (grant no. BL2014019 to Dr Dong Hua).
References
|
1
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheung KL, Evans AJ and Robertson JF: The
use of blood tumour markers in the monitoring of metastatic breast
cancer unassessable for response to systemic therapy. Breast Cancer
Res Treat. 67:273–278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weigel MT and Dowsett M: Current and
emerging biomarkers in breast cancer: Prognosis and prediction.
Endocr Relat Cancer. 17:R245–R262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holme PA, Solum NO, Brosstad F, Røger M
and Abdelnoor M: Demonstration of platelet-derived microvesicles in
blood from patients with activated coagulation and fibrinolysis
using a filtration technique and western blotting. Thromb Haemost.
72:666–671. 1994.PubMed/NCBI
|
|
6
|
Hess C, Sadallah S, Hefti A, Landmann R
and Schifferli JA: Ectosomes released by human neutrophils are
specialized functional units. J. Immunol. 163:4564–4573. 1999.
|
|
7
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: Artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
György B, Szabó TG, Pásztói M, Pál Z,
Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al:
Membrane vesicles, current state-of-the-art: Emerging role of
extracellular vesicles. Cell Mol Life Sci. 68:2667–2688. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harding CV, Heuser JE and Stahl PD:
Exosomes: Looking back three decades and into the future. J Cell
Biol. 200:367–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma X, Chen Z, Hua D, He D, Wang L, Zhang
PJ, Cai Y, Gao C, Zhang X, et al: Essential role for
TrpC5-containing extracellular vesicles in breast cancer with
chemotherapeutic resistance. Proc Natl Acad Sci USA. 111:6389–6394.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noguchi K, Katayama K and Sugimoto Y:
Human ABC transporter ABCG2/BCRP expression in chemoresistance:
Basic and clinical perspectives for molecular cancer therapeutics.
Pharmgenomics Pers Med. 7:53–64. 2014.PubMed/NCBI
|
|
12
|
Brangi M, Litman T, Ciotti M, Nishiyama K,
Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T and Bates SE:
Camptothecin resistance: Role of the ATP-binding cassette (ABC),
mitoxantrone-resistance half-transporter (MXR), and potential for
glucuronidation in MXR-expressing cells. Cancer Res. 59:5938–5946.
1999.PubMed/NCBI
|
|
13
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: Revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Staubach S, Razawi H and Hanisch FG:
Proteomics of MUC1-containing lipid rafts from plasma membranes and
exosomes of human breast carcinoma cells MCF-7. Proteomics.
9:2820–2835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brouckaert O, Laenen A, Wildiers H, Floris
G, Moerman P, Van Limbergen E, Vergote I, Billen J, Christiaens MR
and Neven P: The prognostic role of preoperative and (early)
postoperatively change in CA15.3 serum levels in a single hospital
cohort of primary operable breast cancers. Breast. 22:254–262.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gion M, Mione R, Leon AE and Dittadi R:
Comparison of the diagnostic accuracy of CA27.29 and CA15.3 in
primary breast cancer. Clin Chem. 45:630–637. 1999.PubMed/NCBI
|
|
17
|
Wang X, Yang Q, Guo L, et al: Flotillin-2
is associated with breast cancer progression and poor survival
outcomes. J Transl Med. 11:1902013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burger H, Foekens JA, Look MP, van Gelder
Meijer ME, Klijn JG, Wiemer EA, Stoter G and Nooter K: RNA
expression of breast cancer resistance protein, lung
resistance-related protein, multidrug resistance-associated
proteins 1 and 2 and multidrug resistance gene 1 in breast cancer:
correlation with chemotherapeutic response. Clin Cancer Res.
9:827–836. 2003.PubMed/NCBI
|
|
19
|
Marić P, Ozretić P, Levanat S, Oresković
S, Antunac K and Beketić-Oresković: Tumor markers in breast cancer
- evaluation of their clinical usefulness. Coll Antropol.
35:241–247. 2011.
|