Introduction
In recent years, the incidence of testicular cancer
has increased, particularly in industrialized countries (1). For example, in the USA, the incidence of
testicular germ cell tumors increased from 5.7 to 6.8 cases per
100,000 individuals between 1992 and 2009 (1,2).
Testicular yolk sac tumors are a type of non-seminomatous germ cell
tumor (NSGCT) (1,3). Testicular pure yolk sac tumors are rare
in adults (4). Cases of multiple
metastases, originating from a testicular pure yolk sac tumor
descending from a testis, which descended from the intra-abdominal
cavity, are extremely rarely diagnosed (4). By performing a search of PubMed, it was
identified that <20 cases of adult pure yolk sac tumor have been
reported (4–11), and <5 cases of multiple metastasis
have been reported (5,10,11).
Fludeoxyglucose F 18-positron emission tomography computed
tomography is a useful tool to evaluate systematic conditions prior
to further treatment. Males without offspring may choose to undergo
sperm cryopreservation prior to chemotherapy. Follow-up and
evaluation are necessary for advanced adult testicular cancer. The
current study presents the case of a 30-year-old man, with a
diagnosis of testicular pure yolk sac tumor accompanied by multiple
metastases. Written informed consent was obtained from the
patient's family. Relevant literature concerning incidence,
diagnosis, treatment and prognosis of testicular pure yolk sac
tumors are also reviewed. The current case report aims to increase
knowledge with regard to this rare type of testicular cancer, which
may improve diagnosis and treatment.
Case report
A 30-year-old man presented in September 2014 with a
novel and progressively increasing swelling of the left scrotal
contents for ~7 months. The patient was referred to the Department
of Urology of The Second Xiangya Hospital (Changsha, China),
following an ultrasound that indicated the presence of a testicular
tumor.
The medical history of the patient was as follows:
i) Cryptorchidism and indirect inguinal hernia in the left scrotum
for 30 years; ii) secondary tuberculosis in 2005, and a directly
observed treatment short-course for 6 months. The family history
did not demonstrate anything notable.
Physical examination revealed an enlarged, hard and
slightly tender mass in the left scrotal sac. No sinuses, ulcers or
varicose veins were identified on the skin of the scrotum. A
transillumination test was negative, indicating that the
intra-scrotal mass was a tumor as opposed to a hydrocele.
Lactate dehydrogenase (LDH) measured 469.6 U/l
(normal value, <245 U/l); β-human chorionic gonadotropin (β-hCG)
was 103,541 mIU/ml (normal value, <2 mIU/ml) and α-fetoprotein
(AFP) measured 6,252 ng/ml (normal value, <5.8 ng/ml).
Tuberculin purified protein derivative (PPD)-immunoglobulin (Ig) G,
PPD-IgM and MycoDot testing were positive. C-Reactive protein
measured 20.10 mg/l (normal value, <8 mg/l) and erythrocyte
sedimentation rate was 23 mm/h (normal value, <15 mm/h). A chest
X-ray revealed pulmonary tuberculosis with fibrous bundles and
calcification in the upper lung regions. An ultrasound revealed a
solid, heterogeneous mass, ~40×34 mm in the left scrotum. In
addition, dot- and strip-like echo was detected in the mass,
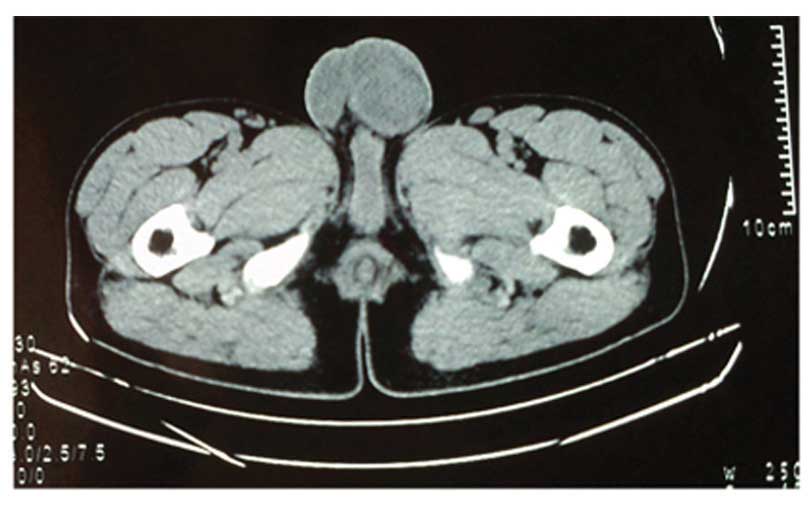
indicating poor vascular flow. Pelvic computed tomography (CT)
scanning additionally revealed a heterogeneous mass characterized
by ring-like shape and flake high density (Fig. 1).
In order to diagnose the scrotal mass, which had
descended from the abdominal cavity, left inguinal exploration and
radical orchiectomy was performed. The excised specimen exhibited a
hard, red surface, and some necrosis and hemorrhaging were observed
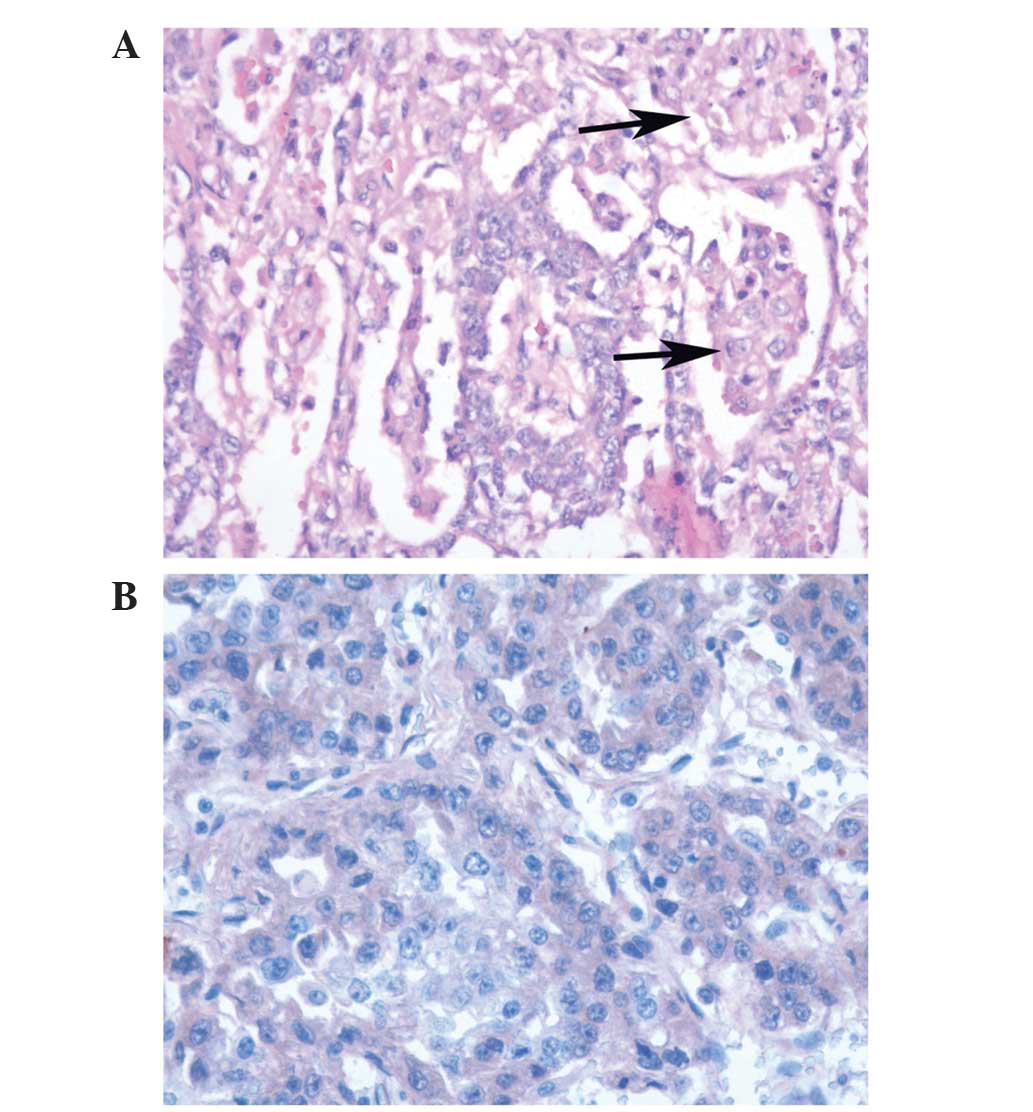
upon sectioning. Pathological results indicated that the specimen
was a germ cell tumor. Subsequent immunohistochemistry revealed AFP
(+), hCG (−), creatine kinase (+), Ki-67 (30%+), octamer-binding
transcription factor 3/4 (−) and placental alkaline phosphatase
(partly+). The final pathological diagnosis reached was a
testicular pure yolk sac tumor (Fig.
2).
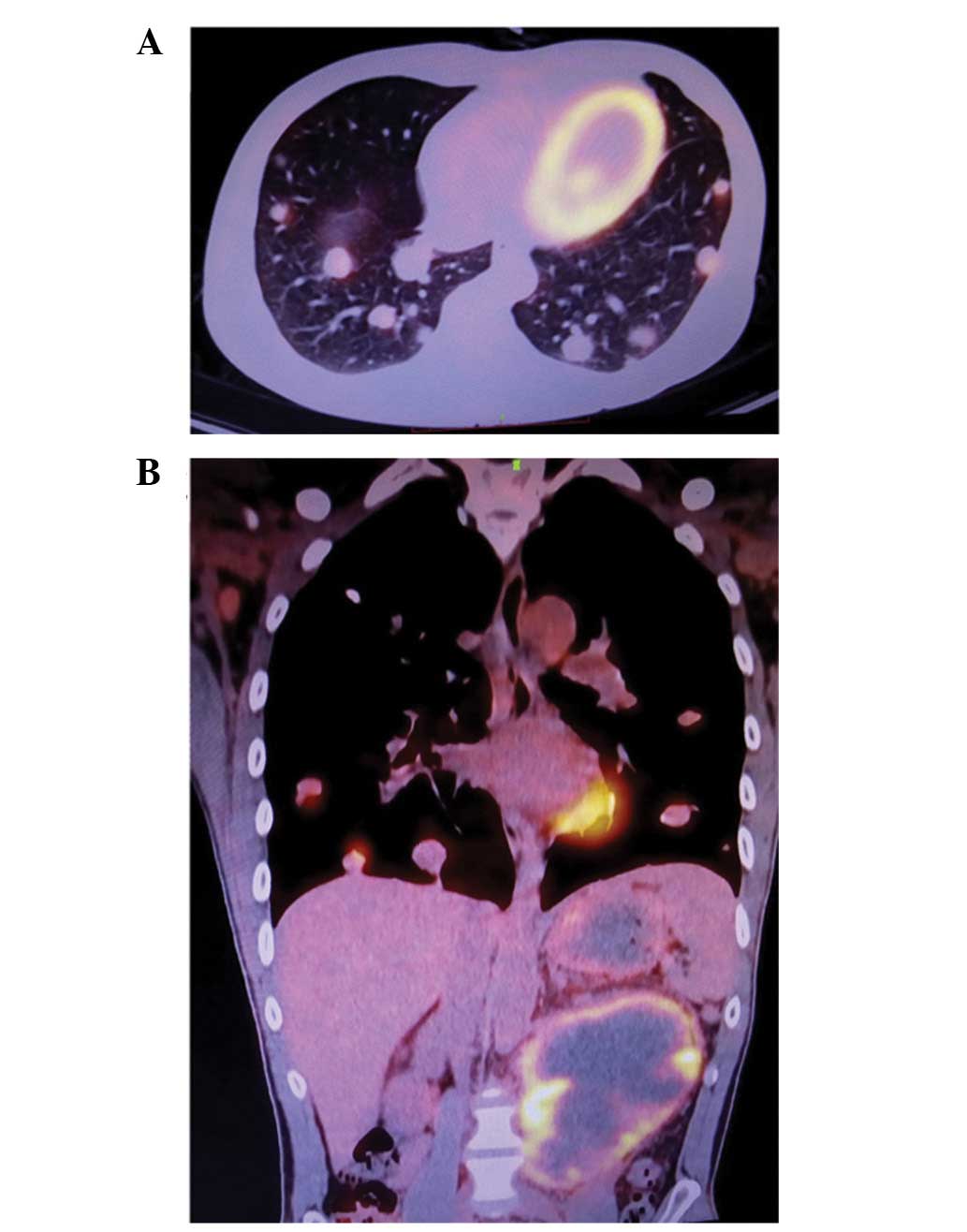
Prior to advanced treatment, fludeoxyglucose F 18
(FDG)-positron emission tomography (PET) assessment was performed
in order to evaluate the general condition of the patient and the
presence of any distal metastases. FDG-PET indicated the presence
of lung and inguinal lymph node metastases, as well as a mass in
the abdominal cavity (Fig. 3). The
patient was advised to undergo semen cryopreservation, and
subsequent cisplatin, etoposide and bleomycin (PEB) combination
chemotherapy regimen (20 mg/m2 cisplatin, days 1–5; 100
mg/m2 etoposide, days 1–5; 30 mg bleomycin, days 1, 8
and 15). However, the patient and his family have currently
rejected the advice, and the patient did not receive any further
therapy.
Discussion
A testicular yolk sac tumor is a type of NSGCT
(1,3).
Testicular yolk sac tumors are common in children exhibiting
testicular cancer, however, they are rarely observed in adults
(4). According to a global
epidemiological investigation, the incidence of testicular cancer
has been increasing in a number of countries between the 1980s and
the 2000s; however, the overall mortality rate has remained low
between the 1970s and the 2000s (12). The same survey additionally revealed
that New Zealand, Australia and Sweden exhibited the three highest
age-standard incidence rates of testicular cancer globally; China
demonstrated one of the lowest mortality rates for testicular
cancer (12). However, the annual
percentage increase in testicular cancer incidence has been
observed to be 3.5% in China (12).
In a Japanese report (13), seminoma
accounted for 80.8% of testicular cancer cases; by contrast yolk
sac tumor cases accounted for 18.0% in 2005 and 2008, respectively
(mean age of patients, 37.0±10.6). PubMed was searched using the
keywords ‘adult testicular cancer’ or ‘yolk sac tumor’. The results
indicated that <20 adult pure yolk sac tumors have been reported
(4–11). Furthermore, multiple organ metastases
associated with yolk sac tumors are even rarer (5,10,11).
Cryptorchidism is a significant risk factor for
testicular cancer (14,15). In the present case, the patient had
possessed a history of cryptorchidism for the previous 30 years.
The present study hypothesized that carcinogenesis occurred prior
to the retained testis descending from the abdominal cavity. The
patient palpated a hard mass and detected a bulge in the left
scrotum 7 months prior to hospital admittance. Indirect inguinal
hernia may have altered the local structure of the inguinal canal,
forming a passage that allowed the intra-abdominal testis to
descend. Alternatively, repeated descending and recurrence of the
hernia may have caused inflammation of the inguinal canal and
intra-abdominal testes. Thus, adhesion may form between the hernia
and testis, causing the hernia sac to pull the intra-abdominal
testis down as it descended from the abdominal cavity.
LDH, β-hCG and AFP are the recommended serum markers
for the evaluation of testicular cancer (1). AFP is not elevated in seminoma; however,
AFP elevation is detectable in 40–60% advanced NSGCT cases
(16). hCG may be elevated in all
types of NSGCT (16). In the present
case, all three serum markers were elevated, particularly AFP and
hCG. This was useful as it is difficult for urologists to evaluate
the pathological patterns of NSGCTs prior to surgery. In the
present case, pathological analysis did not detect trophoblastic
cells, and hCG was negative in immunohistochemical testing in
numerous sample sections. According to Ulbright et al
(17), the immunohistochemistry
results were consistent with a yolk sac tumor. A total of 7 days
following orchiectomy, significant levels of LDH, β-hCG and AFP
were detected, similar to the levels measured prior to surgery. The
elevated hCG levels may not have been due to the testicular cancer;
a coexisting tumor may have been causing the persistent elevation
(16). An FDG-PET procedure was
proposed by the treating urologists in order to evaluate the
general condition of the patient, as well as the presence of
metastases or coexisting tumors. A potential abdominal malignancy
may have been a factor causing the significant positivity of hCG.
LDH, β-hCG and AFP serum markers are generally effective tools for
the evaluation of risk stratifications and treatment decisions,
however, combination analysis and general evaluation may
additionally be necessary (16,18).
Spermatogenesis and semen quality are altered
following chemotherapy for the treatment of testicular cancer, and
the degree of alteration is associated with the type and intensity
of chemotherapy (19,20). According to a survey conducted by
Molnar et al (19), sperm
concentration is reduced in men exhibiting testicular tumors
compared with matched healthy men. In a French national
investigation, sperm concentration and total sperm number were
significantly decreased in NSGCT patients following orchiectomy,
therefore sperm cryopreservation should be considered prior to
orchiectomy (21). In a number of
retrospective studies (22–24), sperm cryopreservation has been
recommended for testicular cancer patients who intend to have a
child in the future. The man in the present case had been married
for 2 years, and intended to have a child in the future, therefore
sperm cryopreservation may be an option.
According to testicular cancer guidelines (1,3,25), the current patient presented with a
stage III tumor, therefore a PEB chemotherapy regimen was proposed
for treatment. Follow-up assessments including general condition,
serum markers and PET-CT should be regularly performed following
chemotherapy. In a Japanese multicenter study (13), the prognosis of testicular cancer was
positive; 1-year and 3-year overall survival rates were 98.3 and
96.8%, respectively. European and USA data indicated that the
5-year relative survival rate of NSGCT patients varied from 90–95%
in 15–29- and 30–49-year olds (26).
However, in the case of the patient in the current study, due to
the presence of multiple metastases and an abdominal tumor, regular
follow-up visits remained necessary in order to evaluate long-term
survival.
In the present case of multiple metastases
originating from a pure testicular yolk sac tumor, serum markers
and FDG-PET may assist with the achievement of a differential
diagnosis. Sperm cryopreservation is necessary for adults who
intend to have a child in the future. Chemotherapy is recommended
in order for patients to achieve tumor remission. In terms of
ongoing evaluation of the remission of metastases, serum marker
evaluation may provide an indicator of patient prognosis.
Acknowledgements
The authors would like to thank Dr Daiqiang Li and
Dr Xiaoling She in the Department of Pathology (The Second Xiangya
Hospital, Changsha, China), for their assistance with the analysis
of pathological results.
References
|
1
|
Albers P, Albrecht W, Algaba F, et al:
Guidelines on Testicular Cancer: 2015 Update. Eur Urol. Aug
18–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nigam M, Aschebrook-Kilfoy B, Shikanov S
and Eggener S: Increasing incidence of testicular cancer in the
United States and Europe between 1992 and 2009. World J Urol.
33:623–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vasdev N, Moon A and Thorpe AC:
Classification, epidemiology and therapies for testicular germ cell
tumours. Int J Dev Biol. 57:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan S, Jetley S, Pujani M and Neogi S:
Pure yolk sac tumor of testis in an adult: A rare occurrence. J
Postgrad Med. 60:351–353. 2014.PubMed/NCBI
|
|
5
|
Balzer BL and Ulbright TM: Spontaneous
regression of testicular germ cell tumors: An analysis of 42 cases.
Am J Surg Pathol. 30:858–865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medica M, Germinale F, Giglio M, Stubinski
R, Campodonico F, Raggio M and Carmignani G: Adult testicular pure
yolk sac tumor. Urol Int. 67:94–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foster RS, Hermans B, Bihrle R and Donohue
JP: Clinical stage I pure yolk sac tumor of the testis in adults
has different clinical behavior than juvenile yolk sac tumor. J
Urol. 164:1943–1944. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Munver R, Kronz JD and Polascik TJ: HIV
infection presenting as an unusually large pure yolk sac tumor of
the testis. J Urol. 164:1653–1654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montserrat Orri V, López-Bonet E, Garijo
G, et al: Pure yolk sack tumor of the testis in adults: Report of a
case. Actas Urol Esp. 20:659–661. 1996.PubMed/NCBI
|
|
10
|
Hashimoto Y, Iwase Y, Mogami T, Hayashi Y,
Sasaki S, Kato M, Tugaya M and Kohri K: A case of adult pure yolk
sac tumor of the testis achieving pathological complete response by
chemotherapy. Hinyokika Kiyo. 41:813–816. 1995.(In Japanese).
PubMed/NCBI
|
|
11
|
Izumi H, Shiokawa H, Shibata Y, Kurokawa J
and Ohbu M: Pure yolk sac tumor of the testis with brain
metastasis: Report of an adult case. Hinyokika Kiyo. 38:1071–1074.
1992.(In Japanese). PubMed/NCBI
|
|
12
|
Shanmugalingam T, Soultati A, Chowdhury S,
Rudman S and Van Hemelrijck M: Global incidence and outcome of
testicular cancer. Clin Epidemiol. 5:417–427. 2013.PubMed/NCBI
|
|
13
|
Miki T, Kamoi K, Fujimoto H, Kanayama HO,
Ohyama C, Suzuki K, Nishiyama H, Eto M, Naito S, Fukumori T, et al:
Clinical characteristics and oncological outcomes of testicular
cancer patients registered in 2005 and 2008: The first large scale
study from the Cancer Registration Committee of the Japanese
Urological Association. Int J Urol. 21:S1–S6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cook MB, Akre O, Forman D, Madigan MP,
Richiardi L and McGlynn KA: A systematic review and meta-analysis
of perinatal variables in relation to the risk of testicular cancer
- experiences of the son. Int J Epidemiol. 39:1605–1618. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pinczowski D, McLaughlin JK, Läckgren G,
Adami HO and Persson I: Occurrence of testicular cancer in patients
operated on for cryptorchidism and inguinal hernia. J Urol.
146:1291–1294. 1991.PubMed/NCBI
|
|
16
|
Gilligan TD, Seidenfeld J, Basch EM,
Einhorn LH, Fancher T, Smith DC, Stephenson AJ, Vaughn DJ, Cosby R
and Hayes DF: American Society of Clinical Oncology: American
Society of Clinical Oncology Clinical Practice Guideline on uses of
serum tumor markers in adult males with germ cell tumors. J Clin
Oncol. 28:3388–3404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ulbright TM, Tickoo SK, Berney DM and
Srigley JR: Members of the ISUP Immunohistochemistry in Diagnostic
Urologic Pathology Group: Best practices recommendations in the
application of immunohistochemistry in testicular tumors: Report
from the International Society of Urological Pathology consensus
conference. Am J Surg Pathol. 38:e50–e59. 2014.PubMed/NCBI
|
|
18
|
Ruf CG, Sachs S, Khalili-Harbi N, Isbarn
H, Wagner W, Matthies C, Meineke V, Fisch M, Chun FK and Abend M:
Prediction of metastatic status in non-seminomatous testicular
cancer. World J Urol. 32:1205–1211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molnar Z, Mokanszki A, Kassai Bazsane Z,
Bhattoa HP, Benyo M, Olah E and Jakab A: Sperm concentration,
hyaluronic acid-binding capacity, aneuploidy and persistent
histones in testicular cancer. Hum Reprod. 29:1866–1874. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paoli D, Gallo M, Rizzo F, et al:
Testicular cancer and sperm DNA damage: Short-and long-term effects
of antineoplastic treatment. Andrology. 3:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rives N, Perdrix A, Hennebicq S,
Saïas-Magnan J, Melin MC, Berthaut I, Barthélémy C, Daudin M,
Szerman E, Bresson JL, et al: The semen quality of 1158 men with
testicular cancer at the time of cryopreservation: Results of the
French National CECOS Network. J Androl. 33:1394–1401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung JP, Haines CJ and Kong GW: Sperm
cryopreservation for Chinese male cancer patients: A 17-year
retrospective analysis in an assisted reproductive unit in Hong
Kong. Hong Kong Med J. 19:525–530. 2013.PubMed/NCBI
|
|
23
|
Molnár Z, Berta E, Benyó M, Póka R, Kassai
Z, Flaskó T, Jakab A and Bodor M: Fertility of testicular cancer
patients after anticancer treatment - experience of 11 years.
Pharmazie. 69:437–441. 2014.PubMed/NCBI
|
|
24
|
Záková J, Lousová E, Ventruba P, Crha I,
Pochopová H, Vinklárková J, Tesařová E and Nussir M: Sperm
cryopreservation before testicular cancer treatment and its
subsequent utilization for the treatment of infertility.
ScientificWorldJournal. 2014:5759782014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oldenburg J, Fosså SD, Nuver J,
Heidenreich A, Schmoll HJ, Bokemeyer C, Horwich A, Beyer J and
Kataja V: ESMO Guidelines Working Group: Testicular seminoma and
non-seminoma: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 24(Suppl 6): vi125–vi132. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Verhoeven RH, Gondos A, Janssen-Heijnen
ML, Saum KU, Brewster DH, Holleczek B, Crocetti E, Rosso S,
Hakulinen T, Aareleid T and Brenner H: Testicular cancer in Europe
and the USA: Survival still rising among older patients. Ann Oncol.
24:508–513. 2013. View Article : Google Scholar : PubMed/NCBI
|