Introduction
Oral malignant melanomas (MMs) are extremely rare,
accounting for <2% of all reported MMs. MMs are known to have a
high potential for malignancy, invasiveness and metastasis
(1,2).
Although MMs with characteristic melanin-pigmented tumor cells are
easy to diagnose, amelanotic MMs (AMMs) are extremely difficult to
diagnosis due to the absence of these cells (3,4). AMM can
be divided into the following five types based on the varied
clinical features observed: The pigmented nodular type, the
non-pigmented nodular type, the pigmented macular type, the
pigmented mixed type and the non-pigmented mixed type (5,6). The
non-pigmented nodular type is an amelanotic tumor that lacks a
radial growth phase, while the non-pigmented mixed type is an
amelanotic nodular tumor that is surrounded by a radial growth
phase. It is hard to distinguish between amelanotic epithelioid
melanoma and poorly-differentiated carcinoma or large cell lymphoma
(4,7).
Furthermore, the differentiation between spindle cell melanomas and
sarcomas and sarcomatoid carcinomas is also difficult (7,8). In such
cases, immunohistochemistry with antibodies directed against
certain melanocytic differentiation antigens is typically required
for the final diagnosis (4,7,8).
Currently, the three most useful immunomarkers for
the identification of melanocytes and melanomas are HMB-45, S-100
and Melan-A (4,7–11). The
S-100 protein is an acidic, calcium-binding protein that Moore
(12) first extracted from the bovine
brain in 1965. Although it is an extremely sensitive marker for
nevi and melanoma cells (9), the
central and peripheral nervous systems of all vertebrates also
contain a wide distribution (13). A
number of previous studies have used the anti-S-100 antibody to
immunohistochemically diagnose the primary and metastatic MMs of
various sites in the human body (14).
Anti-HMB-45 was initially described by Gown et
al (15) in 1986. The antibody
recognizes gp100, a melanosomal glycoprotein. The HMB-45 antigen
has previously been shown to be localized in pre-melanosomes, with
HMB-45-positive staining associated with active early melanosome
formation (9).
In 1985, Melan-A was cloned by Coulie et al
(16) from the human melanoma
SK-MEL-29 cell line. Melan-A encodes a melanoma antigen that
autologous cytotoxic T cells recognize. The Melan-A-derived peptide
recognized by the T cells has been identified as the nonamer
AAGIGILTV, which shows HLA-A2 restriction. Isolation of T cells
with specificity for Melan-A is possible in normal individuals, as
well as melanoma patients (17,18). A
previous study revealed that Melan-A-positive staining was
associated with initially developed and massively proliferated
lesions. In the present study, the expression of three melanocytic
differentiation markers, HMB-45, S-100 and Melan-A, was examined by
immunohistochemistry in order to determine whether HMB-45, S-100
and Melan-A were useful for diagnosing primary oral AMM. The study
also attempted to identify which marker was the most sensitive.
Written informed consent was obtained from the patient's
family.
Case report
Patient
In October 2009, an 80-year-old male presented to
the Second Department of Oral and Maxillofacial Surgery, Osaka
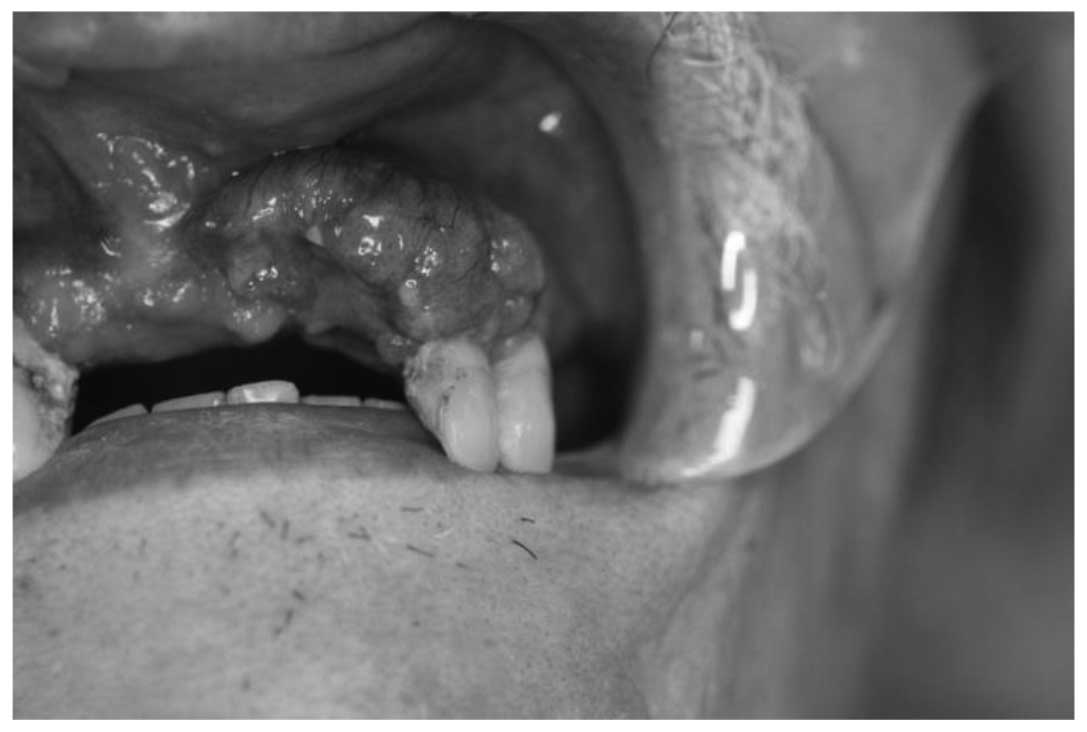
Dental University (Chuo-ku, Japan) with a painful swelling in the
left maxillary region that had been apparent for 1 month. The
patient had undergone surgery for esophageal cancer three months
earlier at a medical hospital. A physical examination revealed a
4×5-cm enlargement in the left maxillary gingiva that extended from
the midline to the first molar. The lesion was mildly erythematous
with ill-defined borders and an intact, smooth mucosal surface
(Fig. 1). A radiographic
investigation using orthopantomography revealed slightly horizontal
bone resorption in the alveolar ridge. There was no palpable
lymphadenopathy. An incisional biopsy was performed under local
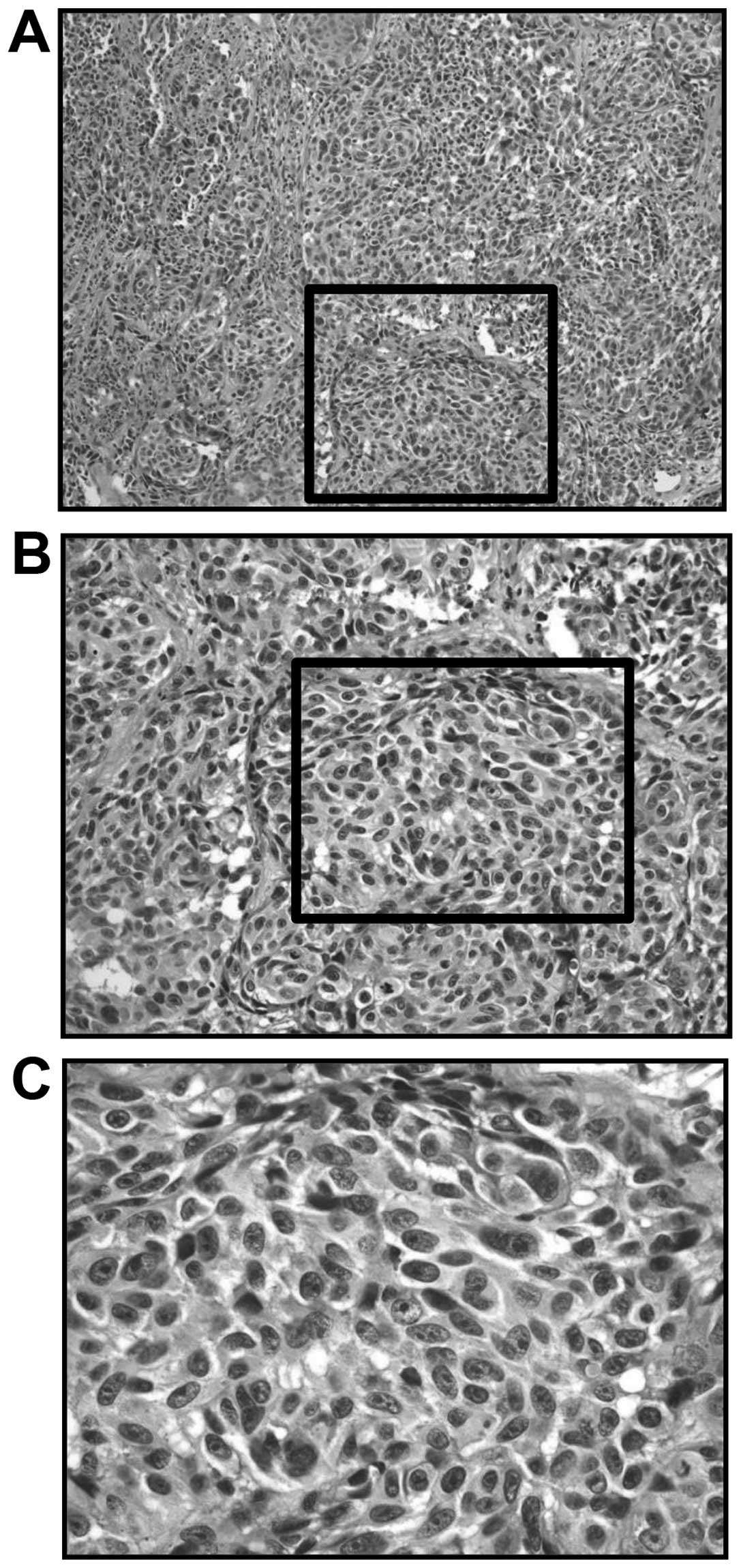
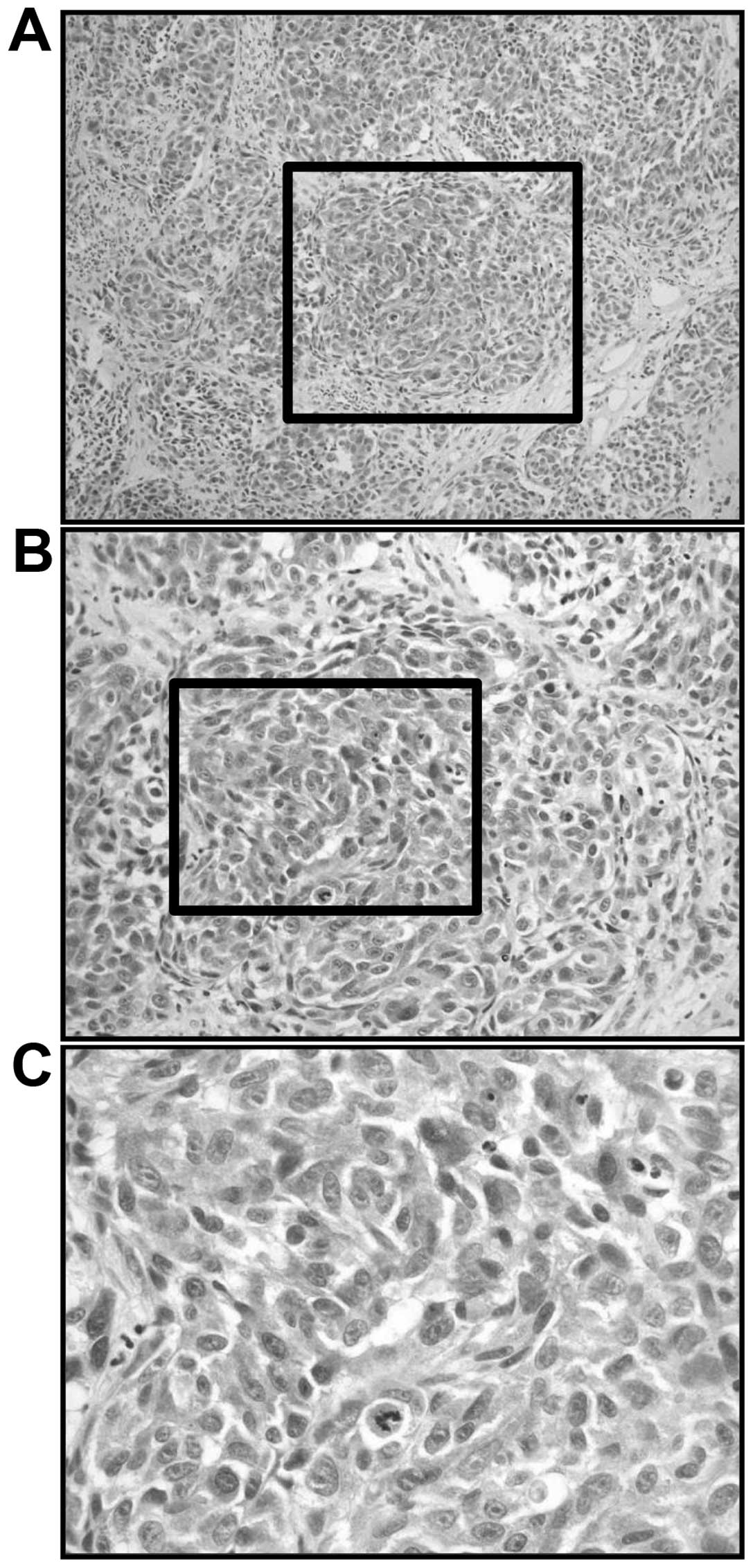
anesthesia. Microscopically, the cellularity was high, with an
increased density of epithelioid-type and spindle-type cells.
Moreover, there were also cells with bizarre nuclei, and notable
mitosis (Fig. 2). Immunoreactivity
was detected in the tumor cells for S-100, HMB-45 and Melan-A
(Figs. 3–5). Therefore, the pathological diagnosis of
the lesion was AMM. In December 2009, the patient underwent surgery
(partial maxillectomy and bilateral modified radical neck
dissection) and in January 2010 received systemic dacarbazine,
nimustine and vincristine, and local intracutaneous interferon β
therapy (DAV-Feron) (19) at the
Department of Otolaryngology and Head and Neck Surgery, Osaka City
University Graduate School of Medicine (Osaka, Japan). After
DAV-Feron, in August 2010, dentures were created at the Department
of Geriatric Dentistry, Osaka Dental University.
Clinical investigation
Non-pigmented tumors without a radial growth phase
have clinically been classified as the non-pigmented nodular type
(4,20). This type of tumor has often been
misdiagnosed as an epulis, squamous cell carcinoma or benign tumor
due to the lack of pigments and a radial growth phase. In the
present case, the non-pigmented nodular type tumor was formed from
spindle cells and a majority of polygonal cells. Only a small
number of pigmented neoplastic melanocytes were observed.
Hematoxylin and eosin staining indicated a histopathological
diagnosis of MM. Immunohistochemical staining with antibodies
against HMB-45, S-100, and Melan-A was positive, thus confirming
the diagnosis.
Immunohistochemistry
Tissue samples were fixed in 10% neutral buffered
formalin solution immediately after resection and then embedded in
paraffin. Sections (4-µm thick) were cut and mounted on
silane-coated glass slides. The sections were deparaffinized in
L-limonene and dehydrated through a graded ethanol series. Antigen
retrieval was performed by autoclaving at 121°C for 15 min in Histo
VT One® (pH 7.0; Nacalai Tesque, Kyoto, Japan). Endogenous
peroxidase activity was blocked with 3% H2O2
for 10 min. Tissue sections were then incubated with the
anti-HMB-45 monoclonal antibody (Dako, Tokyo, Japan), anti-S-100
polyclonal antibody (Nichirei Bioscience, Tokyo, Japan) and
anti-Melan-A monoclonal antibody (Dako) at room temperature for 1
h. The tissue slides were incubated with peroxidase
micropolymer-conjugated secondary antibodies (Vector Laboratories,
Burlingame, CA, USA) at room temperature for 30 min, and visualized
by incubation with the 3,3′-diaminobenzidine tetrahydrochloride
liquid system (Dako) at room temperature for 5 min. The sections
were subsequently counterstained with hematoxylin and observed by
light microscopy (Olympus Corporation, Tokyo, Japan). Cutaneous
melanoma and nevus tissues, obtained during surgeries performed
between April and October 2011 at Gokeikai Osaka Kaisei Hospital
(Osaka, Japan), were provided by Dr. Masaki Akane (Gokeikai Osaka
Kaisei Hospital) and served as positive controls, and Tris-buffered
saline was used instead of primary antibody as the negative
control. Brown cytoplasmic staining was considered as a positive
result for HMB-45 and Melan-A, and brown nuclear and cytoplasmic
staining was considered to be positive for the S-100 protein.
Positively immunostained cells were easy to distinguish from
melanin-pigmented cells when the sections were carefully compared
with negative control sections. Staining intensities (SIs) were
graded as follows: 0, no staining; 1, weak staining; 2, moderate
staining; and 3, strong staining, when compared with the cutaneous
melanoma positive control sections, in which the SI was graded as
3. Labeling indices (LIs) were calculated by initially scanning the
sections at low power, then selecting at least three high-power
fields and counting 1,000 tumor cells in each of the three fields.
HMB-45, S-100, and Melan-A LIs were counted as the ratio of
positively immunostained tumor cells to the total number of tumor
cells counted.
Statistical analysis
A Mann-Whitney U test was performed using the SPSS
software package (version 13.0; SPSS, Inc., Chicago, IL, USA) to
assess statistically significant differences between samples. Data
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
The majority of HMB-45, S-100 and Melan-A
immunostaining in the melanoma cells was cytoplasmic, but nuclear
staining was also found in the anti-S-100-stained tumor cells. The
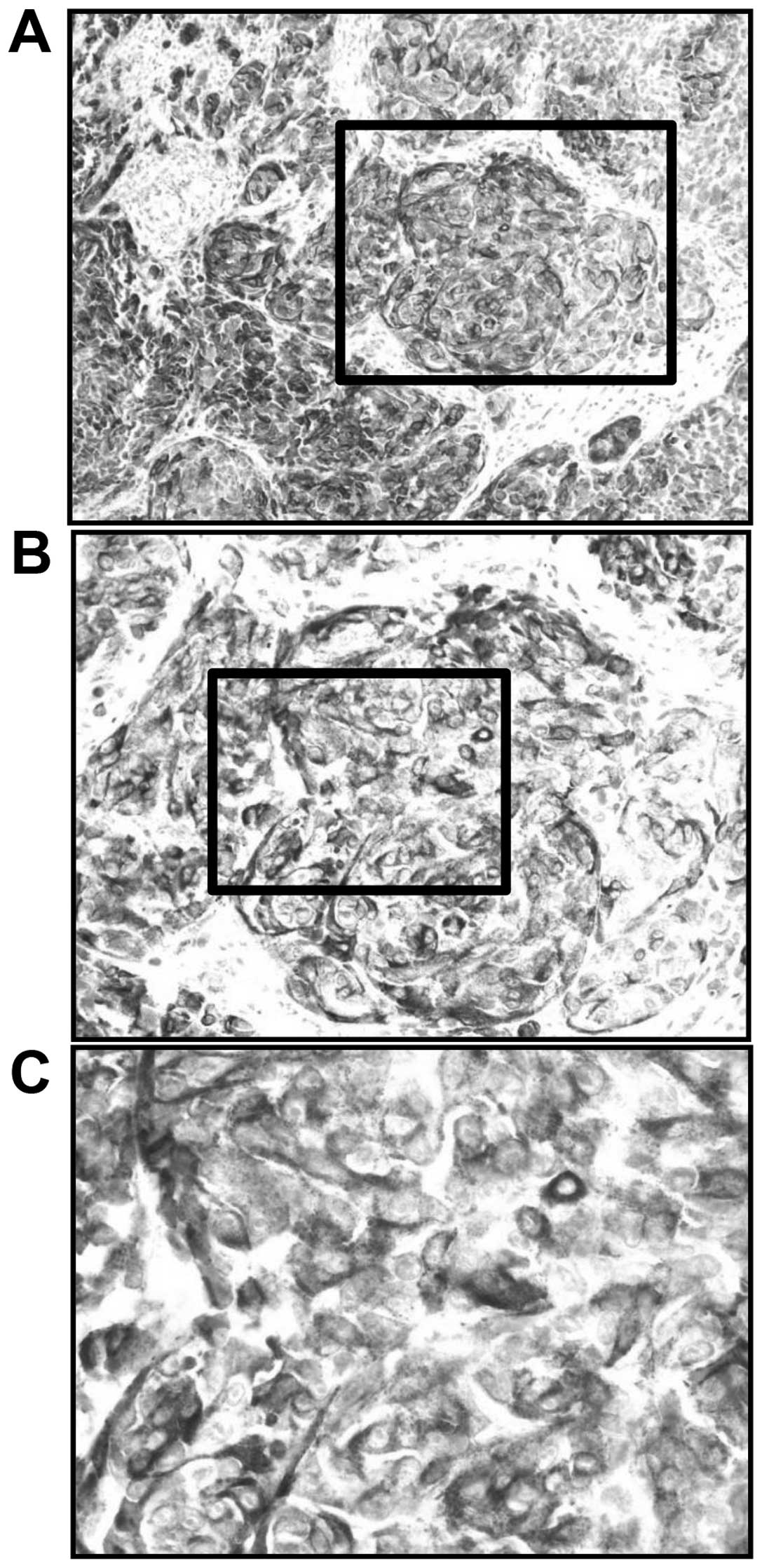
SIs for HMB-45-positive AMM were graded as 3 (Fig. 3). The LI for HMB-45-positive AMM was
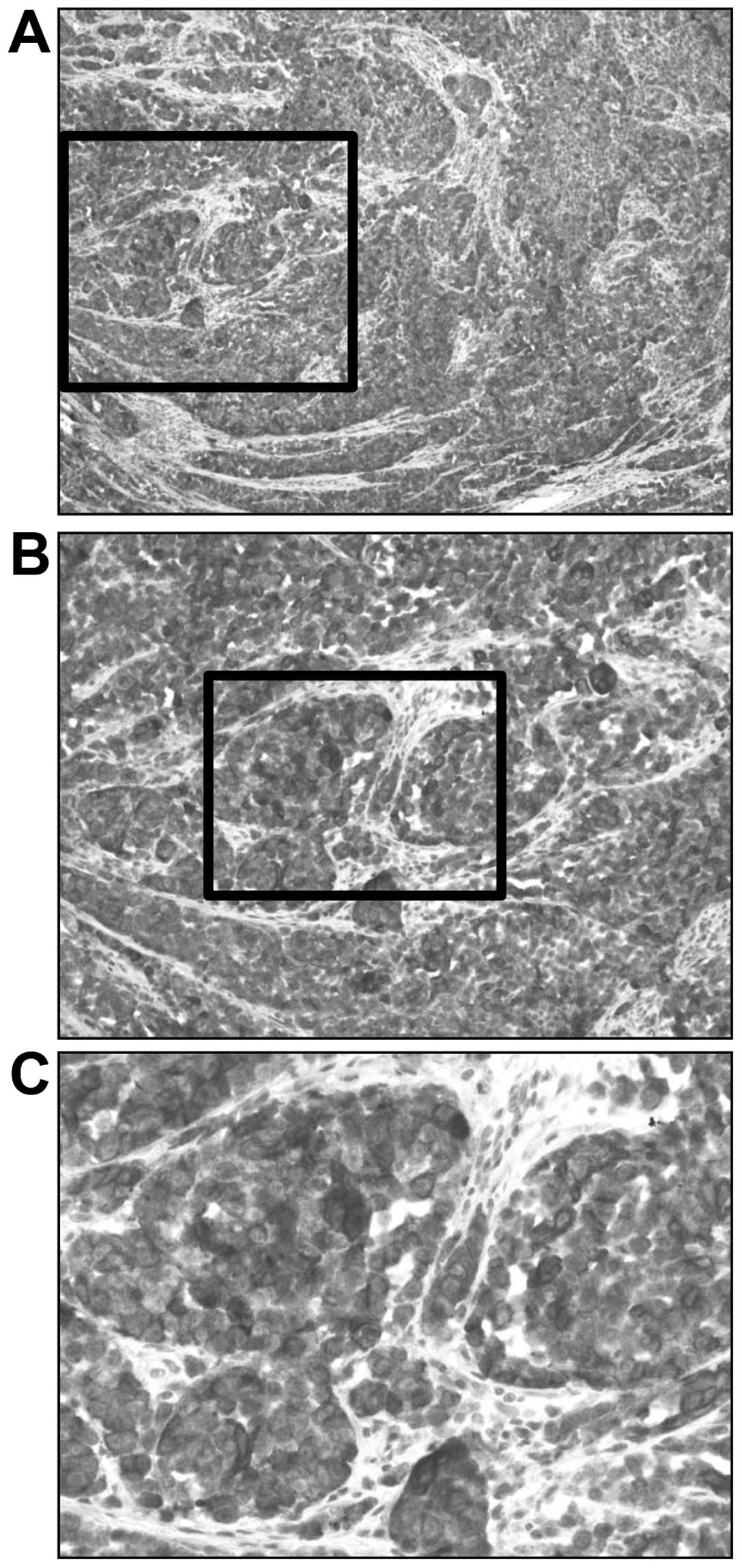
85%. The S-100 SI was graded as 2 (Fig.
4) and the LI for S-100-positive AMM was 71%. Additionally, the
SIs for Melan-A-positive AMM were graded as 3 (Fig. 5) and the LI was 95%.
Discussion
Oral MM accounts for only 0.2 to 8.0% of all MMs
(1). The 5-year survival rate has
previously been reported to be between 15 and 38% (21). The origins of AMM reside in the
malignant transformation of proliferating melanocytes existing in
the epithelial basal layer in the mucosa. The palate is the most
commonly affected site, followed by the maxillary gingiva (18). The differential diagnosis for
pigmented lesions on the oral mucosa includes melanotic macule,
nevi, melanoma, physiological pigmentation and tattoos (21). Moreover, as AMM accounts for only 2.3%
of all MMs (22), it is extremely
rare.
Clinically, the diagnosis of MM is commonly
dependent on the presence of a pigmented lesion (23). In its absence, there is typically no
other parameter to alert a clinician to the possibility of MM,
particularly in the oral region due to its rarity in this location.
Upon histopathological examination, a mixture of polygonal and
spindle cells are present in differing proportions. Amelanotic or
only slightly pigmented tumors can, however, be diagnosed correctly
with immunohistochemistry. In the present study,
immunohistochemical staining with antibodies against the HMB-45,
S-100 and Melan-A proteins successfully diagnosed this amelanotic
case. Anti-S-100, anti-HMB-45 and anti-Melan-A are the three most
commonly used antibodies for the immunohistochemical diagnosis of
primary oral MM. Previous studies have recorded a positive rate of
100% for anti-S-100 (3,7,11) and ~79%
(range, 71–100%) for anti-HMB-45 (1,3,7,11). These
results indicated that S-100 is a more sensitive marker than HMB-45
and Melan-A for immunohistochemically diagnosing MM. However, Naoki
et al (24) reported that
Melan-A-positive staining was associated with both initially
developed and massively proliferated lesions. However, HMB-45 and
S-100 were positive in initially developed lesions and negative in
massively proliferated lesions. Furthermore, the SI of Melan-A was
higher than those of HMB-45 and S-100 in the present study. Taken
together, these results suggest that HMB-45, S-100, and Melan-A may
be good markers for immunohistochemically diagnosing primary oral
MMs. Moreover, Melan-A may be a more sensitive and specific marker
than S-100 and HMB-45.
In conclusion, oral MMs are extremely rare and
difficult to diagnosis due to the absence of melanin-pigmented
tumor cells. The three most useful immunomarkers for the
identification of melanocytes and melanomas appear to be HMB-45,
S-100 and Melan-A. Furthermore, Melan-A may be a more sensitive and
specific marker than S-100 and HMB-45.
References
|
1
|
Manganaro AM, Hammond HL, Dalton MJ and
Williams TP: Oral melanoma: Case reports and review of the
literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
80:670–676. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rapini RP: Oral melanoma: Diagnosis and
treatment. Semin Cutan Med Surg. 16:320–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gazit D and Daniels TE: Oral melanocytic
lesions: Differences in expression of HMB-45 and S-100 antigens in
round and spindle cells of malignant and benign lesions. J Oral
Pathol Med. 23:60–64. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirano T: Immunohistochemical study of
malignant melanoma. Acta Pathol Jpn. 36:733–743. 1986.PubMed/NCBI
|
|
5
|
Tanaka N, Amagasa T, Iwaki H, Shioda S,
Takeda M, Ohashi K and Reck SF: Oral malignant melanoma in Japan.
Oral Surg Oral Med Oral Pathol. 78:81–90. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka N, Mimura M, Ichinose S and Odajima
T: Malignant melanoma in the oral region: Ultrastructural and
immunohistochemical studies. Med Electron Microsc. 34:198–205.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prasad ML, Jungbluth AA, Iversen K, Huvos
AG and Busam KJ: Expression of melanocytic differentiation markers
in malignant melanomas of the oral and sinonasal mucosa. Am J Surg
Pathol. 25:782–787. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kilpatrick SE, White WL and Browne JD:
Desmoplastic malignant melanoma of the oral mucosa. An
underrecognized diagnostic pitfall. Cancer. 78:383–389. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blessing K, Sanders DS and Grant JJ:
Comparison of immunohistochemical staining of the novel antibody
melan-A with S100 protein and HMB-45 in malignant melanoma and
melanoma variants. Histopathology. 32:139–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jungbluth AA, Busam KJ, Gerald WL,
Stockert E, Coplan KA, Iversen K, MacGregor DP, Old LJ and Chen YT:
A103: An anti-Melan-a monoclonal antibody for the detection of
malignant melanoma in paraffin-embedded tissues. Am J Surg Pathol.
22:595–602. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barrett AW, Bennett JH and Speight PM: A
clinicopathological and immunohistochemical analysis of primary
oral mucosal melanoma. Eur J Cancer B Oral Oncol. 31B:100–105.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore BW: A soluble protein characteristic
of the nervous system. Biochem Biophys Res Commun. 19:739–744.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakajima T, Watanabe S, Sato Y, Kameya T,
Shimosato Y and Ishihara K: Immunohistochemical demonstration of
S100 protein in malignant melanoma and pigmented nevus and its
diagnostic application. Cancer. 50:912–918. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nobuyuki T, Teruo A, Hiroshi I, Shigetoshi
S, Masamune T, Kenichi O and Steven F: Oral malignant melanoma in
Japan. Oral Surg Oral Med Oral Pathol. 78:81–90. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gown AM, Vogel AM, Hoak D, Gough F and
McNutt MA: Monoclonal antibodies specific for melanocytic tumors
distinguish subpopulations of melanocytes. Am J Pathol.
123:195–203. 1986.PubMed/NCBI
|
|
16
|
Rapini RP, Golitz LE, Greer RO Jr,
Krekorian EA and Poulson T: Primary malignant melanoma of the oral,
cavity. A review of 177 cases. Cancer. 55:1543–1551. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sensi M, Traversari C, Radrizzani M, Salvi
S, Maccalli C, Mortarini R, Rivoltini L, Farina C, Nicolini G,
Wolfel T, et al: Cytotoxic T-lymphocyte clones from different
patients display limited T-cell-receptor variable-region gene usage
in HLA-A2-restricted recognition of the melanoma antigen
Melan-A/MART-1. Proc Natl Acad Sci USA. 92:5674–5678. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salvi S, Segalla F, Rao S, Arienti F,
Sartori M, Bratina G, Caronni E, Anichini A, Clemente C, Parmiani
G, et al: Overexpression of the T-cell receptor beta-chain variable
region TCRBV14 in HLA-A2-matched primary human melanomas. Cancer
Res. 55:3374–3379. 1995.PubMed/NCBI
|
|
19
|
Ishida H, Nagai T, Sato S, Honda M, Uotani
T, Samejima K, Hanaoka T, Akahori T, Takai Y and Seki H:
Concomitant sentinel lymph node biopsy leading to abbreviated
systematic lymphadenectomy in a patient with primary malignant
melanoma of the vagina. Springerplus. 4:1022015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka N, Mimura M, Kimijima Y and Amagasa
T: Clinical investigation of amelanotic malignant melanoma in the
oral region. J Oral Maxillofac Surg. 62:933–937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ariel IM: Amelanotic melanomas: An
analysis of 77 patients. Curr Surg. 38:151–155. 1981.PubMed/NCBI
|
|
22
|
Fujita S, Takahashi H, Tsuda N and Okabe
H: Immunohistochemical localization of S-100 protein and its
subunits in melanotic lesion in the oral mucosa and skin. J Oral
Pathol Med. 20:429–432. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naoki O Kawada: The stage of melanogenesis
in amelanotic melanoma. Melanoma in the Clinic - Diagnosis,
Management and Complications of Malignancy. Murph M: (InTech).
277–283. 2011.
|
|
24
|
Naoki O, Masuki Y, Shigeru K and Akira K:
Amelanotic vulvar melanoma with intratumor histological
heterogeneity. J Dermatol. 37:537–5412010.
|