Introduction
Glioblastoma multiforme (GBM) is the most common and
fatal primary brain tumor in adults. Despite available treatments,
including surgical resection, chemotherapy and radiotherapy, the
vast majority of individuals only survive 1–2 years following
diagnosis (1). Poor prognosis is
attributed to extensive infiltration to brain tissue and intrinsic
resistance to conventional treatments. Aggressive invasion of GBM
cells into brain tissue often limits complete surgical resection
and contributes to therapeutic resistance, which results in fatal
tumor recurrence (2). Thus, the
development of strategies targeting the invading cells or
restraining their invasive capacity is likely to provide effective
therapies.
Increasing evidence has supported the hypothesis
that a rare subpopulation of cancer cells sharing stem cell
characteristics within a GBM has potent capacity of tumor
propagation (3,4). These cells are termed glioma stem cells
(GSCs); they demonstrate greater tumorigenic potential compared
with matched non-stem tumor cells when xenotransplanted into the
brains of immunocompromised rodents (5–8). The work
of Singh et al (9) firmly
established the existence of a subpopulation of cells with a ‘stem
cell-like’ phenotype, expressing CD133 cell surface marker, within
malignant brain tumors. CD133 and nestin are currently the most
accepted markers for identification of GSCs. However, certain
studies have proposed that there is not a hierarchical association
between CD133+ and CD133− cells composing
neurospheres (10).
A2B5 antigen is recognized as a marker of neural
progenitor cells, and explants from A2B5+ tumor cells
displayed a typical progenitor morphology and clearly indicated
their immature state (11). In a
previous study, the majority of A2B5+ multipotential
progenitor cells differentiated to oligodendrocytes and a minority
of these cells differentiated to neurons (12). A2B5+ cells exhibit the
potential to differentiate into oligodendrocytes and type-1 and
type-2 astrocytes, and all xenografts containing A2B5+
cells generated migrating cells with distinctive functional
properties according to glioma subtypes (11). The A2B5+ cells, but not the
A2B5− cells isolated from GBM, have neural stem-like
cell properties. Thus the A2B5+ initiating cells may be
sorted into two populations, the A2B5+/CD133+
and A2B5+/CD133− cells, according to
expression of CD133 antigen.
At present, there have been no studies directly
examining the migratory and invasive potential of glioma-initiating
cells (GICs), expressing CD133−/A2B5+ surface
markers, compared with matched differentiated cells. The invasive
potential of CD133−/A2B5+ GICs and
differentiated non-initiating tumor cells were investigated in
vitro and in vivo in a mouse tumor xenograft model.
Materials and methods
GICs culture
Tumor tissues from a human GBM surgical specimen,
which were collected from a 23-year-old man in The First Affiliated
Hospital of Soochow University (Suzhou, China), were washed,
deprived of vessels, acutely dissociated in phosphate buffered
saline (PBS) and subjected to enzymatic dissociation. The patient
provided written informed consent to participate in the study,
which was approved by the Ethics Review Board of the First
Affiliated Hospital of Soochow University (no. 2012070). Cells were
cultured in high glucose Dulbecco's modified Eagle's medium (DMEM)
with 10% fetal bovine serum (FBS) for ~2 months, then the glioma
cells were subsequently placed in serum-free DMEM/F12 medium
supplemented with 1% N2 (Gibco; Thermo Fisher Scientific Waltham,
MA, USA), 20 ng/ml epidermal growth factor [EGF (Invitrogen; Thermo
Fisher Scientific)], and 20 ng/ml basic fibroblast growth factor
[bFGF (Invitrogen; Thermo Fisher Scientific)] for ~7 days and
formed non-adhesive neurospheres. Neurospheres were maintained by
changing half of the medium every 3 days and collected by
centrifugation at 1,000 × g for 10 min. Subspheres were formed for
3~4 days after primary spheres were dissociated mechanically to
single cell suspension. Neurospheres of ~12 passages were used for
sorting. Magnetic isolation of CD133−/A2B5+
GICs population was performed using the Miltenyi Biotec A2B5 and
CD133 Cell Isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany). Cells were cultured at 37°C in a humidified 5%
CO2/95% air atmosphere.
Immunofluorescence staining for GICs
markers
Tumor spheres were plated onto poly-L-lysine-coated
glass coverslips. Cells were fixed with 4% paraformaldehyde for 20
min at room temperature, washed three times with PBS, blocked with
2% goat serum (Boster Bio-Engineering, Wuhan, China) for 30 min and
permeabilized with 0.1% Triton X-100 (Beyotime Institute of
Biotechnology, Shanghai, China). Cells were incubated with primary
monoclonal anti-human mouse CD133 (1:200; cat. no. 130-090-422;
Miltenyi Biotec GmbH), monoclonal anti-human rabbit nestin (1:300;
cat. no. ab105389; Abcam, Cambridge, MA, USA) and monoclonal
anti-human mouse A2B5 (1:200; cat. no. ab53521; Abcam) antibodies
overnight at 4°C. Subsequently, cells were washed three times with
PBS. Secondary anti-mouse IgGs conjugated with Alexa fluor 555
(1:1,000; cat. no. A-21422; Molecular Probes; Thermo Fisher
Scientific) or anti-rabbit IgGs conjugated with Alexa fluor 488
(1:1,000; cat. no. A-11008; Molecular Probes; Thermo Fisher
Scientific) were used in darkness for 30 min at room temperature.
The nuclei were counterstained with anti-fade sealant containing
4′6-diamidino-2-phenylindole (DAPI) (5 µg/ml; SouthernBiotech,
Birmingham, AL, USA). Fluorescence images were captured using a
fluorescence microscope (Olympus BX40; Olympus Corporation, Tokyo,
Japan).
Flow cytometric analysis of
CD133−/A2B5+ cells
GICs were dissociated into single cell suspension,
then incubated with PE-conjugated anti-human mouse IgG A2B5
antibody (1:100; Miltenyi Biotech GmbH) and APC-conjugated
anti-human mouse IgG CD133 antibody (1:100; Miltenyi Biotech GmbH).
Labeled cells were analyzed by a flow cytometer Beckton Dickinson
FACScan (BD Biosciences, San Jose, CA, USA) (13). Unsorted GICs were also analyzed as a
control.
Differentiation of GICs into
non-initiating tumor cells
GICs were differentiated into non-initiating tumor
cells in a high glucose DMEM of 10% FBS without N2/EGF/bFGF for 28
days. The cells were blocked with normal goat serum for 30 min.
Next, the cells were incubated with monoclonal anti-human rabbit
SOX2 (GSC marker; 1:200; cat. no. ab97959; Abcam), monoclonal
anti-human mouse Tuj1 (neuron marker; 1:200; cat. no. ab14545;
Abcam), monoclonal anti-human mouse GFAP (astrocyte marker; 1:300;
cat. no. ab10062; Abcam) and monoclonal anti-human mouse Galc
(oligodentrocyte marker; 1:100; cat. no. ab125086; Abcam) primary
antibodies overnight at 4°C. Secondary anti-mouse IgGs conjugated
with Alexa fluor 555 (1:1,000; cat. no. A-21422; Molecular Probes;
Thermo Fisher Scientific) or anti-rabbit IgGs conjugated with Alexa
fluor 488 (1:1,000; cat. no. A-11008; Molecular Probes; Thermo
Fisher Scientific) were used in darkness for 30 min at room
temperature. The nuclei were counterstained with DAPI (5 µg/ml).
Fluorescence images were captured using a fluorescence microscope
(Olympus BX40; Olympus Corporation) to confirm that differentiation
had occurred.
In vitro cell migration and matrigel
invasion assay
The in vitro cell invasion assay was
performed using a Matrigel-coated (3 mg/ml) invasion chamber (BD
Biosciences), while the migration assay was performed by control
inserts. Cell culture medium supplemented with 10% FBS was added to
the lower chamber to act as a chemoattractant. Cells were seeded at
a density of 1×105 cells/well onto the upper inserts.
After incubation for 24 h at 37°C, the non-migratory or
non-invasive cells were removed from the upper side of the filter
by gentle scrubbing with a moist cotton swab. The migrating or
invading cells in the reverse side of the filter were fixed in 100%
methanol and stained with crystal violet (Beyotime Institute of
Biotechnology), then counted under an inverted microscope (Olympus
CKX41; Olympus Corporation) in 6 fields at random for analysis.
Red fluorescence labeling of
cells
Cells were transfected with red fluorescence protein
(RFP) gene using a lentivirus mediated gene transfection kit
(Shanghai GenePharma Co.,Ltd., Shanghai, China) according to the
manufacturer's instructions. More than 90% of GICs and
non-initiating tumor cells expressed RFP under fluorescence
microscope (Olympus IX51; Olympus Corporation), the RFP gene
integrated stably in target cell genome and maintained a stable RFP
expression in the transfected tumor cells. Invasive or migratory
potential of GICs-RFP and non-initiating tumor cells-RFP cells
in vitro was detected by matrigel assay or polycarbonate
filter, respectively, and was observed directly under fluorescence
microscope (Olympus IX51; Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-PCR)
A total of 3×105 cells/well were seeded
into 6-well plates in triplicate and incubated at 37°C for 24 h.
The cells were lysed, and total RNA was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific) according to the
manufacturer's instructions. First strand cDNA synthesis was
performed using reverse transcriptase and random hexanucleotide
primers (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
complementary DNA was subsequently used to perform qPCR on a
LightCycler480 Thermal Cycler (Roche Diagnostics, Basel,
Switzerland) using SYBR Green (Molecular Probes; Thermo Fisher
Scientific) with gene-specific primers (Gene Pharma, Shanghai,
China) and JumpStart™ Taq DNA polymerase (Invitrogen; Thermo Fisher
Scientific). The crossing threshold value was normalized to GAPDH,
and quantitative changes in mRNA were expressed as fold-change
relative to the control ± standard error of the mean (SEM)
value.
Western blot analysis
Cell extracts were prepared by lysing cells in RIPA
buffer containing a mixture of protease and phosphatase inhibitor
cocktails (Thermo Fisher Scientific, Inc.) followed by sonication
and centrifugation at 10,000 × g for 10 min at 4°C. Protein
concentration was determined using a BCA protein assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). A total of 50 µg of
protein was loaded onto a 10% SDS-PAGE gel and transferred to
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked in 5% non-fat dry milk for 1 h and probed
overnight with primary antibodies at 4°C. Subsequently the
membranes were incubated with secondary antibodies conjugated with
horseradish peroxidase for 1 h at room temperature. The blots were
visualized using enhanced chemiluminescence (ECL) reagents
(Invitrogen; Thermo Fisher Scientific) and densitometric
quantification was analyzed using Launch Sensi Ansys software
(Shanghai Peiqing Science & Technology Co., Ltd., Shanghai,
China). The primary antibodies including anti-epithelial cadherin
(E-cadherin, Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-intercellular adhesion molecule 1 (ICAM-1, Santa Cruz
Biotechnology, Inc.), anti-matrix metalloproteinase 2 (MMP-2,
Abcam), anti-MMP-9 (Abcam) and anti-tissue inhibitor of
metalloproteinase 3 (TIMP3, Abcam) were used. β-actin (Santa Cruz
Biotechnology, Inc.) expression was evaluated as a control for
protein loading.
Intracranial transplantation to
establish GBM xenografts
BALB/c mice (Shanghai Laboratory Animal Center, CAS,
Shanghai, China) were divided into 2 groups, and each consisted of
8 mice. Animal care and experimental procedures described in the
study were performed in accordance with the Guidelines for Animal
Experiments at Soochow University from Institutional Animal Care
and Use Committee with the approval of Ethics Committee of the
university [Certificate no. SYXK (Su) 2007-0035]. Athymic/nude
immunocompromised mice at 6–8 weeks of age were maintained under
pathogen-free conditions within the institutional animal facility.
Food and water were provided ad libitum. Surgical procedures
were performed in a sterile fashion. Mice were anesthetized by
intraperitoneal injection of chloral hyfrate (400 mg/kg), then
positioned in a rodent stereotaxic frame. A total of
2×105 CD133−/A2B5+ GICs or matched
non-initiating tumor cells were stereotactically injected into the
right putamen (1 mm forward, 2 mm right lateral from the bregma,
and 2.5 mm down from the dura) by use of a Hamilton syringe to
establish GBM xenografts. The mice were sacrificed when symptoms of
brain tumor, including sustained weight loss, ataxia and
periorbital bleeding, were observed. Prior to the collection of
mouse brains bearing GBM tumors, cardiac perfusion with PBS
followed by perfusion with 4% paraformaldehyde was performed. The
whole brain was harvested and continuously sectioned at a thickness
of 5 µm, then either stained with hematoxylin and eosin (H&E;
Beyotime Institute of Biotechnology) using routine
histopathological procedures, or directly observed under
fluorescent microscopy. Red fluorescence indicated the presence of
tumor cells, whereas normal brain cells exhibited no
fluorescence.
Statistical analysis
All determinations were performed from ≥3
independent experiments as indicated. The data are presented as the
mean ± SE. Statistically significant differences in mean values
between the 2 groups were tested by Student's t test. A
value of P<0.05 was considered to indicate a statistically
significant difference. All statistical calculations were performed
using SPSS software, version 11.0 (SPSS Inc., Chicago, IL,
USA).
Results
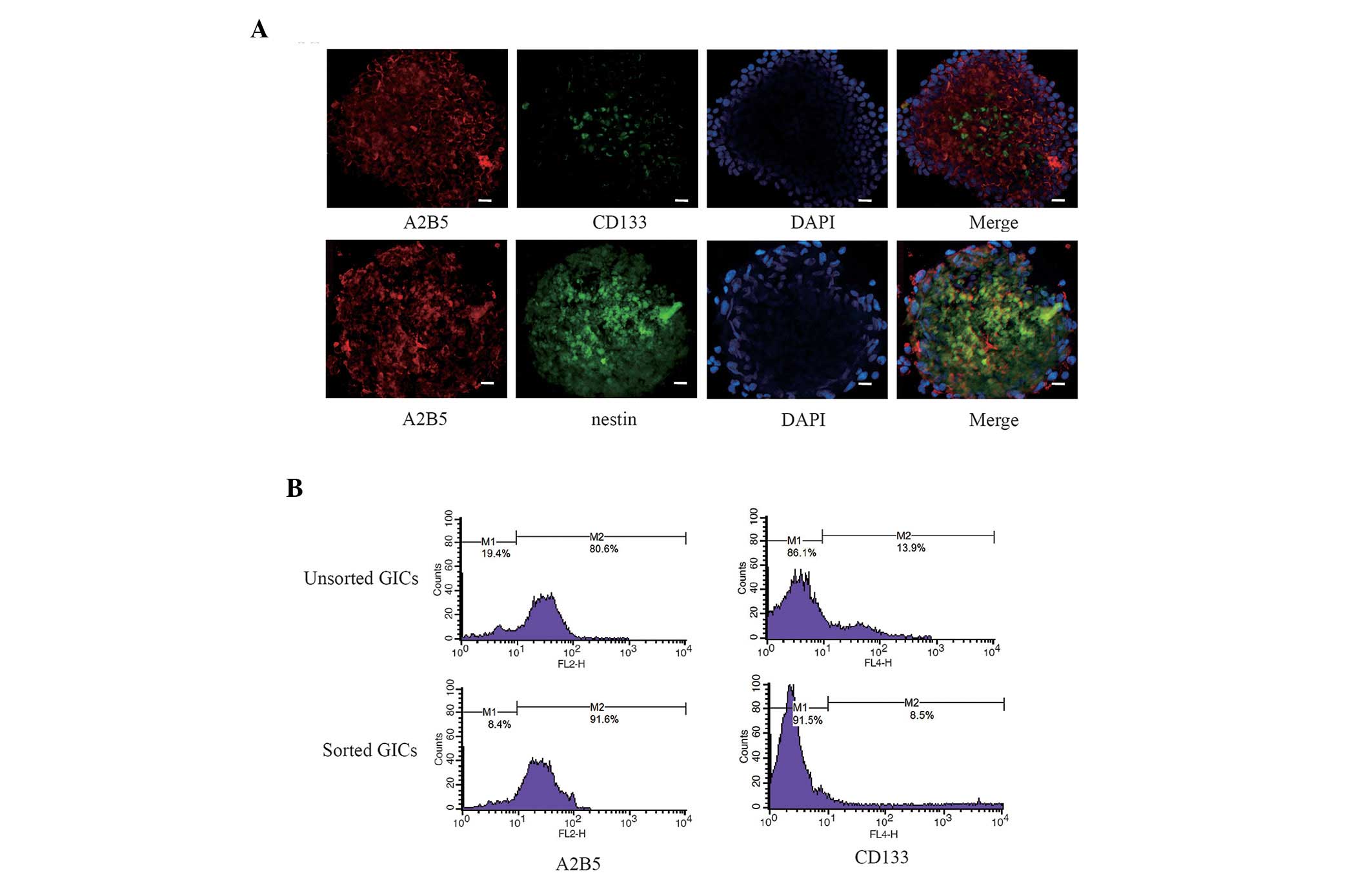
Identification of GICs
Following magnetic sorting, a majority of GICs were
shown to exhibit negative expression of glioma stem cell marker
CD133 and positive expression of marker A2B5
(CD133−/A2B5+ cells), which were involved in
the self-renewal and proliferation of stem cells (Fig. 1A). Co-expression of A2B5 and nestin
was observed in the majority of GICs (Fig. 1A). To determine the percentage of
cells that expressed the CD133−/A2B5+
phenotype and the sorting efficiency of the magnetic sorting
method, quantitative analysis of CD133 and A2B5 positive cells was
performed by flow cytometry. The results demonstrated that the GICs
sorted by magnetic beads consisted of 8.5% CD133+ cells
and 91.6% A2B5+ cells; the unsorted GICs consisted of
13.9% CD133+ cells and 80.6% A2B5+ cells
(Fig. 1B).
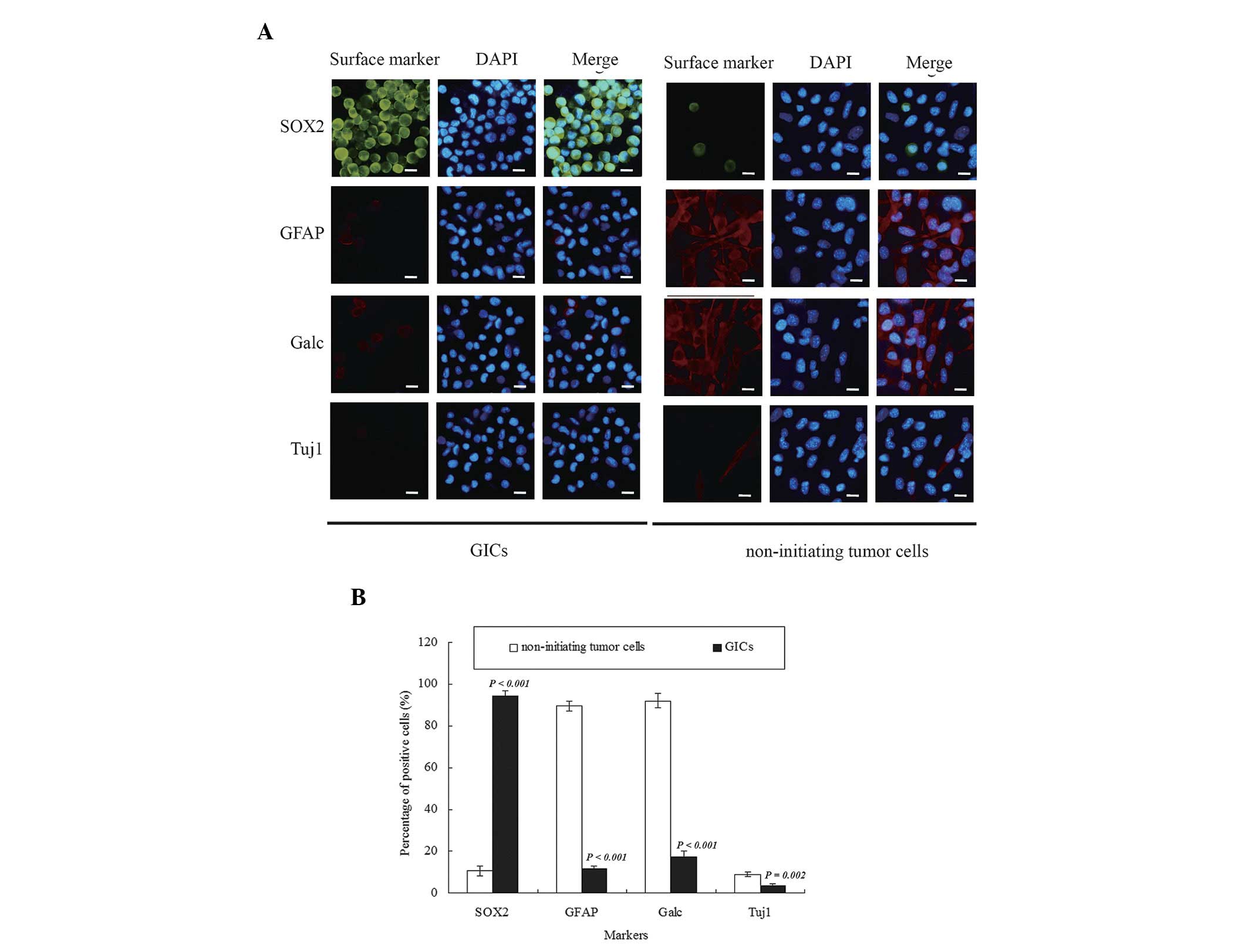
Characterization of GICs and
differentiated non-initiating tumor cells
A high level of human SOX2, a stemness marker of
GSCs, and a low expression level of human Tuj1, GFAP and Galc,
differentiation markers of tumor cells, were observed in the GICs.
In contrast, a reduced number of SOX2+ cells and an
increased number of Tuj1+, GFAP+ and
Galc+ cells were observed in the non-initiating tumor
cell population compared with GICs (Fig.
2A). There were significant differences in the expression
levels of the stemness marker SOX2 (P<0.001) and differentiation
markers GFAP (P<0.001), Galc (P<0.001) and Tuj1 (P=0.002)
between GICs and matched non-initiating tumor cells (Fig. 2B).
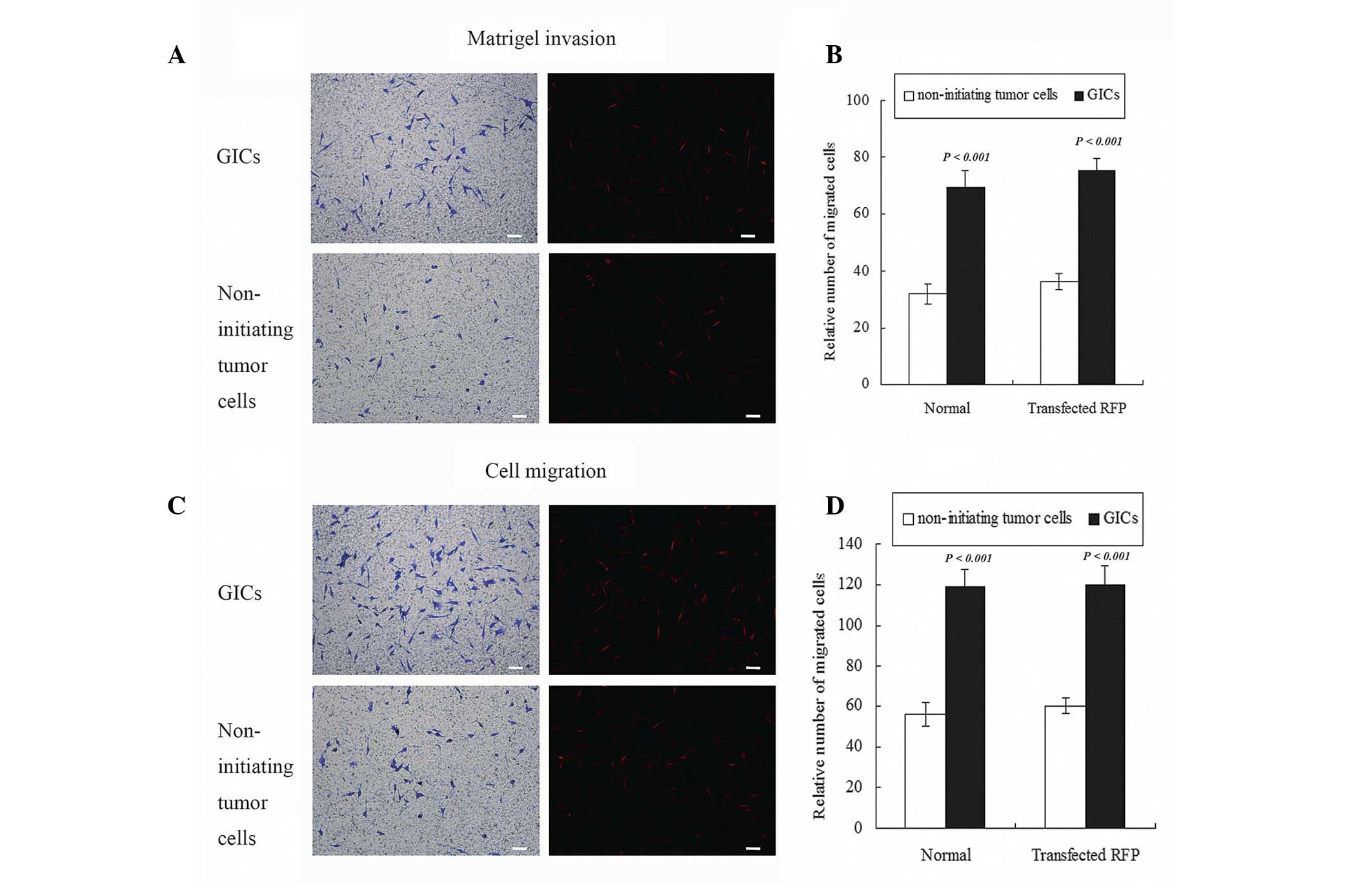
Migration and invasion were greater in
vitro for CD133−/A2B5+ GICs compared with
matched non-initiating tumor cells
CD133−/A2B5+ GICs and matched
non-initiating tumor cells were assessed for their invasive
potential by a matrigel invasion assay and migratory capability by
polycarbonate filters. The results revealed that an increase in
invasive cells through the matrigel and more migratory cells
through filters were observed in GICs comparing with matched
non-initiating tumor cell populations. A total number of 72.7±6.7
GICs in a field passed the matrigel, compared with 30.3±4.6
non-initiating tumor cells (Fig. 3A).
The number of migratory cells was 118.3±8.5 in the GIC group when
compared with 56.7±5.7 in the non-initiating tumor cells group. The
same events occurred in migratory assay (Fig. 3C). Quantified data demonstrated 2.42
folds more invasive (Fig. 3B) and
2.11 folds more migratory (Fig. 3D)
cells in GICs comparing to matched non-initiating tumor cells
populations. These findings indicated that the migratory and
invasive potential of CD133−/A2B5+ GICs was
greater than that of the matched non-initiating tumor cells in
vitro (P<0.001). No significant differences in migration or
invasion were observed in GICs-RFP compared with GICs or
non-initiating tumor cells-RFP (P>0.05). These results indicated
that RFP transfection did not change the migration and invasion of
GICs or matched non-initiating tumor cells, and cells with RFP may
be used for in vivo invasive detection.
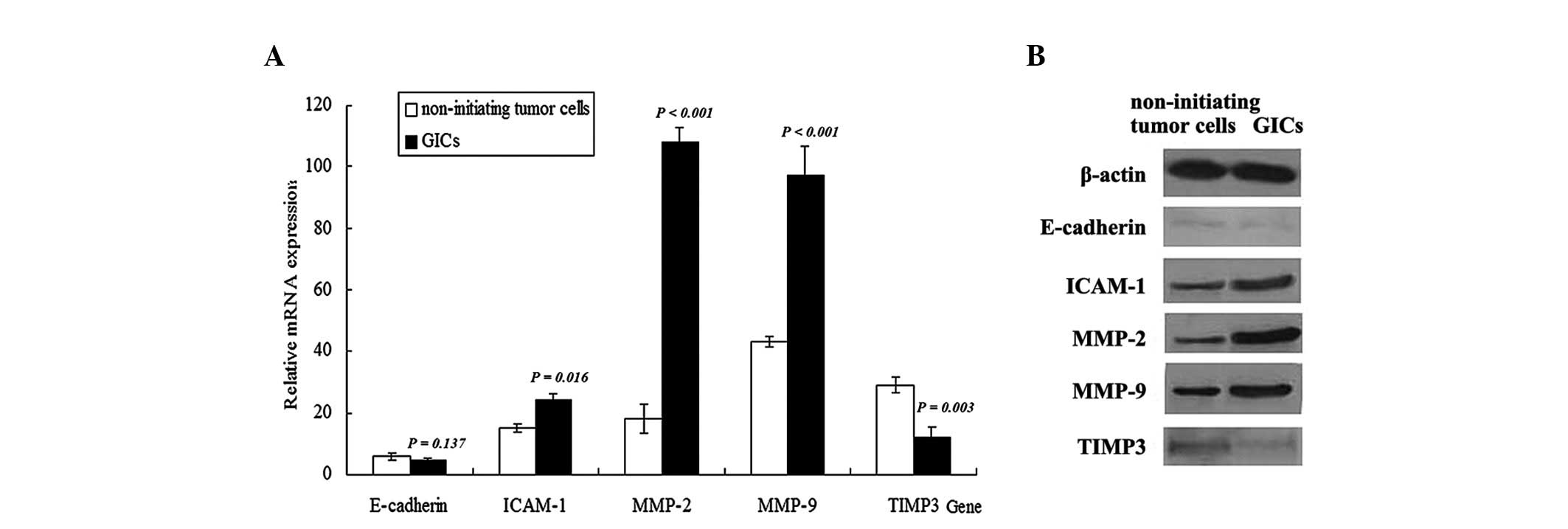
The expression levels of migration or
invasion-associated markers were altered in
CD133−/A2B5+ GICs
In addition to possessing the abilities of
sphere-forming and growing, CD133−/A2B5+ GICs
possessed certain genetic features during differentiation. Given
that E-cadherin, ICAM-1, MMP-2, MMP-9 and TIMP3 are involved in the
acquisition of highly migratory or invasive characteristics by
different subsets of glioma cells, the mRNA and protein expression
levels of these genes were examined using RT-qPCR and western blot
analysis, respectively, to determine whether they were functionally
associated with aggressive migration and invasion of GICs (Fig. 4A). The mRNA levels determined by
RT-qPCR demonstrated that the expression levels of ICAM-1
(P=0.016), MMP-2 (P<0.001) and MMP-9 (P<0.001) were increased
in CD133−/A2B5+ GICs compared with matched
non-initiating tumor cells. The protein expression levels of
ICAM-1, MMP-2 and MMP-9 were assessed using western blotting and
matched the pattern of mRNA expression, demonstrating a significant
increase in these proteins in GICs compared with matched
non-initiating tumor cells (Fig. 4B).
The mRNA and protein expression levels of E-cadherin and TIMP3 were
also detected in GICs. A significant reduction in the mRNA
(P=0.003) and protein expression levels of TIMP3 was observed in
GICs compared with matched non-initiating tumor cells. On the other
hand, no significant difference in E-cadherin mRNA (P=0.137) or
protein levels was observed between GICs and matched non-initiating
tumor cells.
CD133-/A2B5+ GICs display
greater invasive capacity compared with non-initiating tumor cells
in vivo
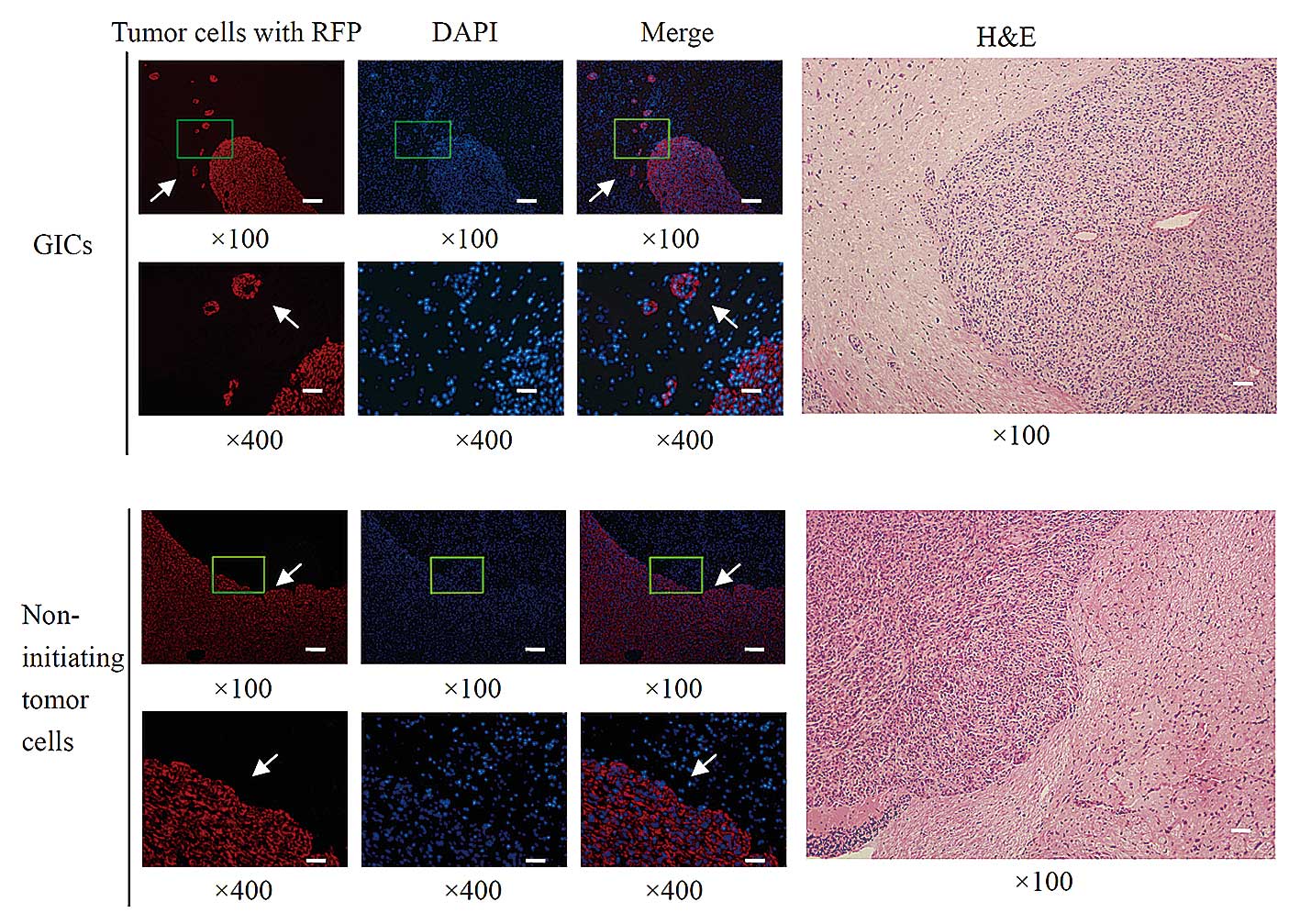
Aggressive invasion of tumor cells into brain tissue
is one of the most malignant characteristics of GBM. To test the
infiltrative potential of GICs in vivo,
CD133−/A2B5+ GICs or matched non-initiating
tumor cells were transplanted into brains of athymic/nude mice
through intracranial injection. Macroscopically, yellow-gray tumors
were observed in mice with intracranial GICs or non-initiating
tumor cells inoculation. Microscopically, the majority of tumor
cells were primitive round or oval, distributed evenly and densely,
after transplantation with GICs or non-initiating tumor cells. As
presented in Fig. 5, in the brains
implanted with GICs, aggressive invasion of tumor cells
infiltrating into surrounding normal tissue was observed in 7 mice,
and these tumor cells spread particularly far away from the
original injection site. Obvious invasion occurred in 87.5% mice
when implanted with CD133−/A2B5+ GICs.
However, in the brains implanted with non-initiating tumor cells,
the majority of tumor cells remained close to the injection site
and no dispersal of tumor cells was detected following cell
transplantation in all 8 mice. These data demonstrated that
CD133−/A2B5+ GICs demonstrated infiltrating
growth patterns and displayed greater invasive potential compared
with matched non-initiating tumor cells.
Discussion
Surface markers of cancer stem cells such as CD133,
CD15, A2B5, CD44 and α6-integrin have been observed on distinct
human glioblastoma cell populations (14–16). The
phenotype of cancer stem cells varies substantially between
patients, and tumors may contain multiple cancer stem cells, which
are phenotypically or genetically distinct (17). Human gliomas consistently express A2B5
marker in a large percentage of cells, but CD133 expression is less
abundant and less consistent, and the CD133+ population
is almost entirely contained within the A2B5+ population
(18). Human gliomas are composed of
multiple populations of cells which have the capacity to form
tumors. Notably, Ogden et al (18) identified a specific population of
tumorigenic A2B5+ cells that were phenotypically
distinct from CD133+ cells.
Tchoghandjian et al (19) reported that A2B5+ cells
isolated from human GBM displayed neurosphere-like, self-renewal,
asymmetrical cell division properties and had multipotent
differentiation capability. As few as 1,000 cells derived from
A2B5+ secondary spheres produced a tumor, and
A2B5+/CD133+ and
A2B5+/CD133− cell fractions displayed high
proliferative and migratory properties. For comparison with
non-initiating tumor cells, the authors used the differentiated
progeny of the CD133−/A2B5+ GICs, which
provided an isogenic model system for detecting the influence of
the GICs on migration and invasion. In the present study, the
Transwell experiments indicated that GICs had greater migratory and
invasive potential than matched non-initiating tumor cells in
vitro.
Cell adhesion molecules, MMPs and TIMPs serve
crucial roles in migration, invasion and metastasis and regulate
signaling pathways that control cell growth, survival, invasion,
inflammation and angiogenesis (20–23). In
the present study, higher expression levels of ICAM-1, MMP-2 and
MMP-9 and lower expression of TIMP3 were observed in
A2B5+/CD133− GICs compared with matched
non-initiating tumor cells. These results indicated that the
expression levels of ICAM-1, MMP-2, MMP-9 and TIMP3 may be
important in the process of aggressive GICs invasion. Expression
changes in genes associated with migration and invasion in GICs
compared with matched non-initiating tumor cells indicated that
there may be multiple molecules and processes involved in
stimulating GICs invasion in vivo.
Identification of individual or even small numbers
of invasive tumor cells in the brain adjacent to tumor is difficult
using standard immunohistochemistry or routine H&E techniques.
RFP has been widely applied in the biomedical field as a
non-enzymatic reporter gene. The RFP gene may be stably transduced
into cancer cell lines, which subsequently express RFP at high
levels in vitro and in vivo, including primary and
metastatic tumor deposits. The method of RFP transfection is
superior to H&E staining for the detection and study of
physiologically relevant patterns of brain tumor invasion in
vivo (24). Zhang et al
(25) transferred DsRed2, an RFP
gene, into malignant glioma cells. DsRed2 fluorescence clearly
demarcated the primary tumor margins and readily allowed for the
visualization of local invasion at the single-cell level in the
brain adjacent to tumor. In the present study, in order to detect
brain tumor invasion in an orthotopic glioma model of nude mice by
florescence microscopy, a Lentivirus carrying the RFP gene was
transfected into CD133−/A2B5+ GICs and
matched non-initiating tumor cells. Infiltrating growth patterns
and aggressive invasion potential were demonstrated in xenografts
from CD133−/A2B5+ GICs compared with matched
non-initiating tumor cells. The present results were in accordance
with previous studies, which also demonstrated that glioblastoma
CD133− cells exhibited properties of stem cells, and
generated more aggressive tumors than CD133+ cells when
engrafted intracerebrally into nude mice (26–28).
In conclusion, the present study demonstrated that
these CD133−/A2B5+ cells fulfilled the
criteria of brain tumor initiating cells in vitro. These
cells displayed significant migratory and invasive properties.
These infiltrated cells in the invasive fronts may be responsible
for rapid tumor recurrence after conventional treatments. Current
hypotheses relating to cancer stem cells generally propose
targeting CD133+ cells to inhibit GBM recurrence, but
the present study indicated that CD133−/A2B5+
GICs are also an important cell subpopulation with great invasive
potential that should not be ignored. Targeting both
CD133+ and CD133− invasive GICs may
effectively reduce GBM invasion and recurrence.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81207142 and
81372689), the Major Issues Foundation of the Health Department of
Jiangsu Province (grant no. K201106) and the National ‘Twelfth
Five-Year’ Science and Technology Support Program of China (grant
no. 2013BAI01B08).
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vehlow A and Cordes N: Invasion as target
for therapy of glioblastoma multiforme. Biochim Biophys Acta.
1836:236–244. 2013.PubMed/NCBI
|
|
3
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanai N, Alvarez-Buylla A and Berger MS:
Neural stem cells and the origin of gliomas. N Engl J Med.
353:811–822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Bao S, Wu Q, Wang H, Eyler C,
Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, et al:
Hypoxia-inducible factors regulate tumorigenic capacity of glioma
stem cells. Cancer Cell. 15:501–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
10
|
Brescia P, Ortensi B, Fornasari L, Levi D,
Broggi G and Pelicci G: CD133 is essential for glioblastoma stem
cell maintenance. Stem Cells. 31:857–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colin C, Baeza N, Tong S, Bouvier C,
Quilichini B, Durbec P and Figarella-Branger D: In vitro
identification and functional characterization of glial precursor
cells in human gliomas. Neuropathol Appl Neurobiol. 32:189–202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nunes MC, Roy NS, Keyoung HM, Goodman RR,
McKhann G II, Jiang L, Kang J, Nedergaard M and Goldman SA:
Identification and isolation of multipotential neural progenitor
cells from the subcortical white matter of the adult human brain.
Nat Med. 9:439–447. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miconi G, Palumbo P, Dehcordi SR, La Torre
C, Lombardi F, Evtoski Z, Cimini AM, Galzio R, Cifone MG and Cinque
B: Immunophenotypic characterization of human glioblastoma stem
cells: Correlation with clinical outcome. J Cell Biochem.
116:864–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stieber D, Golebiewska A, Evers L,
Lenkiewicz E, Brons NH, Nicot N, Oudin A, Bougnaud S, Hertel F,
Bjerkvig R, et al: Glioblastomas are composed of genetically
divergent clones with distinct tumourigenic potential and variable
stem cell-associated phenotypes. Acta Neuropathol. 127:203–219.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son MJ, Woolard K, Nam DH, Lee J and Fine
HA: SSEA-1 is an enrichment marker for tumor-initiating cells in
human glioblastoma. Cell Stem Cell. 4:440–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lathia JD, Gallagher J, Heddleston JM,
Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE,
Hjelmeland AB, et al: Integrin alpha 6 regulates glioblastoma stem
cells. Cell Stem Cell. 6:421–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Visvader JE and Lindeman GJ: Cancer stem
cells: Current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogden AT, Waziri AE, Lochhead RA, Fusco D,
Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, et al:
Identification of A2B5+CD133- tumor-initiating cells in adult human
gliomas. Neurosurgery. 62:505–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tchoghandjian A, Baeza N, Colin C, Cayre
M, Metellus P, Beclin C, Ouafik L and Figarella-Branger D: A2B5
cells from human glioblastoma have cancer stem cell properties.
Brain Pathol. 20:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farahani E, Patra HK, Jangamreddy JR,
Rashedi I, Kawalec M, Rao Pariti RK, Batakis P and Wiechec E: Cell
adhesion molecules and their relation to (cancer) cell stemness.
Carcinogenesis. 35:747–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamamoto N, Tsuchiya H and Hoffman RM:
Tumor imaging with multicolor fluorescent protein expression. Int J
Clin Oncol. 16:84–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Zheng X, Jiang F, Zhang ZG,
Katakowski M and Chopp M: Dual-color fluorescence imaging in a nude
mouse orthotopic glioma model. J Neurosci Methods. 181:178–185.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133(+) and CD133(−) glioblastoma-derived cancer stem cells
show differential growth characteristics and molecular profiles.
Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joo KM, Kim SY, Jin X, Song SY, Kong DS,
Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, et al: Clinical and
biological implications of CD133-positive and CD133-negative cells
in glioblastomas. Lab Invest. 88:808–815. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Sakariassen PØ, Tsinkalovsky O,
Immervoll H, Bøe SO, Svendsen A, Prestegarden L, Røsland G, Thorsen
F, Stuhr L, et al: CD133 negative glioma cells form tumors in nude
rats and give rise to CD133 positive cells. Int J Cancer.
122:761–768. 2008. View Article : Google Scholar : PubMed/NCBI
|