Introduction
Breast cancer is a malignant disease derived from
the epithelium of the mammary glands. In 2012, breast cancer was
ranked first in cancer incidence among women worldwide, with an
estimated 1.7 million cases (1). For
cancer-associated mortality, breast cancer was ranked the leading
cause of death among women, with an estimated 521,900 mortalities
worldwide in 2012 (1). It is
therefore important to investigate potential targets for
tumorigenesis and progression of breast cancer. Previous studies
(2–4)
have demonstrated that Akt/mTOR pathways appear to be a prime
strategic target for breast cancer therapeutic development.
Akt and the mammalian target of rapamycin (mTOR)
signaling pathways have regarded to serve critical roles in cell
proliferation, motility, survival and therapy resistance (3). The activation of the Akt/mTOR pathway
has been observed in a variety of tumors, including colon cancer,
kidney cancer, some lymphomas, bone sarcoma and in particular,
breast cancer (3). Inhibition of the
Akt/mTOR pathway results in suppression of cell proliferation and
promotion of cell death, making it an attractive target for
oncology research (4).
MicroRNAs (miRs) are a type of multi-functional
small non-coding RNA, which have numerous number and exists widely
in animals and plants. In recent years, it has been demonstrated
that miRs target mRNA at the transcription level and inhibit the
mRNA translation process or promote the degradation of mRNA
(5). A number of miRs have been
reported to target or affect the Akt/mTOR pathways. Ectopic
expression of miR-7, which has previously been identified as a
tumor suppressor, inhibited tumor growth and metastasis, and
restrained the expression of Akt, mTOR and the downstream P70S6K in
hepatic cancer (6). In addition, it
was demonstrated that mTOR and P70S6K are target genes of miR-7
(6). Other miRNAs including miR-144,
miR-126, miR-199a-3p and miR-718, regulate the Akt/mTOR pathway in
ovarian, oral, colorectal and human non-small-cell lung cancers and
Kaposi's sarcoma (7–11). Few studies identified miRs regulating
Akt/mTOR pathway in breast cancer (12–15).
MiR-122 was observed to inhibit the cell proliferation and
tumorigenesis of breast cancer and to suppress the activation of
the Akt/mTOR pathway by targeting IGF-1R, which is an upstream
molecule of the Akt/mTOR pathway (12). Another study identified three miRs
(miR-147, miR-124, and miR-193a-3p) that inhibit cell-cycle
progression and proliferation by targeting EGFR-driven cell-cycle
network proteins in breast cancer (13). EGFR also serves a crucial role in the
initiation of the Akt/mTOR pathway (14). Furthermore, miR-147 was found to
repress cell invasion and proliferation, induce cell arrest at G1
in colon cancer (15). In addition,
it has been shown that ectopic expression of miR-147 prevented Akt
phosphorylation (15).
Although studies have identified miR-147 as a tumor
suppressor (14,15), the effect and the corresponding
mechanisms of miR-147 in breast cancer remains to be investigated.
Here, the present study aimed to understand the effect of miR-147
on the biological behaviors of breast cancer cells, phosphorylation
of Akt and the potential downstream pathways regulating these
processes in breast cancer.
Materials and methods
Cell culture
The normal mammary epithelial cell line MCF-10A and
the breast cancer cell lines MCF7, SK-BR-3 and MDA-MB-231 were
obtained from the Institute of Basic Medical Sciences of the
Chinese Academy of Medical Sciences (Beijing, China). MCF-10A cells
and MDA–MB–231 cells were cultured in Dulbecco's minimum essential
medium (DMEM; Hyclone, Logan, Utah, USA) with 10% fetal bovine
serum (FBS; Lanzhou National Hyclone Bio-engineering, Lanzhou,
China). MCF7 cells were cultured in RPMI 1640 (Hyclone) with 10%
FBS and SK-BR-3 cells were maintained in RPMI 1640+HEPES with 10%
FBS. All of the above cells were cultured at 37°C in a 5%
CO2 atmosphere incubator.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA samples were isolated using Invitrogen
TRIzol reagent (Thermofisher Scientific, Inc., Carlsbad, CA, USA)
according to the manufacturer's instructions. Reverse transcription
was then conducted using All-in-One miRNA qRT-PCR Detection kit
(GeneCopoeia, Rockville, MD, USA) to obtain cDNA. The total
reaction system was incubated at 42°C for 60 min, then 70°C for 10
min and cooled on ice. The PCR reaction was performed using
All-in-One miRNA qRT-PCR Detection kit (GeneCopoeia) with a Bio-Rad
iCycler single-color real-time detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). RNU6 was used as the
internal control of miRNA. The primers for miR-147 and RNU6 were
designed and sythesized by GeneCopoeia. The relative expression
values of miRNA were calculated by the 2−ΔΔCq method. Cq
is the intensity value of fluorescent signal detected by the
thermal cycler. ΔΔCq=(CqmiRNA - CqU6). Using 2−ΔΔCq as
the expression of miR in each experimental group. The average
expression level of MCF7 cells was set to 1. The expression levels
of the other cancerous groups were normalized to that of the MCF7
cell group, as the MCF7 cell group exibited the least intra-group
difference with regard to miR-147 expression.
Ectopic expression of miR-147 and RNA
interference
miR-147 mimic transfection was performed using
mimics of miR147 or C. elegans miRNA (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) following the manufacturer's protocol. The
final reagent concentration of the mimics was modulated to 50 nM.
The si-Akt expressing plasmid vector was designed, synthesized and
packaged by Shanghai GenePharma Co., Ltd (Shanghai, China). The
quantity of si-Akt expressing plasmid vector used was 2 µg/well (6
well plate). A total of 1.25 µl/well Invitrogen Lipo2000
(Thermofisher Scientific, Inc.) was used to transfect the miR mimic
or siRNA into MDA-MB-231 cells at a density of 2.5×105
cells/well, according to the manufacturer's protocol. Cell
proliferation assays were performed every 24 h for seven days after
transfection. Total RNA and total protein were extracted two days
after transfection, and the cells were dissociated two days after
transfection for the Transwell assays.
Cell proliferation assay
A total of 2.5×103 cells/well were seeded
into 96-well plates with 5 repeated wells. The absorbance values
were detected every 24 h for 7 days. Before the detection, 20 µl
MTT (Sigma-Aldrich, St. Louis, MO, USA) was added in each well and
the cells were incubated for 4 h. After incubation, the upper
liquid was removed and 200 µl DMSO (Chengdu Kelong Chemical Co.,
Ltd., Chengdu, China) was added to each well. The absorbance value
was determined at 490 nm using a Multiskan Spectrum microplate
spectrophotometer (Thermofisher Scientific, Inc.).
Transwell assay
Boyden Well (BD Biosciences, Franklin Lakes, NJ,
USA) was used for the assessment of the migration and invasion of
transfected cells. Polycarbonate Microporous Membrane was placed
between the upper and lower chambers. For the invasion assay, the
membrane of the upper chambers were coated with 6 mg/l Matrigel (BD
Biosciences) and incubated at 37°C for 30 min. For the migration
assay, the wells were not coated with Matrigel. MDA-MB-231 cells
were seeded at a density of 1×105 cells in each upper
chamber and DMEM+10% FBS was placed in the lower chambers. The
plates were then incubated at 37°C, 5% CO2. After 24 h,
the membranes were removed and fixed in 4% paraformaldehyde
(Chengdu Kelong Chemical Co., Ltd.), stained with Giemsa
(Sigma-Aldrich) and washed with PBS buffer. The migration and
invasion of each group were quantified as the mean number of cells
found in ten microscope fields [magnification, ×400; Eclipse E200;
Nikon Instruments (Shanghai) Co., Ltd., Shanghai, China].
Western blot analysis
Cells were dissolved using RIPA buffer to obtain the
cell lysates. Next, 5X Loading Buffer (Chengdu Cetme Science and
Technology Co., Ltd., Chengdu, China) was added to the cell
lysates, and incubated at 70°C for 10 min. Total proteins were
separated on 8–12% gradient SDS-polyacrylamide gels and transferred
to PVDF membranes (EMD Millipore, Billerica, MA, USA). β-actin was
used as loading control. The PVDF membranes were then blocked in
TBST with 5% nonfat milk for 1 h, and incubated with the following
antibodies: monoclonal rabbit anti-human anti-Akt (1:1,000),
anti-p-Akt (1:1,000), anti-P70S6K (1:1,000), anti-p-P70S6K
(1:1,000), anti-4E-BP-1 (1:1,000), anti-p-4E-BP-1 (1:1,000) (CST,
Boston, Massachusetts, USA) and monoclonal mouse anti-human
anti-β-actin (1:1,000) (Santa Cruz Biotechnology, Dallas, Texas,
USA). Following washing with TBST the membranes were incubated with
polyclonal goat anti-rabbit or rabbit anti-mouse IgG secondary
antibodies (1:10,000) (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 1 h then washed with
TBST. The blots were developed using ECL reagents (EMD Millipore).
The exposure and image capture was performed with a Gel Dox XR+
imaging system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All quantitative data are expressed as the mean ±
standard error. The results were analyzed by Prism 6 software,
version 6.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analyses were performed using Student's t-test for
comparisons between each case group and the control group. Linear
regression was used for cell growth curve comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
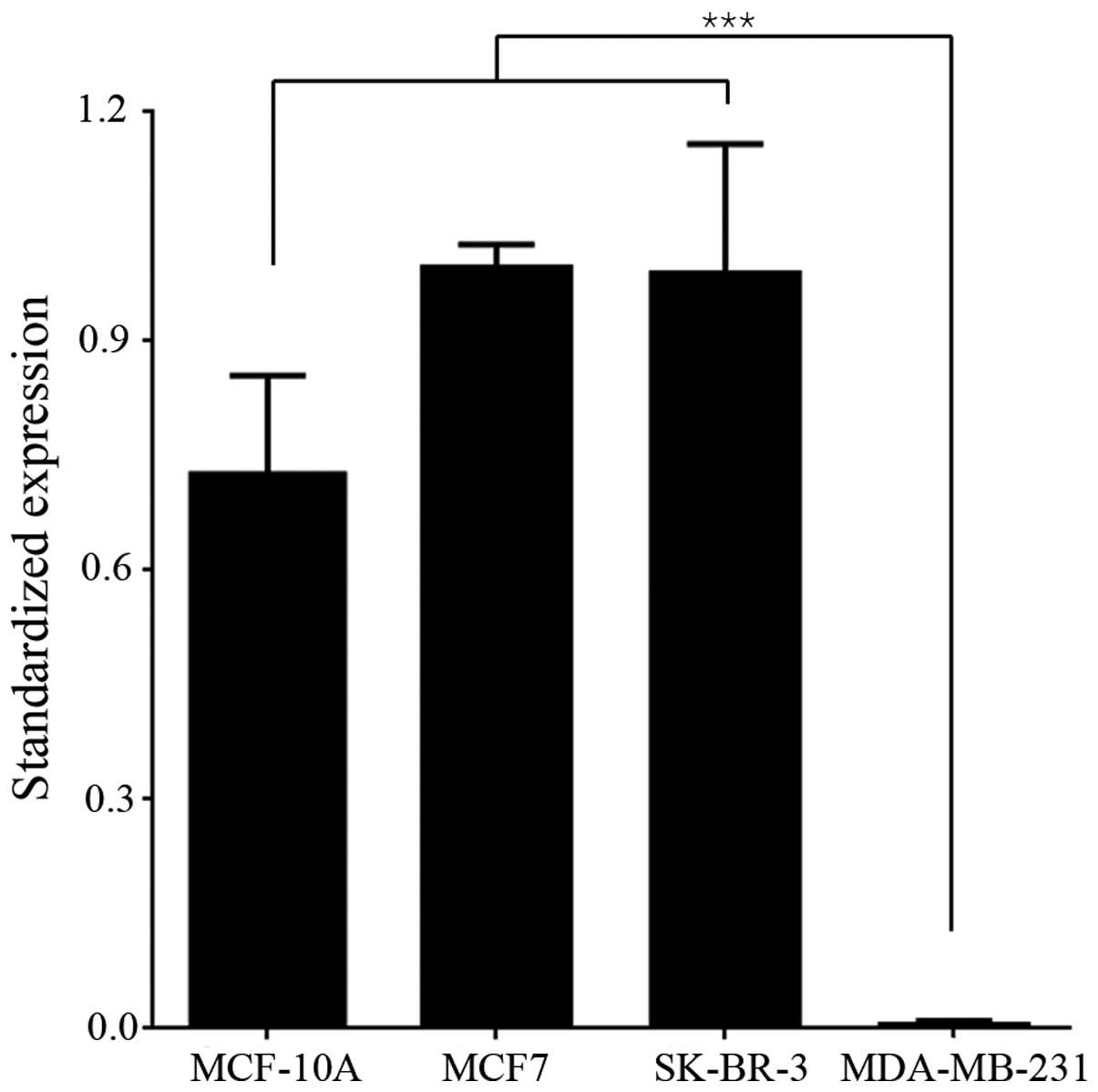
miR-147 expression was downregulated
in highly aggressive breast cancer cell line
To determine the function and mechanism of miR-147
in breast cancer, miR-147 expression was detected in breast cancer
cell lines. Normal mammary epithelial cell line (MCF-10A), two less
aggressive breast cancer cell lines (MCF7 and SK-BR-3), and a
highly aggressive breast cancer cell line (MDA-MB-231) were
cultured in vitro. The endogenous levels of miR-147 in the
above cell lines were examined by RT-PCR. The level of miR-147
expression in MCF-10A, MCF7 and SK-BR-3 cells was relatively high,
while the expression of miR-147 in MDA-MB-231 was too low to be
detected. The difference in miR-147 expression level between
MDA-MB-231 and the other 3 cell lines was statistically significant
(Fig. 1; P<0.001), indicating that
the expression of miR-147 may be negatively correlated with the
aggressiveness of breast cancer. The MDA-MB-231 cell line exhibits
high aggressiveness and low miR-147 expression, and was chosen to
be used in further experiments.
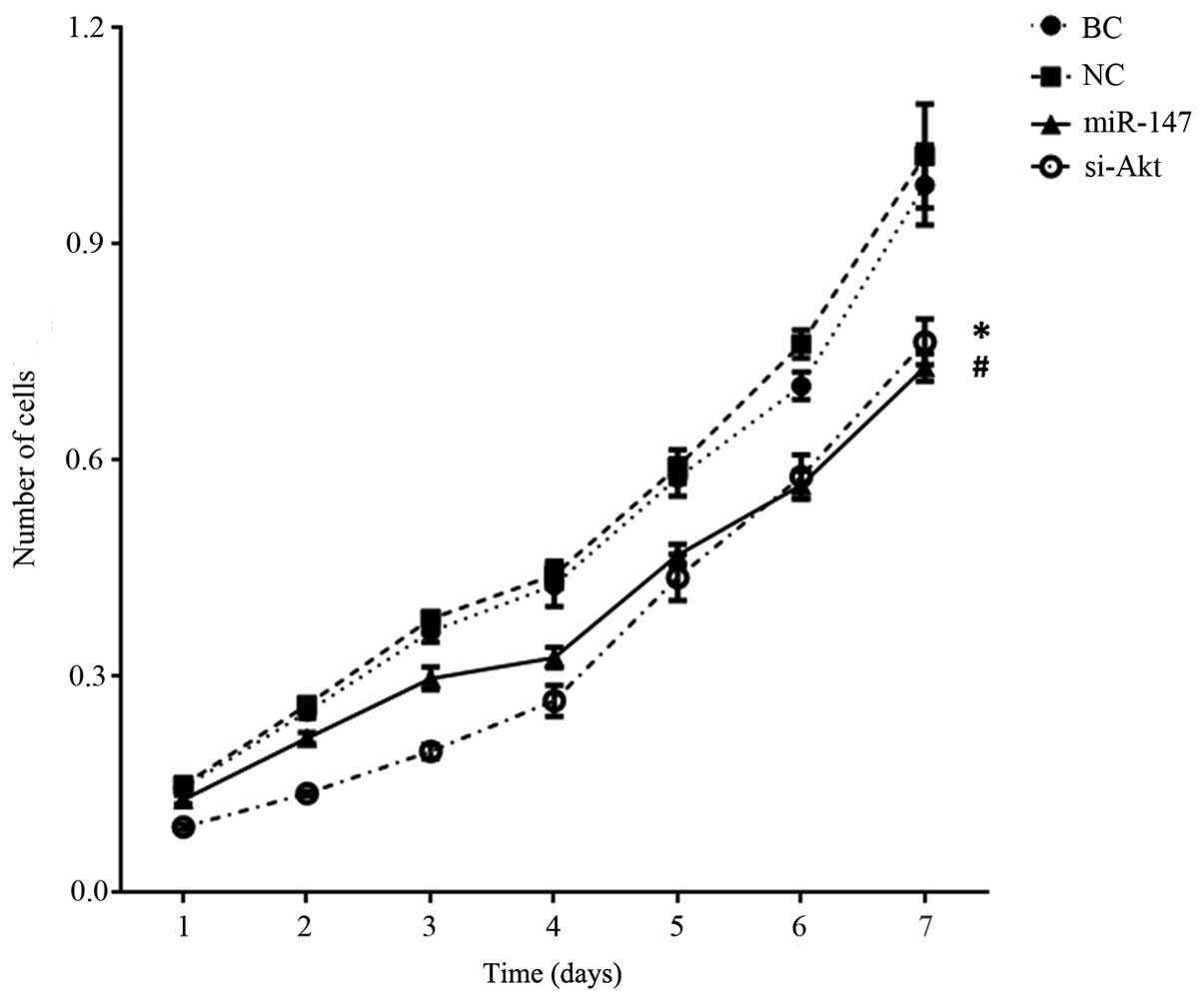
miR-147 repressed cell proliferation,
invasion and migration
Proliferation, invasion and migration are examples
of the characteristic aggressive properties of cancer cells. A
3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide
(MTT) assay was used to investigate the effect of miR-147 on cell
proliferation. MiR-147 was transiently transfected into the cell
line MDA-MB-231 to form the ‘miR-147’ group. In the ‘si-Akt’ group,
siRNA was transfected to knockdown the expression of Akt. Fig. 2 shows that there was a significant
decrease in the MTT value in miR-147 transfected MDA-MB-231 cells
on day 7, in comparison to the blank control cells [MDA-MB-231
cells cultured in DMEM±10% FBS; blank control, (BC)] and negative
control miR transfected cells (P<0.001). In addition, in
comparison with the BC cells, the knockdown of Akt in MDA-MB-231
cells also resulted in a reduced MTT value (P<0.001). Although
miR-147 transfected cells had a higher MTT value compared with
knockdown of Akt cells on day 4 (P=0.007), a statistically
significant difference was not observed between the MTT value of
the miR-147 group and si-Akt group on overall trend (P=0.086).
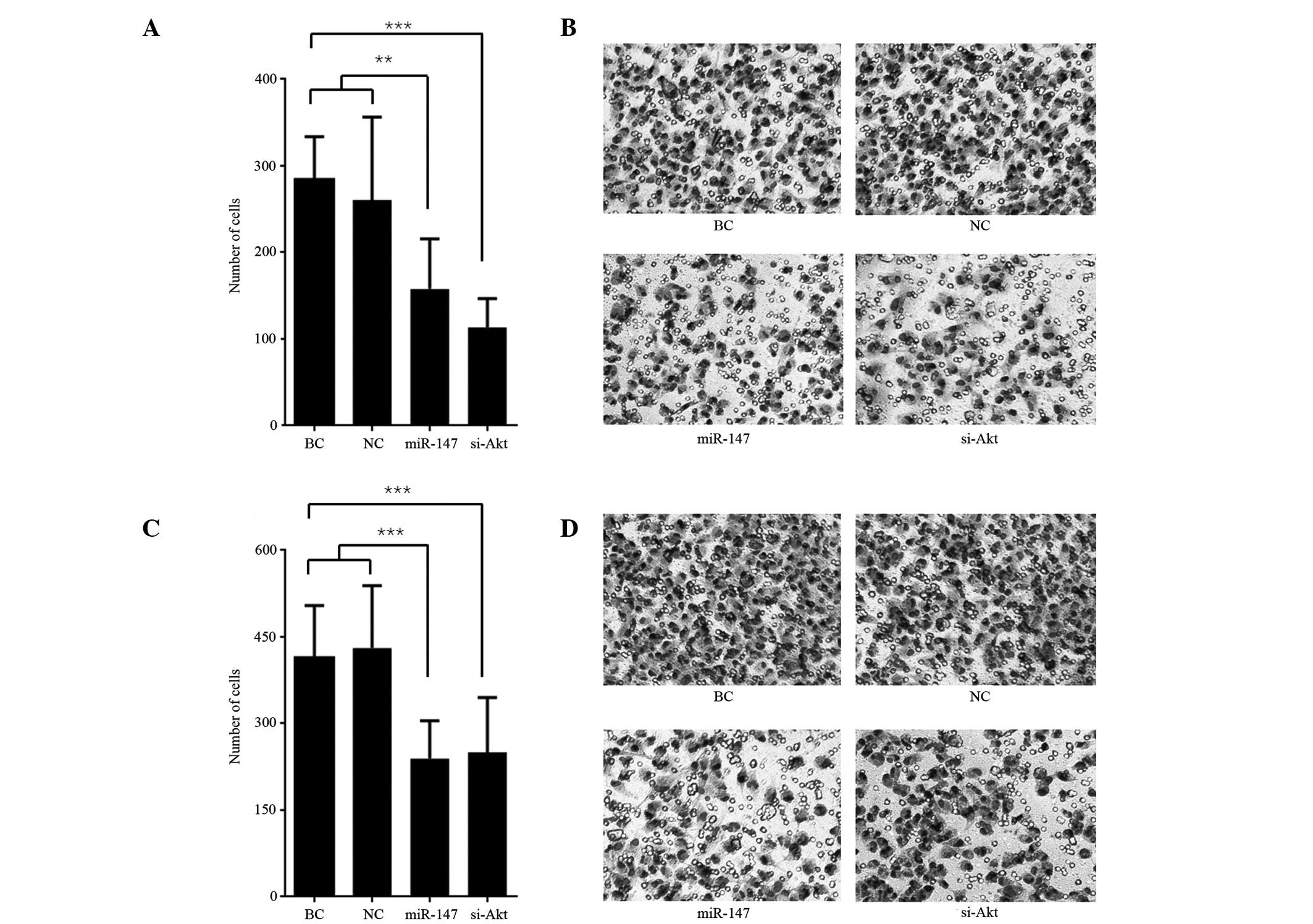
Transwell assays were used to measure the cell
invasive and migrating capability. The number of cells that invaded
the Matrigel or traversed the microporous membrane are presented in
Fig. 3. The number of invasive and
migratory miR-147 transfected cells was significantly less than the
BC and miR transfected negative control cells both in the invasion
assay (P<0.01; Fig. 3A and B) and
migration assay (P<0.001); Fig. 3C and
D). Similarly, the number of invasive and migratory cells
following the knockdown of Akt was less than the BC cells both in
invasion assay (P<0.001; Fig. 3A and
B) and migration assay (P<0.001; Fig. 3C and D). The number of invasive cells
following knockdown of Akt was less than the number of invasive
miR-147 transfected cells in the invasion assay (P=0.047), however
no significant difference between the two in the number of
migrating cells was observed in the migration assay (P=0.769).
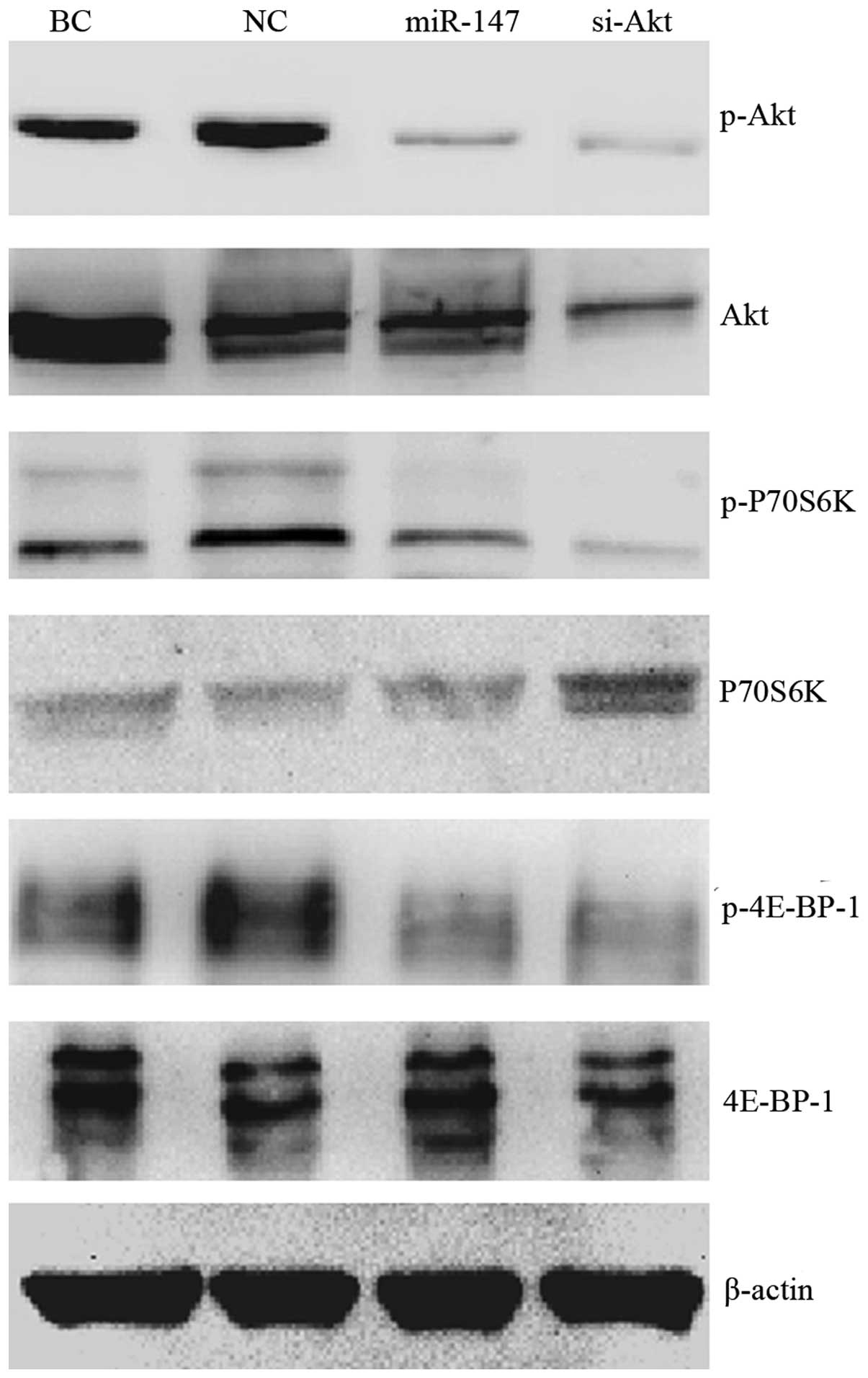
miR-147 reduced the activation of
Akt/mTOR signaling pathway
Previous studies have reported that Akt may be a
potential target of miR-147 (13,15). To
investigate how miR-147 affects AKT, and the role of Akt in miR-147
induced cell proliferation, invasion and migration inhibition,
western blotting was performed to assess the expression and
phosphorylation of the Akt protein and the downstream key effectors
in the Akt/mTOR pathway. Fig. 4
demonstrates that miR-147 expression repressed the phosphorylation
of Akt, P70S6k and 4E-BP-1 in MDA-MB-231 cell, but appeared to have
no effect on the total level of these proteins. Knockdown of Akt
restrained both Akt phosphorylation and total Akt level, and the
downstream phosphorylation of P70S6k and 4E-BP-1. The total P70S6K
level was slightly upregulated in the ‘si-Akt’ group. Generally,
the effect of miR-147 on Akt/mTOR pathway was analogous to that of
siRNA directed at Akt.
Discussion
The present study demonstrated that miR-147 is
relatively highly expressed in the normal breast epithelial cell
line (MCF-10A) and the less aggressive breast cancer cell lines
(MCF7, SK-BR-3) and markedly low expression in the most aggressive
breast cancer cell line studied (MDA-MB-231). Few studies have
reported the expression of miR-147 in breast cancer. In respect to
other types of tumor, Yao et al (16) demonstrated that miR-147 was
overexpressed in gastric cancer compared with normal gastric
tissue; Wong et al (17)
reported that miR-147 has higher expression in squamous cell
carcinoma of the tongue compared with paired normal tissue. miR-147
expression was previously demonstrated to be higher in recurrence
hepatocellular carcinoma following liver transplantation when
compared with non-recurrence hepatocellular carcinoma (18). The result of the present study is
inconsistent with the previous studies, which may be due to
following reasons. First, the tumors in the previous studies are
different types to the current study, and may result in the
differences observed in its detection. In addition, the above
studies analyzed the expression of miR-147 by microarray, which is
high throughput but lower in accuracy (16–18). Yao
et al (16) and Wong et
al (17) did not verify miR-147
expression by PCR, in comparison to the present study that used
RT-PCR to accurately quantify miR levels. The present study
demonstrated that miR-147 was expressed at higher levels in MCF7
and SK-BR-3 cell lines compared with in MCF-10A cells (P<0.05):
This result suggests that less aggressive breast cancers may
express higher levels of miR-147 than that of normal tissue, while
miR-147 expression is significantly reduced in more aggressive
breast cancers. Equally, other types of tumors may also express
high levels of miR-147, as was indicated in the above studies
(16,17). However, the association between
miR-147 expression and the grade malignancy of breast cancer
remains to be defined.
Akt/mTOR signaling pathway is considered to be one
of the most frequently aberrantly activated pathways, which affects
30–50% of human tumors (3). Akt is a
key regulator of survival during cellular stress, which appears to
be crucial in cancer (19). mTOR is a
serine/threonine kinase widely expressed in mammalian cells.
Through its downstream effectors, P70S6K and 4E-BP-1, it is
involved in the initiation of ribosomal translation of mRNA into
proteins necessary for cell proliferation, cell metabolism and cell
cycle progression (20). Akt and the
downstream P70S6K and 4E-BP-1 serve key roles in Akt/mTOR pathway.
The phosphorylation of these molecules represents the activation of
Akt/mTOR pathway. On the contrary, reduced phosphorylation of Akt,
P70S6K and 4E-BP-1 indicates suppression of the pathway (21). The present study demonstrated that
ectopic expression of miR-147 restrained the phosphorylation of Akt
and the downstream P70S6K and 4E-BP-1. This effect was similar to
that of siRNA which specifically silence the expression of Akt. The
result indicates that miR-147 may serve a suppressor role in
Akt/mTOR pathway. A previous study reported that overexpression of
miR-147 may downregulate the phosphorylation of Akt in colon cancer
(15). In addition, Uhlmann et
al (13) even identified Akt2 and
CyclinD1 as direct targets of miR-147. However, these two studies
did not demonstrate the effect of miR-147 in the downstream mTOR
pathway. The present study reported the association between miR-147
and the Akt/mTOR pathway in breast cancer.
The current study demonstrated that miR-147
expression represses the cell proliferation, invasion and migration
in breast cancer, similarly to the effect of siRNA silencing Akt
expression. This result is consistent with Uhlmann et al
(13). miR-147 was also demonstrated
to inhibit the invasion and motility of colon cancer cells
(15). The comparison between miR-147
and knockdown of Akt with siRNA was not conducted in the above
studies. The present study directly compared the effect of miR-147
with that of siRNA downregulating Akt, and confirmed that the
suppressor function of miR-147 was achieved through Akt/mTOR
pathway. miR-147 may provide a novel target for breast cancer
therapy targeting Akt/mTOR pathway.
The endogenous level of miR-147 was downregulated in
highly aggressive breast cancer cell line. Ectopic expression of
miR-147 repressed cell proliferation, invasion and migration by
inhibiting activation of Akt/mTOR signaling pathway in breast
cancer cell. The above effects of miR-147 were analogous to that of
siRNA targeted to specifically silence Akt. Acting as a potential
tumor suppressor, miR-147 indicates a novel avenue for breast
cancer therapy targeting Akt/mTOR pathway.
Acknowledgements
The authors sincerely thank Dr Chen Nianyong for
comments, improvement and submission on the manuscript; and Dr Wang
Yanping and Dr Wang Zhu of Tumor Molecular Diagnostic Laboratory
for their technical support.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mita MM, Mita A and Rowinsky EK: Mammalian
target of rapamycin: A new molecular target for breast cancer. Clin
Breast Cancer. 4:126–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagaraja AK, Creighton CJ, Yu Z, Zhu H,
Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM,
et al: A link between mir-100 and FRAP1/mTOR in clear cell ovarian
cancer. Mol Endocrinol. 24:447–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uesugi A, Kozaki K, Tsuruta T, Furuta M,
Morita K, Imoto I, Omura K and Inazawa J: The tumor suppressive
microRNA miR-218 targets the mTOR component Rictor and inhibits AKT
phosphorylation in oral cancer. Cancer Res. 71:5765–5778. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwaya T, Yokobori T, Nishida N, Kogo R,
Sudo T, Tanaka F, Shibata K, Sawada G, Takahashi Y, Ishibashi M, et
al: Downregulation of miR-144 is associated with colorectal cancer
progression via activation of mTOR signaling pathway.
Carcinogenesis. 33:2391–2397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu T, Li J, Yan M, Liu L, Lin H, Zhao F,
Sun L, Zhang Y, Cui Y, Zhang F, et al: MicroRNA-193a-3p and −5p
suppress the metastasis of human non-small-cell lung cancer by
downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway.
Oncogene. 34:413–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue M, Yao S, Hu M, Li W, Hao T, Zhou F,
Zhu X, Lu H, Qin D, Yan Q, et al: HIV-1 Nef and KSHV oncogene K1
synergistically promote angiogenesis by inducing cellular miR-718
to regulate the PTEN/AKT/mTOR signaling pathway. Nucleic Acids Res.
42:9862–9879. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Wang H and Yang Z: MiR-122
inhibits cell proliferation and tumorigenesis of breast cancer by
targeting IGF1R. PLoS One. 7:e470532012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uhlmann S, Mannsperger H, Zhang JD, Horvat
EÁ, Schmidt C, Küblbeck M, Henjes F, Ward A, Tschulena U, Zweig K,
et al: Global microRNA level regulation of EGFR-driven cell-cycle
protein network in breast cancer. Mol Syst Biol. 8:5702012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo HW, Hsu SC and Hung MC: EGFR signaling
pathway in breast cancers: From traditional signal transduction to
direct nuclear translocalization. Breast Cancer Res Treat.
95:211–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CG, McCarthy S, Gruidl M, Timme C and
Yeatman TJ: MicroRNA-147 induces a mesenchymal-to-epithelial
transition (MET) and reverses EGFR inhibitor resistance. PLoS One.
9:e845972014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Y, Suo AL, Li ZF, et al: MicroRNA
profiling of human gastric cancer. Mol Med Rep. 2:963–970.
2009.PubMed/NCBI
|
|
17
|
Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP
and Wei WI: Mature miR-184 as Potential Oncogenic microRNA of
Squamous Cell Carcinoma of Tongue. Clin Cancer Res. 14:2588–2592.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han ZB, Zhong L, Teng MJ, et al:
Identification of recurrence-related microRNAs in hepatocellular
carcinoma following liver transplantation. Mol Oncol. 6:445–457.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sehgal SN, Baker H and Vézina C: Rapamycin
(AY-22,989), a new antifungal antibiotic. II. Fermentation,
isolation and characterization. J Antibiot (Tokyo). 28:727–732.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|