Introduction
Skin cancer is the most frequently occurring among
all types of human cancers, and cutaneous squamous cell carcinoma
(CSCC) is the second most common of all the skin tumors (1). CSCC is a major health concern in
Caucasian in the United States, with a similar mortality rate to
melanoma (2). In total, >700,000
new cases of CSCC are diagnosed every year, and this assumes that
20% of non-melanoma skin cancers (NMSCs) are CSCCs (3). CSCC is a type of tumor that is more
aggressive than other skin carcinomas, as 12% of cases metastasize
(4,5).
Generally, the prognosis of CSCC is satisfactory, however, 4% of
cases will still develop metastases and 1.5% of patients will
succumb to this disease (6,7). The rising incidence and morbidity rates
of CSCC have generated great interest for researchers with regard
to the progression and mechanisms of metastatic CSCC migration to
the distant organs.
High-mobility group box 1 protein (HMGB1), a
chromatin-binding nuclear protein, is highly expressed in numerous
types of cancer cells and is involved in cell progression (8). Besides its intracellular function, HMGB1
can also act as an extracellular molecule binding to the receptor
for advanced glycation end products and toll-like receptors,
therefore resulting in cell differentiation, cell migration, tumor
progression and inflammation (9,10). The
function of extracellular HMGB1 is important in the metastasis of
several types of tumor cells (11).
However, the role of HMGB1 in the migration of CSCC
remains largely uninvestigated (12).
We hypothesize that HMGB1 is a key regulator that induces
metastatic CSCC to migrate. To investigate this, the present study
compared CSCC cells with human epidermoid carcinoma cells in order
to assess the level of HMGB1. Next, the study determined whether
HNGB1 was able to induce migration in CSCC and investigated the
signaling pathway involved in this process, so that it could be
determined whether HMGB1 is a potential therapeutic target for
preventing the metastasis of CSCC.
Materials and methods
Cell lines and culture
Human CSCC SCC13 and epidermoid carcinoma A431 cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The two cell lines were cultured in Iscove's modified
Dulbecco's medium supplemented with 10% heat-inactivated fetal
bovine serum, 2 mM glutamine and an antibiotic-antifungal mixture
in a humidified incubator with 5% CO2 and 95% air. Each
cell line was confirmed according to the American Type Culture
Collection instructions.
Antibodies and reagents
Recombinant HMGB1 protein was obtained from Eli
Lilly Company (Indianapolis, IN, USA) and glycyrrhizin (GR) was
obtained from Sigma-Aldrich, (St. Louis, MO, USA). The antibodies
for the western blotting were rabbit monoclonal anti-phospho-akt
(Thr308; catalog no. C31E5E; 1:1,000 dilution), rabbit monoclonal
anti-phospho-PI3kinase p85 [(Tyr458)/p55 (Tyr199); 1:1,000
dilution)], rabbit monoclonal anti-phospho-p38 MAPK (Thr180/Tyr182;
catalog no. 3D7; 1:1,000 dilution), mouse monoclonal
anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204; catalog no. E10;
1:1,000 dilution), rabbit monoclonal anti-AKT (1:1,000 dilution),
rabbit monoclonal anti-PI3kinase p85 (1:1000 dilution), rabbit
monoclonal anti-p38 MAPK (1:1,000 dilution) and rabbit monoclonal
anti-p42/44 MAPK (1:1,000 dilution). These antibodies were obtained
from Cell Signaling Technology (Danvers, MA, USA).
Western blotting
Briefly, after treatment with/without HMGB1 or GR,
the whole cell lysates were prepared with ice-cold cell lysis
buffer and cleared by centrifugation at 10,000 × g. The total
protein concentration was measured with the bicinchoninic acid
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal
amount of proteins were loaded onto 8–12% SDS-PAGE gel sand
transferred to polyvinylidene fluoride membranes by
electroblotting. Subsequent to blocking in phosphate-buffered
saline (PBS) plus Tween-20 containing 5% dried milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies at 4°C overnight. The blots were incubated with
appropriate secondary antibodies at room temperature for 1 h the
next day. The signals were detected by enhanced chemiluminescence
reagent (ThermoFisher Scientific, Inc., Rockford, IL, USA).
Chemotaxis assay
The 8-µm polycarbonate membranes were incubated with
5 µg fibronectin in 400 µl PBS overnight at 4°C and blocked with 1%
bovine serum albumin for 2 h prior to use. Briefly, the cells were
detached with trypsin, washed twice with PBS and resuspended in
serum-free Dulbecco's modified Eagle's medium to a density of
5×105 cells/ml. Next, 200 µl of the prepared cells were
seeded into the upper chambers of Transwell inserts (Costar
Transwell; Corning Inc., Corning, NY, USA). The lower chambers were
filled with different concentrations of HMGB1 (10, 30 and 100
ng/ml), as indicated, or with DMEM (control). After 12 h of
incubation at 37°C, the inserts were removed from the Transwells
and thoroughly washed three times with PBS. Cells remaining in the
upper chambers were wiped off with swabs and cells that had
migrated were stained by Hema 3 according to the manufacturer's
instructions (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The adherent cells were then counted under a light microscope
(Olympus BX63 Upright Microscope; Olympus Corporation, Tokyo,
Japan).
Enzyme-linked immunosorbent assay
(ELISA)
Levels of HMGB1 in the supernatant of the samples
were measured using the human HMGB1 ELISA assay kit
(Immuno-Biological Laboratories International, Toronto, ON, Canada)
according to the manufacturer's instructions.
Statistical analysis
Data in this study are presented as the mean ±
standard error of the mean of at least three different independent
experiments. The statistical analysis was performed using GraphPad
Prism software, version 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA). Results were analyzed by Student's t-test for comparisons
between the two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
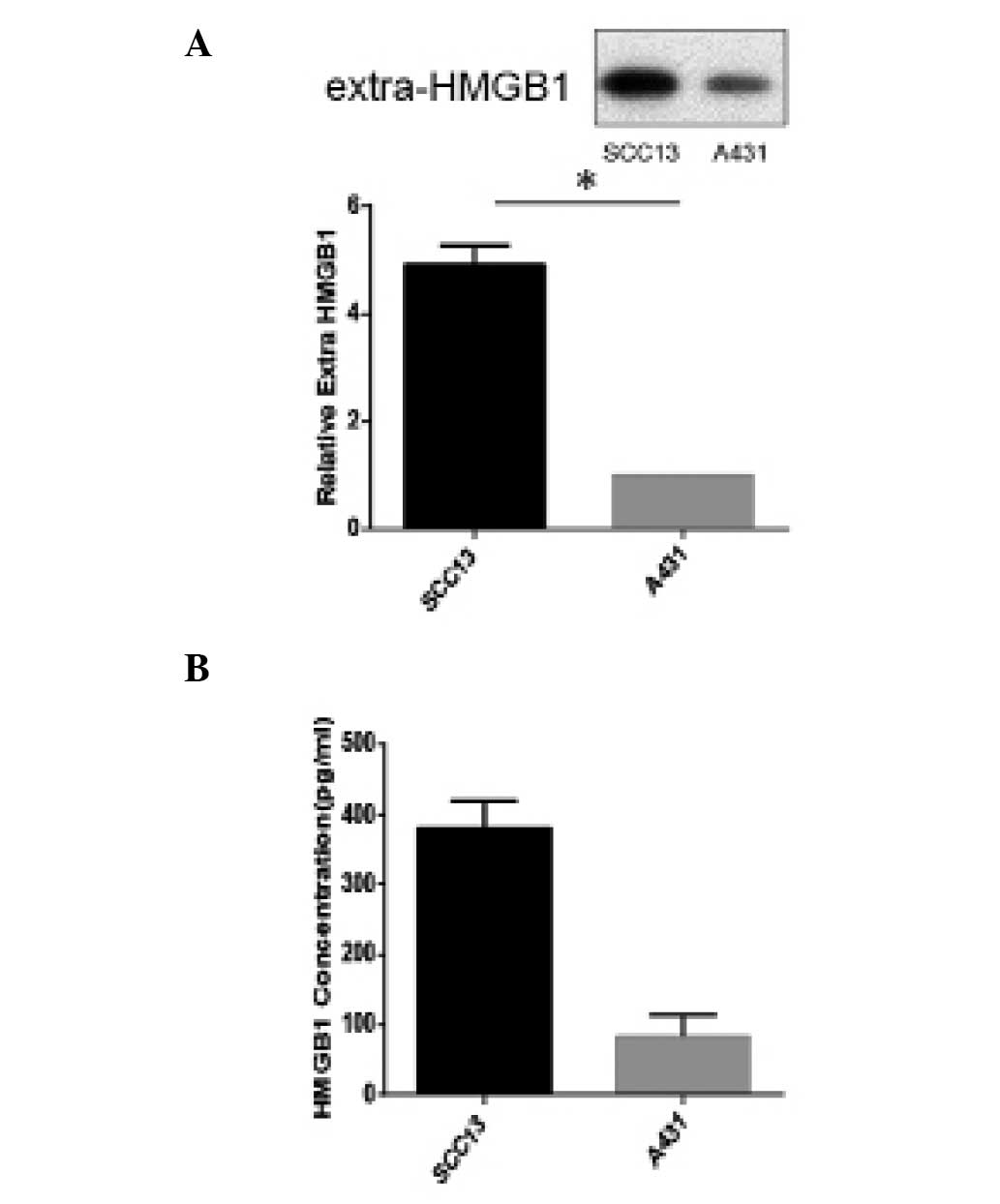
Human CSCC SCC13 cells secrete higher
HMGB1 levels compared with other NMSC cells
Since HMGB1 is well known as an important regulator
in the development, progression and metastasis of certain types of
cancers, the present study aimed to identify whether human CSCC
SCC13 cells can secrete this protein, and the difference between
SCC13 and other NMSC cells, namely A431 cells (9,10). First,
these two human cell lines were cultured in regular medium for 24
h, then the supernatant was collected and a western blotting assay
was performed (Fig. 1A). The level of
HMGB1 in the supernatant of the SCC13 cells was significantly
higher than the level in the A431 cell supernatant (P<0.01;
Fig. 1A). ELISA was also used to
confirm these results (Fig. 1B). The
levels of HMGB1 in the supernatant of these two cell lines were
measured by the human HMGB1 ELISA assay kit. The human CSCC SCC13
cells secreted higher HMGB1 levels compared with the A431 cell
line.
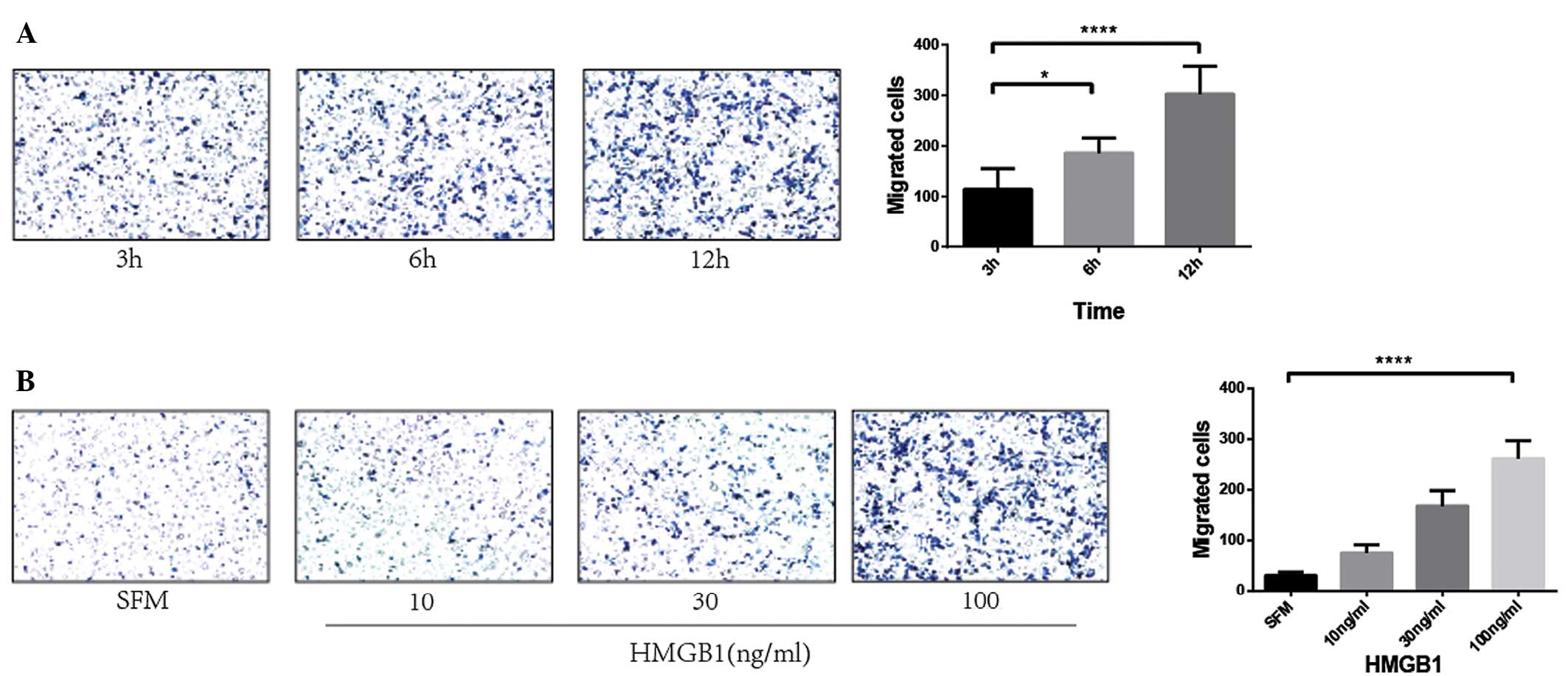
HMGB1 regulates the metastatic
potential of SCC13 cells in a time- and dose-dependent manner
First, the SCC13 cells were treated with HMGB1 (100
ng/ml) for different lengths of time (3, 6 and 12 h), and then the
number of migrated cells was counted under a microscope. A
significantly higher level of cell migration was evident following
incubation for 3 h, however, the highest level was at the 12-h
time-point (P<0.0001; Fig. 2A).
Therefore, the 12-h time-point was chosen for further study. The
SCC13 cells were next treated with different concentrations of
HMGB1 for 12 h. A significantly higher level of cell migration was
evident at 100 ng/ml HMGB1 (P<0.0001; Fig. 2B). These data indicated that an
increase in HMGB1 level induces SCC13 cell migration in a time- and
dose-dependent manner.
PI3K/AKT and MAPK signaling pathways
are involved in HMGB1-induced migration
Since the PI3K/AKT and MAPK signaling pathways are
well known to play an essential role in regulating cell
proliferation, differentiation, migration and the other cell
progression, the present study analyzed the PI3K/AKT and MAPK
signals induced by HMGB1. The SCC13 cells were treated with HMGB1
(100 ng/ml) in the presence or absence of GR (100 µM), which is an
HMGB1 inhibitor, for 24 h. It was found that HMGB1 stimulated the
activation of the p38, p42/44 MAPK, AKT and PI3K signals in the
SCC13 cells by an increased level of phosphorylated p38, P42/44
MAPK, AKT and PI3K. GR was able to almost completely inhibit this
activation (Fig. 3), which indicates
a key role of the AKT/AMPK pathway in regulating HMGB1-induced
metastasis.
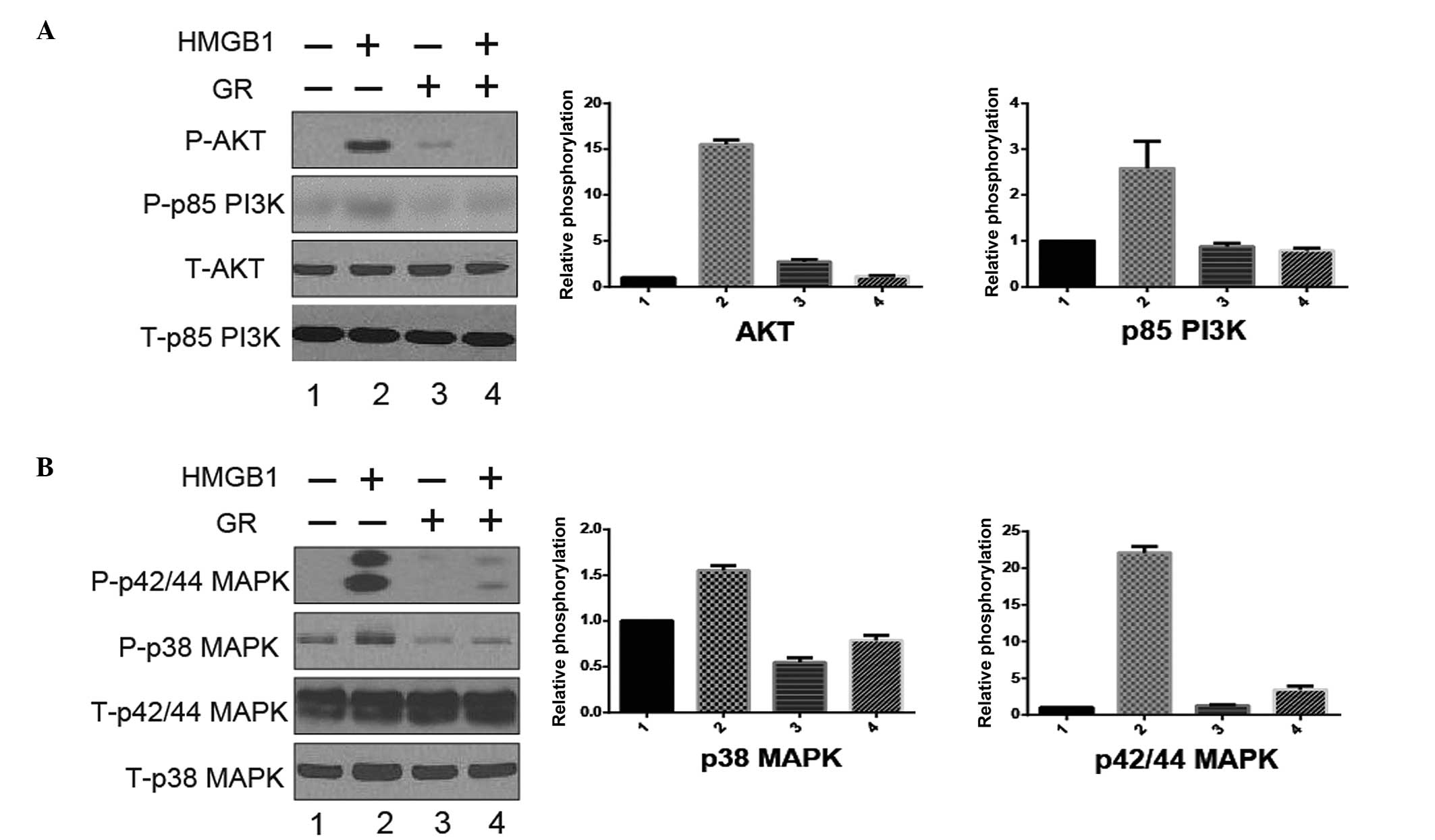 | Figure 3.PI3K/AKT and MAPK signaling pathways
are involved in HMGB1-induced migration. CSCC SCC13 cells were
treated with/without HMGB1 (100 ng/ml) or GR (100 µM) for 24 h. At
the end of treatment, the activation [phosphorylation (P)] of AKT,
p85 PI3K, p38 and p42/44 MAPK was analyzed by western blotting. The
whole cell lysates were prepared, resolved by SDS-PAGE and
subjected to western blot analysis. The total (T)-AKT, −p85 PI3K,
−p38 and −p42/44 MAPK were used as a loading control. Results shown
are representative of three independent experiments and were
qualified by densitometry. (A) AKT and p85 PI3K; (B) p38 MAPK and
p42/44 MAPK. CSCC, cutaneous squamous cell carcinoma; HMGB1,
high-mobility group box 1; PI3K, phosphoinositide 3-kinase; MAPK,
mitogen-activated protein kinase; GR, glycyrrhizin. |
Discussion
CSCC is the second most commonly occurring type of
NMSC, with an incidence rate that is increasing rapidly (13). The metastases of cancer cells are
viewed as a major cause of mortality in the majority of cancer
types. Since the metastasis of the primary cancer causes damage in
other organs, treatment is no longer efficient (14). Metastatic CSCC causes the majority of
fatalities associated with NMSC, but the molecular mechanisms for
CSCC progression and migration remain poorly identified. The
present study aimed to determine the element that plays an
essential role in this progression and to unravel the associated
signaling pathways.
HMGB1 is well known for its chemotactic activity in
certain tumor cells (8–11), however, little is known with regard to
its function in the metastasis of CSCC. Therefore, the present
study sought to determine whether HMGB1 plays a role in this
process. First, the level of HMGB1 was examined in CSCC cells and
compared with normal skin carcinoma cells. It was found that the
level of HMGB1 in the supernatant of the CSCC cells was
significantly higher than that in the A431 cells, which meant that
HMGB1 may be involved in this process. Next, the effects of HMGB1
on the migration of the CSCC cells were examined. The CSCC cells
were treated with HMGB1 at different concentrations and for varying
times. The results suggested that HMGB1 can induce the migration of
CSCC cells in a time- and dose-dependent manner. In addition, it
was found that the PI3K/AKT and MAPK signaling pathways are
involved in the regulation of the HMGB1-induced migration of the
CSCC cells (Fig. 3). Additional
studies are required to clarify the downstream genes with critical
roles.
Overall, the present study showed that HMGB1 is
secreted more in CSCC cells than in human epidermoid carcinoma
cells, and that HMGB1 regulates the migration of CSCC cells by
activating the PI3K/AKT and MAPK signaling pathways. These results
provide convincing evidence that the inhibition of HMGB1 and this
pathway could have a strong effect on preventing the metastasis of
CSCC. More detailed studies are required to determine whether the
inhibitor of HMGB1 can be a pharmacologically safe and efficient
agent for the treatment of CSCC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30860257, 81101188 and
810701297).
Glossary
Abbreviations
Abbreviations:
|
CSCC
|
cutaneous squamous cell carcinoma
|
|
HMGB1
|
high-mobility group box 1
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
GR
|
glycyrrhizin
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Uribe P and Gonzalez S: Epidermal growth
factor receptor (EGFR) and squamous cell carcinoma of the skin:
Molecular bases for EGFR-targeted therapy. Pathol Res Pract.
207:337–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karia PS, Han J and Schmults CD: Cutaneous
squamous cell carcinoma: Estimated incidence of disease, nodal
metastasis and deaths from disease in the United States, 2012. J Am
Acad Dermatol. 68:957–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rogers HW, Weinstock MA, Harris AR, et al:
Incidence estimate of nonmelanoma skin cancer in the United States,
2006. Arch Dermatol. 146:283–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cherpelis BS, Marcusen C and Lang PG:
Prognostic factors for metastasis in squamous cell carcinoma of the
skin. Dermatol Surg. 28:268–273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Czarnecki D, Staples M, Mar A, Giles G and
Meehan C: Metastases from squamous cell carcinoma of the skin in
southern Australia. Dermatology. 189:52–54. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mourouzis C, Boynton A, Grant J, et al:
Cutaneous head and neck SCCs and risk of nodal metastasis-UK
experience. J Craniomaxillofac Surg. 37:443–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brantsch KD, Meisner C, Schonfisch B, et
al: Analysis of risk factors determining prognosis of cutaneous
squamous-cell carcinoma: A prospective study. Lancet Oncol.
9:713–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang D, Kang R, Livesey KM, Zeh HJ III and
Lotze MT: High mobility group box 1 (HMGB1) activates an autophagic
response to oxidative stress. Antioxid Redox Signal. 15:2185–2195.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang D, Kang R, Cheh CW, et al: HMGB1
release and redox regulates autophagy and apoptosis in cancer
cells. Oncogene. 29:5299–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Penzo M, Molteni R, Suda T, et al:
Inhibitor of NF-kappa B kinases alpha and beta are both essential
for high mobility group box 1-mediated chemotaxis (corrected). J
Immunol. 184:4497–4509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lambert SR, Harwood CA, Purdie KJ, et al:
Metastatic cutaneous squamous cell carcinoma shows frequent
deletion in the protein tyrosine phosphatase receptor Type D gene.
Int J Cancer. 131:E216–E226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madan V, Hoban P, Strange RC, Fryer AA and
Lear JT: Genetics and risk factors for basal cell carcinoma. Br J
Dermatol. 154(Suppl 1): 5–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ekblad L and Johnsson A: Cetuximab
sensitivity associated with oxaliplatin resistance in colorectal
cancer. Anticancer Res. 32:783–786. 2012.PubMed/NCBI
|