Introduction
Oral carcinogenesis is considered to be a sequential
and multi-step process that is mediated by the deregulation of
crucial molecular pathways, among which angiogenesis has long been
considered one of the most important mechanisms involved in the
biology of cancer (1,2). Numerous tumours promote growth and
dispersion to form metastases by recruiting host blood vessels to
grow into the vicinity of the tumour (3–5).
Angiogenic switch is determined by the net balance of natural
inhibitors and angiogenic stimulators, out of which vascular
endothelial growth factor (VEGF), also termed VEGF-A, is considered
to be the major stimulator responsible for tumour angiogenesis
(6,7).
Although extensive data are available on the role of VEGF and VEGF
receptors in the biology and development of a variety of human and
animal invasive malignant tumours (8–14), little
information exists concerning oral canine tumours (15).
The present study hypothesises, however, that an
improved understanding of the underlying mechanism of oral cancer
pathogenesis in dogs may be essential to improve the diagnosis of
canine oral cancer. This may also aid the understanding of the
biological behaviour of tumours and the selection of the treatment
of patients (16).
These considerations prompted the investigation of
VEGF expression using immunohistochemistry in a series of various
pathological lesions of oral canine mucosa, raging between normal
epithelium and squamous cell carcinoma, with various grades of
differentiation. VEGF expression was also associated with tumour
cell proliferation, as assessed using the proliferating cell
nuclear antigen (PCNA) marker, and microvessel density (MVD).
Materials and methods
Tissue samples
In total, 30 canine oral tissue specimens were
examined, which consisted of 6 normal oral mucosa and 24 oral
squamous cell carcinoma (OSCC) tissues from 7 Manchester terriers,
8 English setters, 8 huskies and 7 mixed breed dogs. Samples were
obtained following surgery from the Department of Veterinary
Medicine and Animal Production, University of Naples. The specimens
were fixed in 10% neutral buffered formalin, embedded in paraffin
wax, stained with haematoxylin and eosin (Carlo Erba Reagents SAS,
Val de Reuil, France) and classified using the World Health
Organization criteria (17). The OSCC
tissues were graded by two independent observers, according to the
criteria proposed by Pindborg (18),
as well-differentiated (grade 1; 7 out of 24 tissues), moderately
differentiated (grade 2; 9 out of 24 tissues) or poorly
differentiated (grade 3; 8 out of 24 tissues). The samples did not
include SCC of the tonsil, as this lesion is usually considered
separately from other OSCCs, due to the differences in its
geographical distribution and biological behaviour (19,20).
Out of the 24 OSCC tissues considered in the present
study, 8 lesions were analysed in a previous study (8), in which the microvessel density,
indicated by the number of microvessels per mm2 of
neoplastic tissue, was calculated (8).
Immunohistochemistry
The procedure used for immunohistochemical analysis
was the streptavidin-biotin peroxidase method (LSAB kit; Dako,
Glostrup, Denmark), which was the same method used in previous
studies (12,21–23).
Briefly, histological sections (5 µm) were dewaxed in xylene,
dehydrated in graded concentrations of ethanol and washed in 0.01 M
phosphate-buffered saline (PBS; pH 7.2±7.4). Endogenous peroxidase
activity was blocked using 0.3% hydrogen peroxide (Carlo Erba
Reagents SAS) in absolute methanol for 30 min. Prior to incubation
with primary antibodies, the tissue sections were heated in a
microwave oven for 3 cycles of 5 min (500–750 W) each in EDTA
buffer (pH 6.0; Sigma-Aldrich, Irvine, UK). The sections were then
incubated overnight at 4°C with a monoclonal mouse anti-vascular
endothelial growth factor (diluted 1:100 PBS; clone, JH121; cat no.
MS35OPO; Neo Markers, Fremont, CA, USA) (24) and a monoclonal mouse
anti-proliferating cell nuclear antigen (diluted 1:500 PBS; clone,
PC10; cat no. M0879; Dako), which was used to identify
proliferating cells. The immunolabelling procedure included
negative control sections incubated with PBS instead of the primary
antibody. A mixture of biotinylated anti-mouse, anti-rabbit and
anti-goat immunoglobulins (LSAB kit; cat no. K0675, Dako), diluted
in PBS, was used as secondary antibody and was applied for 30 min.
To reveal the immunolabelling, 3,3′-diaminobenzidine
tetrahydrochloride was used as a chromogen and haematoxylin was
used as a counterstain.
Scoring of immunoreactivity
The immunoreactivity for VEGF and PCNA was evaluated
by two observers that selected 20 maximally immunostained regions
for each case at a magnification of ×400 (×40 objective; ×10
ocular). The number of cells expressing VEGF and PCNA were counted
individually. At least 1,000 cells were counted to obtain the
percentage of positive cells. Epithelial cells exhibiting distinct
cytoplasmic staining for VEGF and a distinct brown nuclear reaction
to PCNA labelling were considered to express VEGF and PCNA,
respectively, and were classified based on the intensity of
staining into weak and strong immunoreactivity groups.
Statistical analysis
The percentage of cells expressing VEGF and PCNA in
each group (OSCC grades 1–3) was expressed as the mean ± standard
deviation. Statistical analysis was performed using analysis of
variance. The correlation between the VEGF and PCNA values was
assessed using a linear correlation test. P<0.01 was used to
represent a statistically significant different.
Results
Immunohistochemistry
Results of the immunohistochemical analysis are
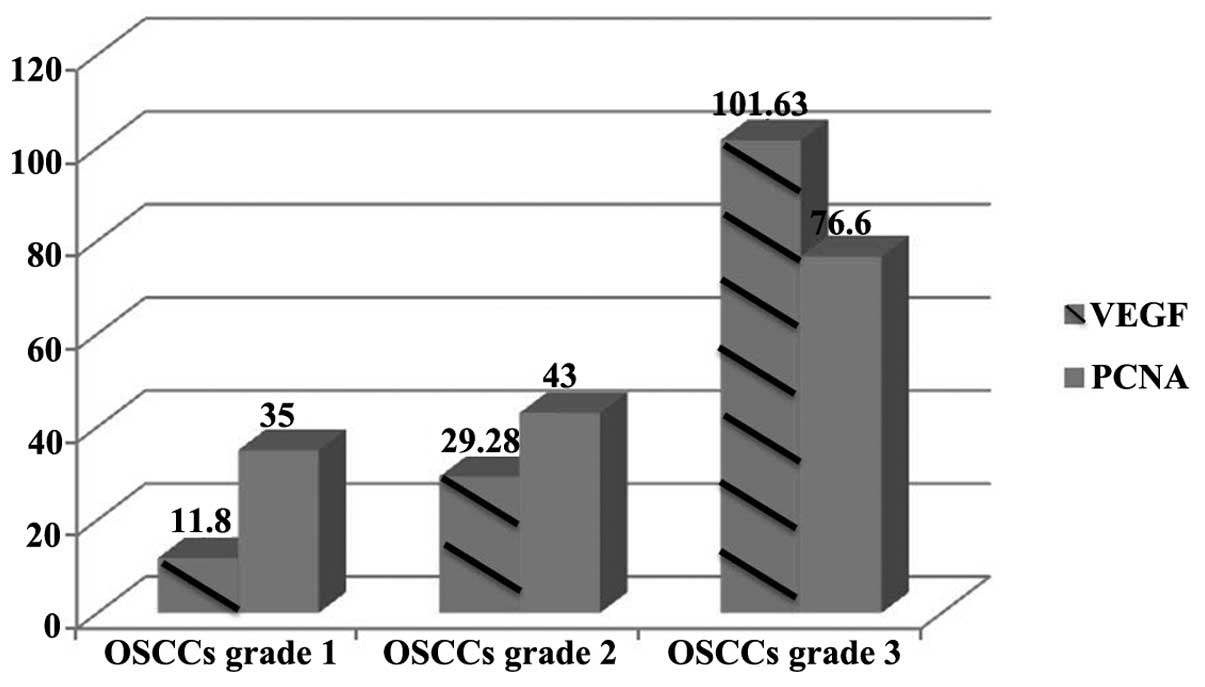
reported in Table I and Fig. 1.
 | Table I.Correlation between the number of
cells expressing VEGF and PCNA and the differentiation grades of
tumours in canine OSCC tissues. |
Table I.
Correlation between the number of
cells expressing VEGF and PCNA and the differentiation grades of
tumours in canine OSCC tissues.
|
| OSCC grade |
|
|---|
|
|
|
|
|---|
| Protein
expressed | 1 | 2 | 3 | P-value |
|---|
| VEGF | 11.80±8.86 |
29.28±20.65 | 101.63±71.96 | 0.0008 |
| PCNA | 35.00±2.23 | 43.00±5.98 |
76.60±10.30 | 0.0010 |
VEGF expression
VEGF immunopositivity was detected in almost all
normal epithelial cells as pale, cytoplasmic staining, which was
predominantly detected in the basal layer. Weak VEGF staining was
also observed in endothelial cells. In the majority of tumour
cells, VEGF expression was indicated by a cytoplasmic staining
pattern that ranged between extremely weak and strong, with
cytoplasmic granules of variable size and amount. In the majority
of grade 1 OSCC tissues (5/7 tissues), VEGF was weakly expressed by
epithelial cells, which demonstrated small cytoplasmic granules
that were frequently restricted beneath the cytoplasmic membrane
(Fig. 2A). In all grade 2 (9/9) and 3
(8/8) OSCC tissues, a heterogeneous pattern of VEGF staining that
ranged between moderate and strong in intensity was observed
(Fig. 2B). Strong staining was
particularly observed in less differentiated regions (Fig. 2C). VEGF granules were large and
frequently polarized in grade 2 OSCC tissues and diffuse in the
cytoplasm in grade 3 OSCC tissues.
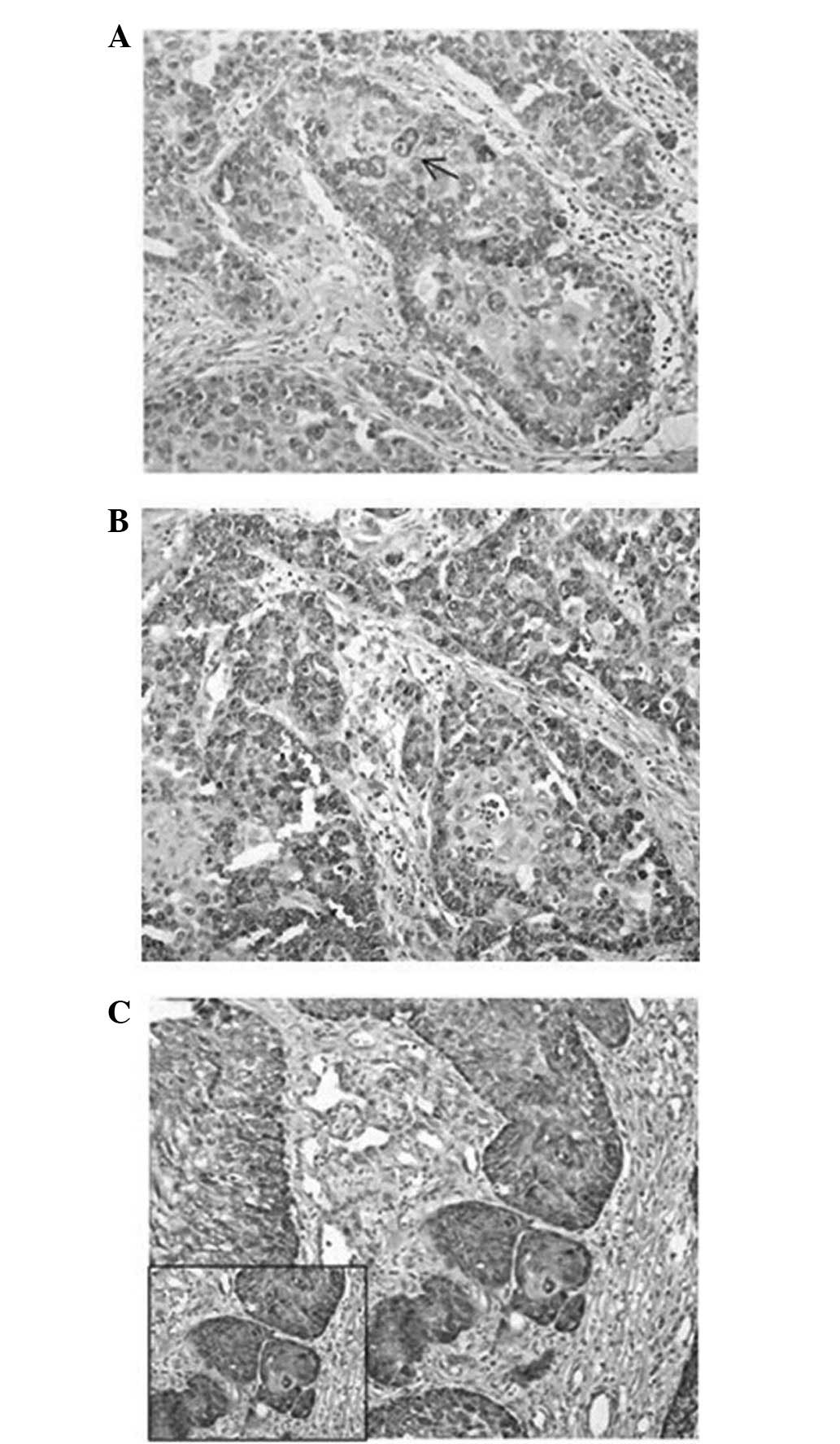 | Figure 2.(A) Well-differentiated (grade 1)
OSCC tissue demonstrating weak expression of the VEGF protein in
few neoplastic cells. The immunolabelling is often restricted often
beneath the cytoplasmic membrane (arrow; stain, streptavidin-biotin
peroxidase; magnification, ×20). (B) Moderately differentiated
(grade)OSCC tissue demonstratign moderate expression of the VEGF
protein in numerous neoplastic cells. The VEGF expression was
localized in almost all the cytoplasm, and was occasionally
polarized (stain, streptavidin-biotin peroxidase; magnification,
×20). (C) Poorly differentiated (grade 3) OSCC tissue demonstrating
strong expression of the VEGF protein in almost all neoplastic
cells, with diffuse large granules in the cytoplasm (stain,
streptavidin-biotin peroxidase; magnification, ×20; insert
magnification, ×40). VEGF, vascular endothelial growth factor;
OSCC, oral squamous cell carcinoma. |
The number of labelled cells increased progressively
between grade 1 (11.8±8.86) and grade 2 (29.28±20.65) OSCC tissues,
and an additional increase was observed between grade 2 and grade 3
(101.63±71.96) tissues. A significant difference was identified
between the number of cells labelled for VEGF expression and the
degree of differentiation in OSSC lesions (P=0.0008).
PCNA expression
PCNA-immunolabelled nuclei were clearly identifiable
in normal oral epithelium tissues, as well as in OSCC tissues. In
the normal epithelium, weak immunostaining was predominantly
observed in the basal layer of the tissue and moderate staining was
observed in the parabasal layers, 2–3 layers from the basal layer.
In grade 1 and 2 OSCC tissues, moderate nuclear staining was more
frequently detected in the peripheral region of the tumour islands
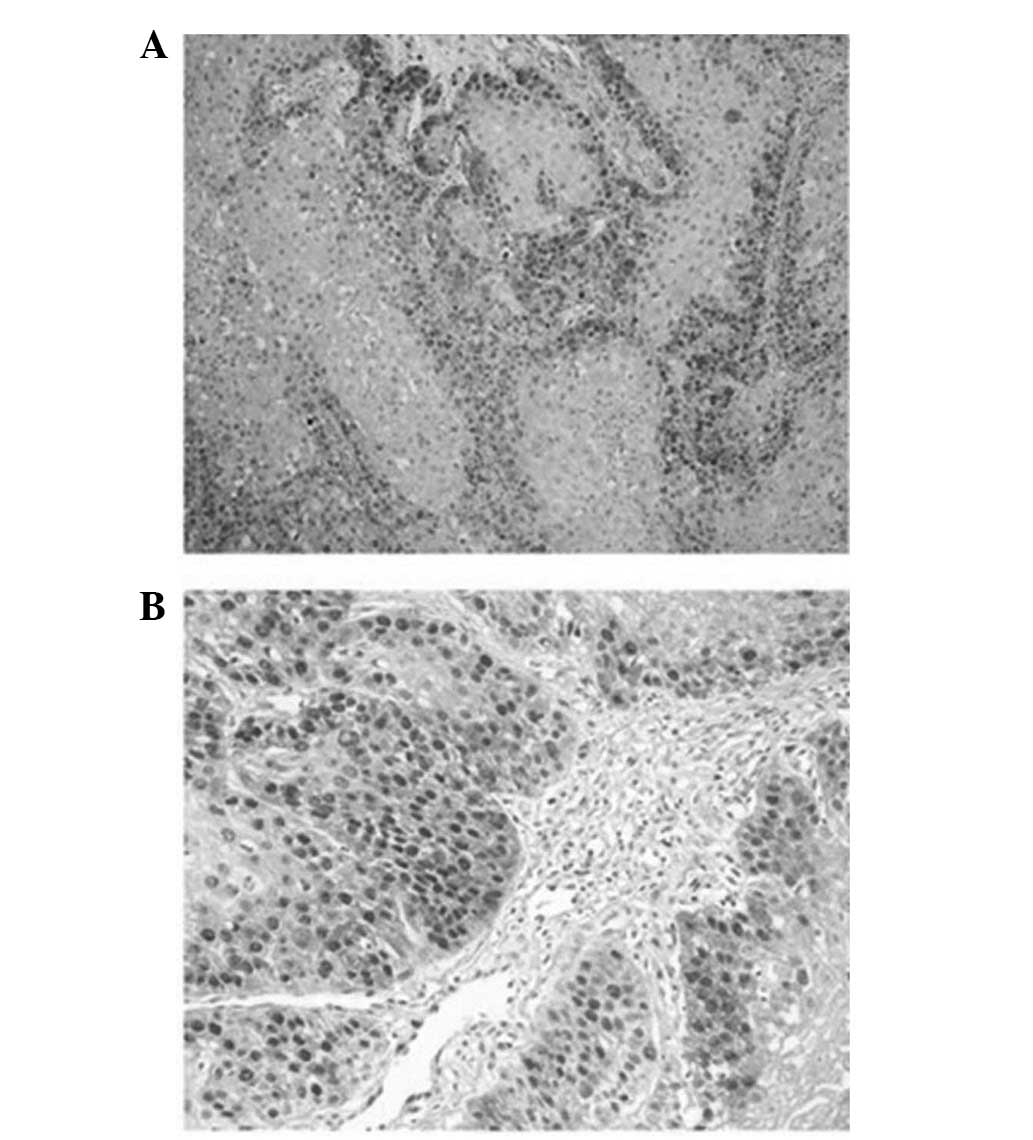
and was almost absent in the central keratinized region (Fig. 3A). Grade 3 OSCCs demonstrated strong
nuclear immunostaining with a diffuse pattern of staining (Fig. 3B). The number of cells expressing PCNA
increased progressively between grade 1 (35±2.23) and 2 (43±5.98)
OSCC tissues and grade 3 OSCC tissues (76.6±10.30). A significant
difference was identified between the number of cells labelled for
PCNA expression and the degree of differentiation of the tumours
(P=0.001).
Correlation between the expression of
VEGF and PCNA and MVD
The expression of VEGF and PCNA strongly correlated
with the degree of differentiation of OSCC lesions (r=0.999). In
addition, in 8/24 OSCCs, the MVD (8)
strongly correlated with the expression of VEGF (r=0.993) and PCNA
(r=0.993).
Discussion
Squamous cell carcinoma of the oral cavity (OSCC) is
the second most common malignant neoplasm of the oral cavity in
dogs, following melanoma, as it accounts for 17–25% of canine oral
tumours (19). Despite the relatively
low metastatic rate (20%), OSCC grows rapidly and typically invades
nearby bone and tissue, in addition to possessing an extremely high
frequency of local recurrence (18,25). In
order to improve the understanding of the pathogenesis of this
tumour, the present study examined the immunohistochemical
expression of VEGF in canine OSCCs and the association of VEGF
expression with neoplastic proliferation and microvessel
density.
VEGF is a multifunctional cytokine that is produced
by neoplastic cells, macrophages, plasma cells and lymphocytes
(26,27). This cytokine acts on endothelial cells
as a highly specific mitogen, binding to specific class III
receptor tyrosine kinases, termed VEGFR-1 (Flt-1) and VEGFR-2
(Flk-1) (28,29). The activation of VEGFR-1 promotes
endothelial cell migration, but does not induce cell proliferation
(30–32). However, VEGFR-2 is the major mediator
of endothelial cell proliferation and survival, and it is also
essential for the differentiation of endothelial cells and the
induction of microvessel permeability (11,33).
Previous studies investigating the role of VEGF in
human oral cancer progression are contradictory. Numerous human
cancers have demonstrated increased VEGF expression compared with
normal tissue (29,34–36). By
contrast, certain studies have reported opposite results,
demonstrating that VEGF expression levels were increased in the
corresponding normal mucosa epithelium and early stage of
premalignant lesions compared with later stages of cancerogenesis
(37,38). These previous studies hypothesized
that VEGF may perform a physiological role in oral mucosa
epithelium, or that the protein may play an important role only in
the early stage of oral tumorigenesis, while other genetic factors
are involved in the later stages. These discrepancies were
considered, and it was suggested that they may be due to the use of
different antibodies or quantification methods (39).
In the present study, a significant increase in VEGF
expression was identified during the transition between normal and
OSCC tissues, in association with an increasing grade of
differentiation. This was indicated by a high number of
VEGF-positive cells associated with the accumulation and consequent
loss of cytoplasmic polarization of VEGF granules in less
differentiated tumours. These results are in agreement with those
of Sobczyńska-Rak et al (15),
who studied the level of VEGF in the serum of dogs with OSCC by
ELISA, and identified an elevated presence of VEGF in the blood
serum of all examined malignant tumours compared with the benign
tumours and control tissues, confirming a significant role of VEGF
in the disease progression of canine oral tumours. Upregulation of
VEGF, which is particularly found in moderately or poorly
differentiated tumours, may be induced mainly by HIF-1α under the
hypoxic conditions present in the necrotic compartments of tumours
(40). By contrast, in numerous types
of tumours, elevated levels of VEGF production may often be
detected in tumour cells located at the extreme periphery of the
tumour where there is no hypoxia (12,41,42).
Therefore, hypoxia-independent production of VEGF by neoplastic
cells may be associated with the activation of oncogenes encoding
VEGF, such as the oncogenes that are components of the
ras/mitogen-activated protein kinase signal transduction
pathway, the inactivation of tumour suppressors, including p53, and
exogenous factors, such as hormones and growth factors (30,43).
In addition, in the present study, VEGF expression
was found to be significantly correlated with PCNA expression.
Tumour growth is associated with the actions of proliferative
proteins such as proliferating cell nuclear antigen (PCNA), a
non-histamine nuclear protein that is considered a specific marker
of cell division (9,44). The expression of PCNA has been found
to be correlated with the degree of malignancy and progression of
cancer (45). The strong correlation
between PCNA and VEGF detected in the present study indicates that
VEGF, which is secreted by neoplastic cells, may induce
self-proliferation in an autocrine manner, contributing to
neoplastic growth, and induce endothelial cell proliferation and
tumour angiogenesis through a paracrine mechanism. Therefore,
malignant neoplastic cells may act simultaneously as VEGF producers
and VEGF target cells, as demonstrated in previous studies
(46,47).
Additionally, in the present study, tumour cell
proliferation was strongly correlated with microvessel density,
demonstrating that increased vascularity occurs to support actively
proliferating and transforming oral epithelial cells, in order to
permit tumour growth. This finding is in agreement with numerous
studies, which have already established that tumour cell
proliferation decreases with the increasing distance between the
tumour and blood vessels, confirming that tumour growth is strongly
dependent on angiogenesis (9,38,48).
Overall, the present results revealed that VEGF
levels are elevated in canine OSCC compared with control specimens.
Increased expression of this angiogenic factor in less
differentiated tumours may determine an increase in neoplastic and
blood vessel proliferation, as determined by the strong correlation
that was identified between VEGF expression, cell proliferation and
microvessel density. This is potentially of significance since
neoangiogenesis is necessary for the nutrition of the neoplastic
cells that demonstrated an increased proliferative rate, and
neoangiogenesis also enhances the ability of tumour cells to
migrate, and therefore the potential of the cells to metastasize
(2,32). As a result, an increase in VEGF
synthesis is associated with increased endothelial and neoplastic
cell proliferation, and the risk of tumour growth and metastasis is
increased. To confirm this hypothesis, additional studies are
required to fully elucidate the role of VEGF and VEGF receptors in
tumour-associated angiogenesis and to determine whether receptors
and ligands were each expressed in the same cells, indicating an
autocrine mechanism, or the receptor was expressed in one
compartment and the ligand in one of the other compartments,
indicating a paracrine loop (49).
In conclusion, VEGF evaluation may provide
additional criteria for assessing the intrinsic malignancy and
growth potential of canine oral tumours, and may have a potential
application as an indicator of prognosis. In addition,
understanding the biology of the VEGF ligand and receptor may
facilitate the development of therapeutic strategies to promote
revascularization of ischemic tissue or to inhibit angiogenesis in
neoplastic pathologies (50).
Observations in vivo suggest that the inhibition of
VEGF-induced angiogenesis results in suppression of tumour growth
in animal studies (51) and a
reduction in tumour size and metastasis in vivo (52).
References
|
1
|
Hasina R and Lingen MW: Angiogenesis in
oral cancer. J Dent Educ. 65:1282–1290. 2001.PubMed/NCBI
|
|
2
|
Ascani G, Balercia P, Messi M, Lupi L,
Goteri G, Filosa A, Stramazzotti D, Pieramici T and Rubini C:
Angiogenesis in oral squamous cell carcinoma. Acta Otorhinolaryngol
Ital. 25:13–17. 2005.PubMed/NCBI
|
|
3
|
Blood CH and Zetter BR: Tumor interactions
with the vasculature: Angiogenesis and tumor metastasis. Biochim
Biophys Acta. 1032:89–118. 1990.PubMed/NCBI
|
|
4
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fox SB, Gatter KC and Harris AL: Tumour
angiogenesis. J Pathol. 179:232–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara N and Keyt B: Vascular endothelial
growth factor: Basic biology and clinical implications. EXS.
79:209–232. 1997.PubMed/NCBI
|
|
7
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martano M, Maiolino P, Cataldi M and
Restucci B: Evaluation of angiogenesis by morphometric analysis of
blood vessels in dysplastic and neoplastic lesions of canine
gingiva. Vet Res Commun. 28(Suppl 1): 299–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tipoe GL, Jin Y and White FH: The
relationship between vascularity and cell proliferation in human
normal and pathological lesions of the oral cheek epithelium. Eur J
Cancer B Oral Oncol. 32B:24–31. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khademi B, Soleimanpour M, Ghaderi A and
Mohammadianpanah M: Prognostic and predictive value of serum
vascular endothelial growth factor (VEGF) in squamous cell
carcinoma of the head and neck. Oral Maxillofac Surg. 18:187–196.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Dissi AN, Haines DM, Singh B and Kidney
BA: Immunohistochemical expression of vascular endothelial growth
factor and vascular endothelial growth factor receptor associated
with tumor cell proliferation in canine cutaneous squamous cell
carcinomas and trichoepitheliomas. Vet Pathol. 44:823–830. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Restucci B, Borzacchiello G, Maiolino P,
Martano M, Paciello O and Papparella S: Expression of vascular
endothelial growth factor receptor Flk-1 in canine mammary tumours.
J Comp Pathol. 130:99–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Restucci B, Maiolino P, Paciello O,
Martano M, De Vico G and Papparella S: Evaluation of angiogenesis
in canine seminomas by quantitative immunohistochemistry. J Comp
Pathol. 128:252–259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maiolino P, De Vico G and Restucci B:
Expression of vascular endothelial growth factor in basal cell
tumours and in squamous cell carcinomas of canine skin. J Comp
Pathol. 123:141–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sobczyńska-Rak A, Polkowska I and
Silmanowicz P: Elevated vascular endothelial growth factor (VEGF)
levels in the blood serum of dogs with malignant neoplasms of the
oral cavity. Acta Vet Hung. 62:362–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martano M, Damiano S, Restucci B, Paciello
O, Russo V and Maiolino P: Nuclear morphometry in canine
acanthomatous ameloblastomas and squamous cell carcinomas. Eur J
Histochem. 50:125–130. 2006.PubMed/NCBI
|
|
17
|
Head KW, Cullen JM, Dubielzig RR, Else RW,
Misdorp W, Patnaik AK, Tateyama S and van der Gaag I: Histological
classification of tumors of the alimentary system of domestic
animals (WHO). Armed Forces Institute of Pathology (Washington,
DC). 2003.
|
|
18
|
Pindborg JJ: Definition of terms related
to oral cancer and precancer. Oral cancer and precancer (Bristol).
John Wright & Sons. 2–19. 1980.Todoroff RJ and Brodey RS: Oral
and pharyngeal neoplasia in the dog: A retrospective survey of 361
cases. J Am Vet Med Assoc 175: 567–571, 1979.
|
|
19
|
Todoroff RJ and Brodey RS: Oral and
pharyngeal neoplasia in the dog: A retrospective survey of 361
cases. J Am Vet Med Assoc. 175:567–571. 1979.PubMed/NCBI
|
|
20
|
Mas A, Blackwood L, Cripps P, Murphy S, De
Vos J, Dervisis N, Martano M and Polton GA: Canine tonsillar
squamous cell carcinoma-a multi-centre retrospective review of 44
clinical cases. J Small Anim Pract. 52:359–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Restucci B, Maiolino P, Martano M, et al:
Expression of beta-catenin, E-cadherin and APC in canine mammary
tumors. Anticancer Res. 27:3083–3089. 2007.PubMed/NCBI
|
|
22
|
Restucci B, Martano M, De Vico G, et al:
Expression of E-cadherin, beta-catenin and APC protein in canine
colorectal tumours. Anticancer Res. 29:2919–2925. 2009.PubMed/NCBI
|
|
23
|
Martano M, Carella F, Squillacioti C, et
al: Metallothionein expression in canine cutaneous apocrine gland
tumors. Anticancer Res. 32:747–752. 2012.PubMed/NCBI
|
|
24
|
Turley H, Scott PA, Watts VM, et al:
Expression of VEGF in routinely fixed material using a new
monoclonal antibody VG1. J Pathol. 186:313–318. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Head KWER and Dubielzig RR: Tumors of the
alimentary tract. Tumors in Domestic Animals. Press IS: (Ames). DJ
Meuten. 401–481. 2002.
|
|
26
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dvorak HF, Detmar M, Claffey KP, Nagy JA,
van de Water L and Senger DR: Vascular permeability factor/vascular
endothelial growth factor: An important mediator of angiogenesis in
malignancy and inflammation. Int Arch Allergy Immunol. 107:233–235.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Denhart BC, Guidi AJ, Tognazzi K, Dvorak
HF and Brown LF: Vascular permeability factor/vascular endothelial
growth factor and its receptors in oral and laryngeal squamous cell
carcinoma and dysplasia. Lab Invest. 77:659–664. 1997.PubMed/NCBI
|
|
30
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
31
|
Margaritescu C, Pirici D, Simionescu C,
Mogoantă L, Raica M, Stîngă A, Ciurea R, Stepan A, Stîngă A and
Ribatti D: VEGF and VEGFRs expression in oral squamous cell
carcinoma. Rom J Morphol Embryol. 50:527–548. 2009.PubMed/NCBI
|
|
32
|
Johnstone S and Logan RM: The role of
vascular endothelial growth factor (VEGF) in oral dysplasia and
oral squamous cell carcinoma. Oral Oncol. 42:337–342. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cross MJ and Claesson-Welsh L: FGF and
VEGF function in angiogenesis: Signalling pathways, biological
responses and therapeutic inhibition. Trends Pharmacol Sci.
22:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maeda T, Matsumura S, Hiranuma H, Jikko A,
Furukawa S, Ishida T and Fuchihata H: Expression of vascular
endothelial growth factor in human oral squamous cell carcinoma:
Its association with tumour progression and p53 gene status. J Clin
Pathol. 51:771–775. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shintani S, Li C, Ishikawa T, Mihara M,
Nakashiro K, et al: Expression of vascular endothelial growth
factor A, B, C and D in oral squamous cell carcinoma. Oral Oncol.
40:13–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shang ZJ and Li JR: Expression of
endothelial nitric oxide synthase and vascular endothelial growth
factor in oral squamous cell carcinoma: Its correlation with
angiogenesis and disease progression. J Oral Pathol Med.
34:134–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salven P, Heikkila P, Anttonen A, Kajanti
M and Joensuu H: Vascular endothelial growth factor in squamous
cell head and neck carcinoma: Expression and prognostic
significance. Mod Pathol. 10:1128–1133. 1997.PubMed/NCBI
|
|
38
|
Tae K, El-Naggar AK, Yoo E, Feng L, Lee
JJ, Hong WK, Hittelman WN and Shin DM: Expression of vascular
endothelial growth factor and microvessel density in head and neck
tumorigenesis. Clin Cancer Res. 6:2821–2828. 2000.PubMed/NCBI
|
|
39
|
Baillie R, Harada K, Carlile J, Macluskey
M, Schor SL and Schor AM: Expression of vascular endothelial growth
factor in normal and tumour oral tissues assessed with different
antibodies. Histochem J. 33:287–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Levy NS, Chung S, Furneaux H and Levy AP:
Hypoxic stabilization of vascular endothelial growth factor mRNA by
the RNA-binding protein HuR. J Biol Chem. 273:6417–6423. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grugel S, Finkenzeller G, Weindel K,
Barleon B and Marmé D: Both v-Ha-Ras and v-Raf stimulate expression
of the vascular endothelial growth factor in NIH 3T3 cells. J Biol
Chem. 270:25915–25919. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rak J, Filmus J, Finkenzeller G, Grugel S,
Marmé D and Kerbel RS: Oncogenes as inducers of tumor angiogenesis.
Cancer Metastasis Rev. 14:263–277. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rak J, Mitsuhashi Y, Bayko L, Filmus J,
Shirasawa S, Sasazuki T and Kerbel RS: Mutant ras oncogenes
upregulate VEGF/VPF expression: Implications for induction and
inhibition of tumor angiogenesis. Cancer Res. 55:4575–4580.
1995.PubMed/NCBI
|
|
44
|
Tsai ST and Jin YT: Proliferating cell
nuclear antigen (PCNA) expression in oral squamous cell carcinomas.
J Oral Pathol Med. 24:313–315. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kato K, Kawashiri S, Yoshizawa K, et al:
Expression form of p53 and PCNA at the invasive front in oral
squamous cell carcinoma: Correlation with clinicopathological
features and prognosis. J Oral Pathol Med. 40:693–698. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Millanta F, Silvestri G, Vaselli C, et al:
The role of vascular endothelial growth factor and its receptor
Flk-1/KDR in promoting tumour angiogenesis in feline and canine
mammary carcinomas: A preliminary study of autocrine and paracrine
loops. Res Vet Sci. 81:350–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Restucci B, Papparella S, Maiolino P and
De Vico G: Expression of vascular endothelial growth factor in
canine mammary tumors. Vet Pathol. 39:488–493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Breward CJW, Byrne HM and Lewis CE:
Modelling the interactions between tumour cells and a blood vessel
in a microenvironment within a vascular tumour. Eur J Appl Math.
12:529–556. 2001. View Article : Google Scholar
|
|
49
|
de Jong JS, van Diest PJ, van der Valk P
and Baak JP: Expression of growth factors, growth-inhibiting
factors and their receptors in invasive breast cancer. II:
Correlations with proliferation and angiogenesis. J Pathol.
184:53–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rosen LS: VEGF-targeted therapy:
Therapeutic potential and recent advances. Oncologist. 10:382–391.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bjorndahl M, Cao R, Eriksson A and Cao Y:
Blockage of VEGF-induced angiogenesis by preventing VEGF secretion.
Circ Res. 94:1443–1450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, et al: Inhibition of vascular endothelial growth factor-induced
angiogenesis suppresses tumour growth in vivo. Nature.
362:841–844. 1993. View Article : Google Scholar : PubMed/NCBI
|