Introduction
Thymoma is an epithelial tumor of the thymus that is
characterized by indolent growth with local invasiveness and is the
most common type of tumor in the anterior portion of the
mediastinum (1). The overall
incidence of thymoma is rare, with ~0.15 cases per 100,000
cases/year (2,3). Thymoma had previously been regarded as a
benign disease, but more recent evidence indicated that it is a
potentially malignant tumor requiring prolonged follow-up (4). However, biomarkers for thymoma diagnosis
and prognosis have not yet been established.
Slug is a member of the Snail family of zinc-finger
transcription factors and was first identified in the neural crest
and developing mesoderm of chicken embryos (5). Slug induces the downregulation of
E-cadherin, an adhesion molecule, leading to the breakdown of
cell-cell adhesions and the acquisition of invasive growth
properties in cancer cells (6). These
changes facilitate an increase in spindle morphology and cellular
invasion (7–9). Previous studies have demonstrated that
Slug expression is associated with poor prognosis in colorectal
carcinoma, esophageal cancer and breast carcinoma (10–13);
however, the clinical relevance of Slug expression in thymoma
remains unknown.
The aims of the present study were to examine Slug
expression in thymoma and to determine whether the degree of Slug
expression correlates with prognosis.
Patients and methods
Patients and specimens
Ethical approval for the present study was obtained
from the medical ethics committee of the Affiliated Hospital of
Qingdao University (Qingdao, China), and the study was performed in
accordance with the institutional guidelines. Written informed
consent was obtained from each patient prior to tissue
acquisition.
Tissue specimens were obtained from 100 patients
with thymoma who underwent thymectomy between January 1997 and
December 2003 at the Affiliated Hospital of Qingdao University.
Thymomas were classified according to the World Health Organization
(WHO) criteria (14), and clinical
stages are based on the Masaoka staging system (15). In addition, normal thymus tissues were
obtained from 60 patients with mediastinal cysts who underwent
cystectomy at the same institution between January 1997 and
December 2003, and were set as the control group (Tables I and II). The normal tissue specimens were
obtained from adjacent thymus tissues based on histological
evidence of the surgically resected mediastinal cyst. Follow-up for
all patients following discharge included an X-ray examination or
computed tomography scan every 3–6 months. Postoperative follow-up
data were obtained from all patients, and the median follow-up
period was 99.7 months (range, 3–120 months).
 | Table I.Clinical characteristics of thymoma
patients and controls (patients with mediastinal cysts). |
Table I.
Clinical characteristics of thymoma
patients and controls (patients with mediastinal cysts).
| Characteristic | Thymoma patients
(n=100) | Controls (n=60) | P-value |
|---|
| Mean age, years
(±SD) | 53.42±12.18 | 48.93±10.17 | 0.078a |
| Gender, n |
|
| 0.902b |
| Male | 54 | 33 |
|
|
Female | 46 | 27 |
|
 | Table II.Slug expression in relation to
clinicopathological findings. |
Table II.
Slug expression in relation to
clinicopathological findings.
|
|
| Slug |
|
|---|
|
|
|
|
|
|---|
| Variables | Total, n | High-level group,
n | Low-level group,
n | P-value |
|---|
| Mean age, years
(±SD) | 53.42±12.18 | 50.74±12.76 | 55.53±12.97 | 0.069 |
| Gender |
|
|
| 0.592 |
| Male | 54 | 24 | 30 |
|
|
Female | 46 | 18 | 28 |
|
| Stage |
|
|
| <0.001 |
| I | 48 | 9 | 39 |
|
| II | 19 | 9 | 10 |
|
| III | 26 | 19 | 7 |
|
| IV | 7 | 5 | 2 |
|
| Classification |
|
|
| 0.013 |
| A, AB,
B1 | 55 | 17 | 38 |
|
| B2, B3,
C | 45 | 25 | 20 |
|
| MG |
|
|
| 0.478 |
| No | 80 | 35 | 45 |
|
|
Yes | 20 | 7 | 13 |
|
Immunohistochemical staining and
evaluation
The primary specimens were fixed in 10% formaldehyde
and routinely embedded in paraffin. Next, 4-µm sections were
prepared and immunohistochemical staining was performed using the
streptavidin-biotin peroxidase method, as described previously
(16). Briefly, following
deparaffinization and hydration, the sections were washed in
phosphate-buffered saline (PBS; 3 times, for 3 min each time), and
the washed sections were treated with 3% hydrogen peroxide in the
dark for 15 min. The sections were washed initially in distilled
water and then in PBS [3 times, for 5 min each time (3×5 min)].
Antigen retrieval was performed in Tris-ethylenediaminetetraacetic
acid (EDTA; 10 mM Tris, 1 mM EDTA, pH 8.0) at 120°C for 2 min, and
the sections were preincubated in PBS (3×5 min). Next, the sections
were incubated with a rabbit anti-human Slug polyclonal antibody
(dilution, 1:100; Slug H-140; catalog no. sc-15391; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C. After
washing in PBS (3×5 min), the sections were incubated with the
secondary antibody (dilution, 1:1,000; Polink-2 HRP; catalog no.
d22–110; Golden Bridge International, Inc., Bothell, WA, USA) at
room temperature for 30 min. The sections were washed again in PBS
(3×5 min), treated with 3,3′-diaminobenzidine working solution at
room temperature for 10 min and then washed in distilled water.
Two pathologists performed independent
immunohistochemical evaluation using the following criteria:
Positive Slug expression was defined as a detectable immunoreaction
in either the nucleus or cytoplasm. The staining intensity was
graded between 0 and 3 (0, negative; 1, weak; 2, moderate; 3,
strong), and the extent of staining was graded between 0 and 4 (0,
no staining; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%). The
overall staining was scored by multiplying the intensity of the
staining (when viewed at ×200 magnification; BX41 Olympus
microscope; Olympus Corporation, Tokyo, Japan) by the extent of
staining (when viewed at ×40 magnification). Therefore, the
staining scores ranged between 0 and 12.
Statistical analysis
The χ2 test and t-test were used to
calculate differences between the thymoma group and the control
group. The overall survival rates were determined using the
Kaplan-Meier method, and group differences were calculated using
the log-rank test. Prognostic factors were examined by univariate
analysis using the Kaplan-Meier method and by multivariate analysis
using the Cox proportional hazards regression model. P-values were
two-sided, and P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software, version 18.0 (SPSS Inc., Chicago, IL,
USA).
Results
Expression of Slug in normal thymus
and in thymoma tissues
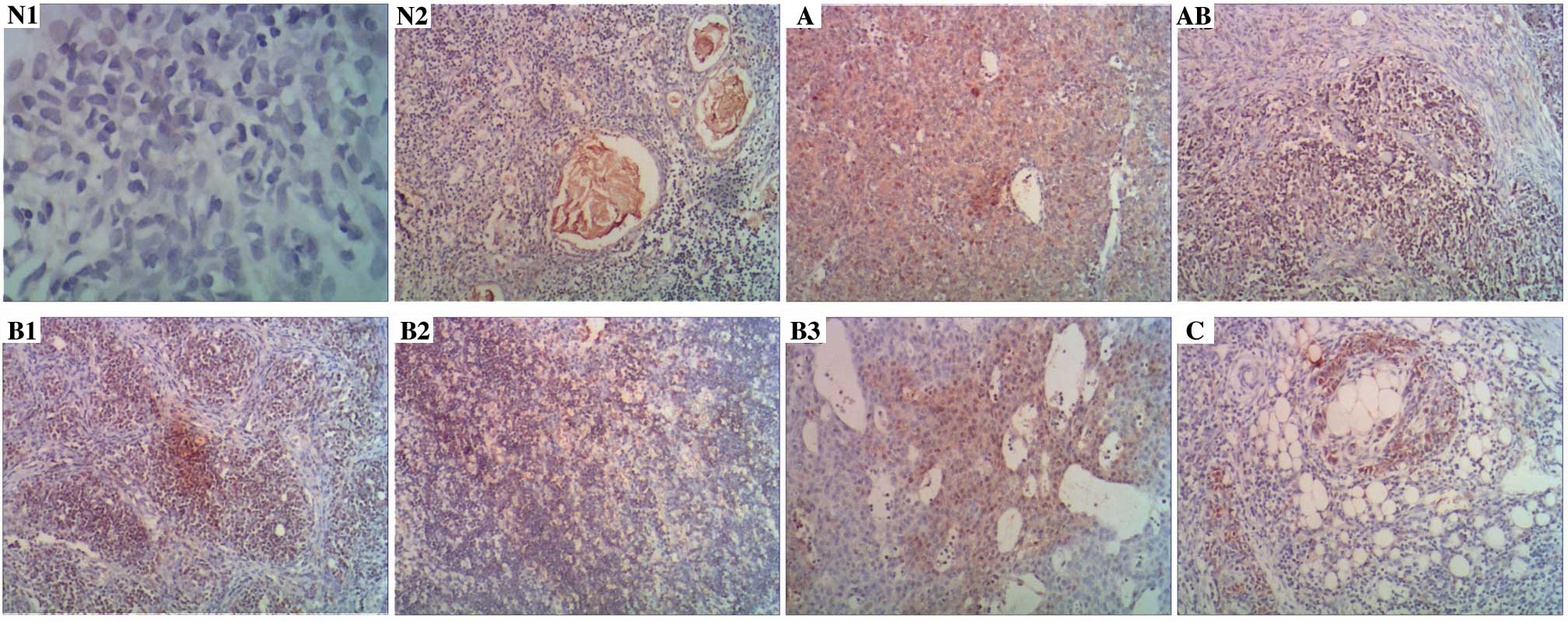
Slug was expressed in the cytoplasm of normal thymus
tissues in 9/60 specimens (15.0%; Fig.
1, tissues N1 and N2), and Slug was expressed in the cytoplasm
or nucleus of thymoma tissues in 51/100 specimens (51.0%; Fig. 1, tissues classified as A, AB, B1, B2,
B3 and C). Notably, cytoplasmic Slug expression was significantly
increased in thymoma tissues compared to that observed in normal
thymus tissues (P<0.001; Table
III).
 | Table III.Slug expression in normal thymus
tissues and thymoma tissues. |
Table III.
Slug expression in normal thymus
tissues and thymoma tissues.
|
| Slug |
|
|
|---|
|
|
|
|
|
|---|
| Tissues | Positive, n
(%) | Negative, n
(%) | Total, n | P-value |
|---|
| Normal thymus | 9
(15.0) | 51 (85.0) | 60 | <0.001 |
| Thymoma | 51 (51.0) | 49 (49.0) | 100 |
|
Slug expression and
clinicopathological characteristics
To evaluate the association between
clinicopathological factors and Slug expression in thymoma, the
specimens were divided based on staining scores into the low-level
(score ≤3, including negative expression) and high-level groups
(score >3; Table II). The Masaoka
stages and WHO classifications of the high-level group were
significantly different compared with those of the low-level group.
Patients with advanced stage thymoma exhibited increased levels of
Slug expression compared with patients with early-stage thymoma
(P<0.001). Furthermore, type B2, B3 and C thymomas exhibited
increased levels of Slug expression compared with type A, AB and B1
thymomas (P=0.013). Slug expression did not correlate with age,
gender or myasthenia gravis (all P>0.05).
Association between prognosis and Slug
expression
The follow-up time was >120 months, during which
1 patient succumbed to a heart attack and another 2 patients did
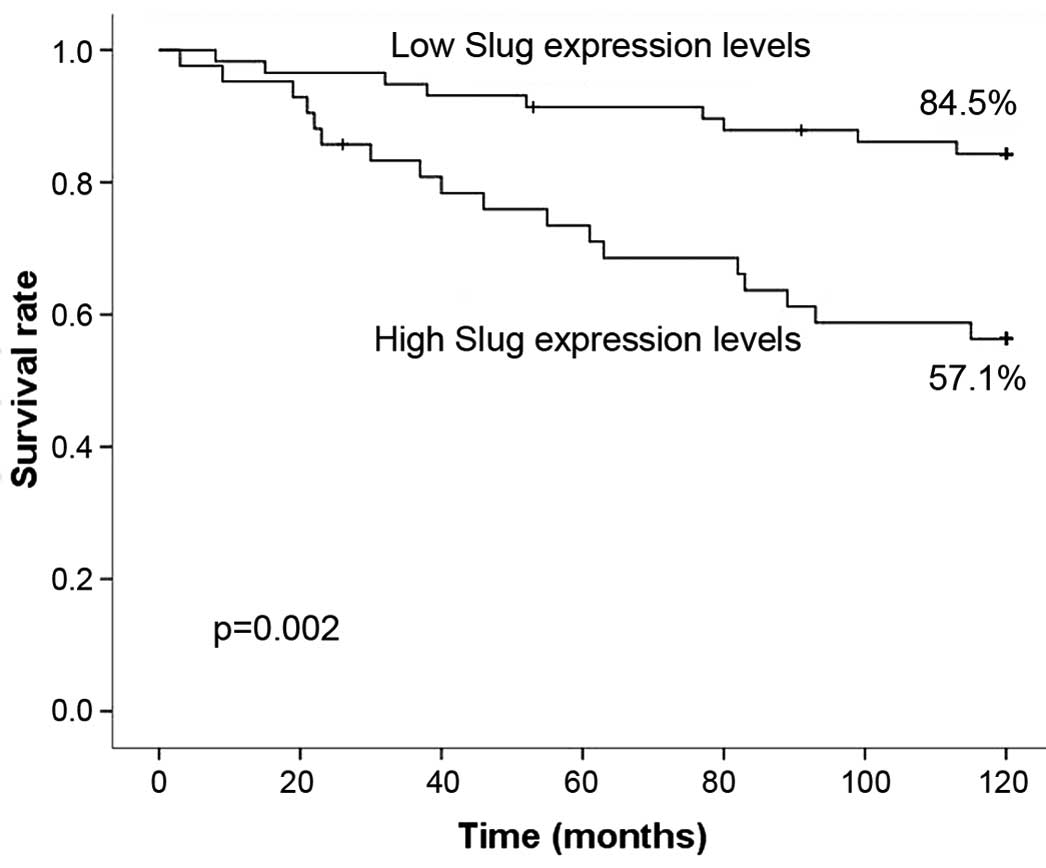
not complete the follow-up. Patients in the low-level group
exhibited a significantly increased 5-year survival rate of 91.4%
(53/58) compared with 73.8% (31/42) for patients in the high-level
group (P=0.016; Fig. 2). Patients
with low Slug expression levels also exhibited a significantly
increased 10-year survival rate of 84.5% (49/58) compared with a
rate of 57.1% (24/42) for patients with high Slug expression levels
(P=0.002; Fig. 2).
Univariate and multivariate analyses
of prognostic factors
Univariate analysis demonstrated that overexpression
of Slug, the Masaoka stage and WHO classification of the tumor were
significantly correlated with patient survival (P<0.05; Table IV). Multivariate analysis using the
Cox proportional hazards regression model indicated that the
Masaoka stage and WHO classification, but not Slug expression, were
independent prognostic factors in patients with thymoma (Table V).
 | Table IV.Univariate analyses of prognostic
factors in thymoma. |
Table IV.
Univariate analyses of prognostic
factors in thymoma.
| Variables | Total, n | 10-year survival
rate, % | P-value |
|---|
| Gender |
|
| 0.642 |
|
Male | 54 | 75.9 |
|
|
Female | 46 | 69.6 |
|
| Group |
|
| <0.001 |
|
Positive | 51 | 56.9 |
|
|
Negative | 49 | 89.8 |
|
| Stage |
|
| <0.001 |
| I and
II | 67 | 89.6 |
|
| III and
IV | 33 | 39.4 |
|
| Classification |
|
| <0.001 |
| A, AB,
B1 | 55 | 94.5 |
|
| B2, B3,
C | 45 | 46.7 |
|
| MG |
|
| 0.812 |
| No | 80 | 75.0 |
|
|
Yes | 20 | 72.5 |
|
 | Table V.Multivariate analyses of prognostic
factors in thymoma patients. |
Table V.
Multivariate analyses of prognostic
factors in thymoma patients.
| Independent
factors | P-value | HR | 95% CI |
|---|
| Stage (I, II/III,
IV) | 0.026 | 0.328 | 0.123–0.872 |
| Classification (A,
AB, B1/B2, B3, C) | 0.003 | 0.143 | 0.040–0.507 |
| Group
(positive/negative) | 0.089 | 0.401 | 0.139–1.151 |
Discussion
At present, thymoma is regarded as a potentially
malignant tumor with a growth pattern that ranges from indolent to
highly invasive and metastatic (4).
Disruption of cellular activities that are important for normal
embryonic development and for the maintenance of proper function
and structure results in the loss of tissue differentiation and
facilitates invasion and metastasis (10). Therefore, certain epithelial markers
and adhesion molecules, including E-cadherin and Slug, are
important for tumor progression and development (17).
Epithelial-mesenchymal transition (EMT), a process
through which epithelial cells lose their polarity and switch to a
migratory sarcomatoid phenotype, is a critical event during
malignant tumor progression and metastasis (18). A hallmark of EMT is the loss of
expression of the cell adhesion molecule E-cadherin, and several
EMT regulators have been identified as E-cadherin repressors
(19). Slug is a member of the Snail
family of transcription factors and is crucial in the regulation of
EMT by suppressing E-cadherin, therefore triggering complete EMT
and the acquisition of invasive and tumorigenic properties
(10,20). The aim of the present study was to
investigate the Slug expression patterns in thymomas in addition to
the association between Slug expression levels and prognosis.
A previous study indicated that Slug is expressed in
the thymus, heart, liver, lung and pancreas in humans (21). Using immunohistochemistry, the present
study demonstrated that 15% of normal thymus tissues expressed Slug
at low levels. Previous studies demonstrated that Slug expression
correlates with prognosis in patients with esophageal squamous cell
carcinoma, gastric cancer and colorectal carcinoma (10,11,22). To
the best of our knowledge, this is the first study on the
expression and clinical significance of Slug in thymoma.
Slug has an anti-apoptotic effect in leukemia cells
(23,24), and downregulates epithelial markers,
including Muc-1, desmoplakin and cytokeratin-18 (25,26). The
downregulation of certain anti-apoptotic cadherins is associated
with poor prognosis in patients. E-cadherin is a major cell-cell
adhesion molecule that is critical for the development and
maintenance of cell polarity and tissue architecture (27,28).
Although E-cadherin expression was not analyzed in the present
study, previous studies have reported that Slug represses
E-cadherin in vivo and that E-cadherin expression is
associated with poor prognosis in esophageal squamous cell and
breast carcinomas (11,12).
The most common disease associated with thymoma is
myasthenia gravis, which is an autoimmune neuromuscular disease
caused by antibodies that block acetylcholine receptors in muscle,
resulting in muscle weakness (29,30). A
previous study revealed that the anti-KV1.4 antibody was
a predictor of prognosis in patients with thymoma-associated
myasthenia gravis (31). However, in
the current study, the expression of Slug was not associated with
myasthenia gravis.
While Slug expression was significantly associated
with patient survival in the univariate analysis, it was not an
independent prognostic factor in the multivariate analysis.
However, the overall survival rate of thymoma patients with high
levels of Slug expression was lower compared with thymoma patients
with low levels of Slug expression.
There are certain limitations in the present study,
including the long observation period (leading to the loss of
patients to follow-up) and the relatively small number of patients.
Thus, these findings should be tested and validated in a larger
scale study that includes more patients.
In conclusion, Slug expression was found to be
associated with prognosis, Masaoka stage and WHO classification,
while overexpression of Slug correlated with poor prognosis in
patients with thymoma. Therefore, Slug may serve as a predictor of
poor prognosis in patients with thymoma and as a diagnostic
biomarker of thymoma. In addition, Slug may be a potential target
for the treatment of thymoma.
References
|
1
|
Chen G, Marx A, Chen WH, Yong J, Puppe B,
Stroebel P and Mueller-Hermelink HK: New WHO histologic
classification predicts prognosis of thymic epithelial tumors: A
clinicopathologic study of 200 thymoma cases from China. Cancer.
95:420–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venuta F, Anile M, Diso D, Vitolo D,
Rendina EA, De Giacomo T, Francioni F and Coloni GF: Thymoma and
thymic carcinoma. Eur J Cardiothorac Surg. 37:13–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wright CD: Management of thymomas. Crit
Rev Oncol Hematol. 65:109–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evoli A, Minisci C, Di Schino C, Marsili
F, Punzix C, Batocchi AP, Tonali PA, Doglietto GB, Granone P,
Trodella L, et al: Thymoma in patients with MG: Characteristics and
long-term outcome. Neurology. 59:1844–1850. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieto MA, Sargent MG, Wilkinson DG and
Cooke J: Control of cell behavior during vertebrate development by
Slug, a zinc finger gene. Science. 264:835–839. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang HW, Menon LG, Black PM, Carroll RS
and Johnson MD: SNAI2/Slug promotes growth and invasion in human
gliomas. BMC Cancer. 10:3012010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vesuna F, van Diest P, Chen JH and Raman
V: Twist is a transcriptional repressor of E-cadherin gene
expression in breast cancer. Biochem Biophys Res Commun.
367:235–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shioiri M, Shida T, Koda K, et al: Slug
expression is an independent prognostic parameter for poor survival
in colorectal carcinoma patients. Br J Cancer. 94:1816–1822. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uchikado Y, Natsugoe S, Okumura H,
Setoyama T, Matsumoto M, Ishigami S and Aikou T: Slug Expression in
the E-cadherin preserved tumors is related to prognosis in patients
with esophageal squamous cell carcinoma. Clin Cancer Res.
11:1174–1180. 2005.PubMed/NCBI
|
|
12
|
Hajra KM, Chen DY and Fearon ER: The SLUG
zinc-finger protein represses E-cadherin in breast cancer. Cancer
Res. 62:1613–1618. 2002.PubMed/NCBI
|
|
13
|
Hasan MR, Sharma R, Saraya A,
Chattopadhyay≈ TK, Gupta Datta S, Walfish PG, Chauhan SS and Ralhan
R: Slug is a predictor of poor prognosis in esophageal squamous
cell carcinoma patients. PLoS One. 8:e828462013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Travis WD, Brambilla E, Müller-Hermelin HK
and Harris CC: Pathology and Genetics. Tumours of the lung, pleura,
thymus and heart. World Health Organisation Classification of
Tumors (Lyon). IARC Press. 142–143. 2004.
|
|
15
|
Masaoka A, Monden Y, Nakahara K and
Tanioka T: Follow-up study of thymomas with special reference to
their clinical stages. Cancer. 48:2485–2492. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugimachi K, Aishima S, Taguchi K, et al:
The role of overexpression and gene amplification of cyclin D1 in
intrahepatic cholangiocarcinoma. J Hepatol. 35:74–79. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barker N and Clevers H: Tumor environment:
A potent driving force in colorectal cancer? Trends Mol Med.
7:535–537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alves CC, Carneiro F, Hoefler H and Becker
KF: Role of the epithelial-mesenchymal transition regulator Slug in
primary human cancers. Front Biosci (Landmark Ed). 14:3035–3050.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hemavathy K, Guru SC, Harris J, Chen JD
and Ip YT: Human Slug is a repressor that localizes to sites of
active transcription. Mol Cell Biol. 20:5087–5095. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchikado Y, Okumura H, Ishigami S, et al:
Increased Slug and decreased E-cadherin expression is related to
poor prognosis in patients with gastric cancer. Gastric Cancer.
14:41–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hemavathy K, Ashraf SI and Ip YT:
Snail/slug family of repressors: Slowly going into the fast lane of
development and cancer. Gene. 257:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inukai T, Inoue A, Kurosawa H, Goi K,
Shinjyo T, Ozawa K, Mao M, Inaba T and Look AT: SLUG, a
ces-1-related zinc finger transcription factor gene with
antiapoptotic activity, is a downstream target of the E2A-HLF
oncoprotein. Mol Cell. 4:343–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guaita S, Puig I, Franci C, Garrido M,
Dominguez D, Batlle E, Sancho E, Dedhar S, De Herreros AG and
Baulida J: Snail induction of epithelial to mesenchymal transition
in tumor cells is accompanied by MUC1 repression and ZEB1
expression. J Biol Chem. 277:39209–39216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frixen UH, Behrens J, Sachs M, Eberle G,
Voss B, Warda A, Löchner D and Birchmeier W: E-cadherin-mediated
cell-cell adhesion prevents invasiveness of human carcinoma cells.
J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirohashi S: Inactivation of the
E-cadherin-mediated cell adhesion system in human cancers. Am J
Pathol. 153:333–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grob D, Brunner N, Namba T and Pagala M:
Lifetime course of myasthenia gravis. Muscle Nerve. 37:141–149.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vincent A, Willcox N, Hill M, Curnow J,
MacLennan C and Beeson D: Determinant spreading and immune
responses to acetylcholine receptors in myasthenia gravis. Immunol
Rev. 164:157–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki S, Nishimoto T, Kohno M, Utsugisawa
K, Nagane Y, Kuwana M and Suzuki N: Clinical and immunological
predictors of prognosis for Japanese patients with
thymoma-associated myasthenia gravis. J Neuroimmunol. 258:61–66.
2013. View Article : Google Scholar : PubMed/NCBI
|