Introduction
Hepatocellular carcinoma (HCC) is the fifth most
frequently diagnosed malignancy and the third most common cause of
cancer-associated mortality globally (1). Previous evidence has suggested the
existence of marked geographic variation, with a high prevalence of
HCC in East Asian countries, particularly China (2). Although significant advances have been
made with regard to surgical resection, ablation and chemotherapy
(3), the prognosis of HCC remains
unfavorable. As our understanding of the molecular mechanisms
underlying the initiation and progression of HCC increases,
targeted therapy has become a promising alternative to currently
used treatments, representing a landmark in drug development and a
significant step towards the development of personalized medicine
for the treatment of HCC (4).
However, the efficacy of existing targeted drugs has plateaued in
recent years due to increasing drug resistance (5). Therefore, the discovery of novel
molecular targets is urgently required for the development of more
effective and efficient therapeutic agents.
In general, receptor tyrosine kinases (RTKs), which
are crucial for signal transduction, are dysregulated in a diverse
range of tumor types (6). The
anaplastic lymphoma kinase (ALK) gene, a proto-oncogene that
encodes a transmembrane RTK, was initially identified in anaplastic
large cell lymphomas carrying an abnormal t(2;5)(p23;q35)
translocation (7). Subsequently, its
fusion variants were additionally identified in inflammatory
myofibroblastic tumors (8) and
diffuse large B-cell lymphomas (9).
The human ALK gene has a significant role in brain and
neuronal development during embryogenesis, however, is
downregulated in adults (10). It is
well-known that genetic abnormalities involving ALK include
translocation (also known as ‘rearrangement’), amplification and
mutation, as well as ALK overexpression (11,12). DNA
amplification and point mutations were originally identified in
neuroblastoma, indicating that the ALK gene may possess high
carcinogenic and neoplastic potential (13). In addition, ALK fusion
oncogenes, resulting from chromosomal translocations, proved to be
the most commonly observed ALK aberrations in cancer, and
were able to induce constant ALK kinase activity via their
own constitutive self-association, as well as acting as an
oncogenic addiction pathway (14). A
number of specific small-molecule ALK inhibitors are capable
of efficiently suppressing such activity (15).
Echinoderm microtubule-associated protein-like 4
(EML4)-ALK fusion variant was initially reported in 2007, as an
oncological driver in non-small cell lung carcinoma (NSCLC)
(16), and this led to a focus on the
development of novel targeted agents for cancer diagnosis and
therapy (11,15). Subsequently, the success of crizotinib
(PF-02341066), a targeted agent against EML4-ALK, in the treatment
of advanced ALK-rearranged NSCLCs, led to an interest in the
significance of ALK status in various other epithelial
malignancies, and represented the beginning of a novel era of the
targeting of ALK abnormalities for therapeutic purposes in
solid tumors (17). Aberrant
ALK genes have been reported in various solid tumors (often
in addition to hematological malignancies), including esophageal
(18), breast (19), colorectal (20) and renal carcinoma (21), indicating an association between
ALK abnormalities and human cancer development. However,
little research has been conducted to evaluate the abnormalities
and clinical significance of the ALK gene in HCC.
In order to improve our current knowledge, the
present study comprehensively detected ALK gene status in a
large HCC cohort, and investigated whether ALK abnormalities
are associated with patient clinicopathological features and
prognosis.
Materials and methods
Clinical specimens and follow-up
A total of 342 HCC tumor specimens and corresponding
normal non-cancerous tissues were obtained from patients who had
undergone surgical resection at Guangdong General Hospital
(Guangzhou, China), between June 2005 and October 2010. For the
performance of immunohistochemistry (IHC) and fluorescence in
situ hybridization (FISH) assays, sections of the resected
malignant tissues were fixed using 10% formalin and subsequently
embedded in paraffin, followed by longitudinal slicing into 4-µm
thick serial sections. The remaining HCC tissues were frozen using
liquid nitrogen and stored at −80°C, until required for intensive
investigation by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and rapid amplification of complementary
DNA (cDNA) ends (RACE)-coupled PCR sequencing. All patients had not
received any anticancer therapy prior to surgery. Micrometastases
were defined in accordance with the criteria proposed by Hu et
al (22). Disease staging was
based on the tumor-node-metastasis staging system of the Union for
International Cancer Control (23).
Clinical history was extracted from patient medical records. All
patients received post-operative follow-up by telephone every 3
months for the initial 2 years of the study, and subsequently every
6 months until mortality or the study endpoint was reached. Overall
survival (OS) and progression-free survival (PFS) were measured
from the date of surgery until mortality/censoring or local
recurrence/distant metastasis/censoring, respectively. The survival
analysis utilized in the present study was designed to compare OS
and PFS among patients. Each participant signed written informed
consent for the use of their resected tissues and personal
information for research purposes, and approval was obtained from
the Ethics Committee of Guangdong General Hospital (Guangzhou,
China).
ALK IHC analysis
ALK IHC staining was performed as previously
described (24). In brief, unstained
slides were successively submerged in xylene (Guangzhou Yikang
Biological Science Technology Co., Ltd., Guangzhou, China), graded
alcohol series and tap water for deparaffinization. Following
deparaffinization, the intrinsic peroxidase activity of samples was
inhibited using 3% hydrogen peroxide. Antigen retrieval was
performed by microwave heating at 95°C, following submersion of the
sections in ethylenediaminetetraacetic acid buffer (pH 8.0;
Shanghai Xibao Biological Technology Ltd., Shanghai, China). The
samples were incubated with rabbit monoclonal anti-human ALK
[D5F3®; Cell Signaling Technology, Inc., Danvers, MA, USA; 1:50
dilution in antibody diluent (Dako, Glostrup, Denmark)] at 4°C
overnight; non-specific protein binding sites were blocked with 5%
goat serum (Shanghai Xibao Biological Technology Ltd.). Subsequent
to being washed, immunoreaction was visualized using biotinylated
goat anti-rabbit IgG secondary polyclonal antibodies (dilution,
1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1
h, and thereafter, streptavidin-horseradish-peroxidase complex
(Shanghai Xibao Biological Technology, Ltd.) and 3-amino-9-ethyl
carbazole (Shanghai Xibao Biological Technology, Ltd.) were added.
Finally, all samples were counterstained using hematoxylin
(Shanghai Xibao Biological Technology, Ltd.), dehydrated and sealed
with cover slips for microscopic examination (CX31; Olympus
Corporation, Tokyo, Japan). Two experienced investigators, who were
blinded to the patient clinicopathological profiles, confirmed the
immunostaining levels separately. The scoring scheme utilized to
assess staining was as follows: 0, absent staining; 1+, weak
cytoplasmic staining; 2+, moderate and smooth cytoplasmic staining,
and 3+, strong and granular cytoplasmic staining in ≥10% of tumor
cells (24).
ALK FISH analysis
FISH, a gold standard for confirming gene status,
was applied to 342 unstained HCC samples with the Vysis ALK
Break Apart FISH Probe kit (Abbott Laboratories, Chicago, IL, USA)
according to standard FISH protocols. Following deparaffinization,
dehydration and treatment with citric acid, the tumor sections were
washed with phosphate-buffered saline, followed by dehydration in
alcohol and air-drying at 37°C. Subsequently, the slides were added
to 5 µl of diluted ALK probe and denatured. The probe was
hybridized and the slides were washed. The reagent was comprised of
two DNA probes labeled with Spectrum Orange (red) and Spectrum
Green (green), which were able to test various genetic
rearrangements of ALK at 2p23, by hybridizing and breakpoint
flanking. FISH signals were evaluated under a fluorescence
microscope (IX72; Olympus Corporation) using an oil immersion
objective. Two pathologists independently analyzed all FISH
results. ALK translocation positivity was defined as the
separation of red and green signals, or a single red signal, in at
least 15% of analyzed cells. However, since the criteria for gain
of ALK copy number have not been established, the present
study adopted the previously published cut-off values for
colorectal cancer in the current analysis (25). Briefly, the positivity for gain of
ALK gene copy number was considered to be 3–5 copies of
ALK per cell in ≥10% of all detected cells, and
amplification was regarded to be ≥6 copies of ALK per cell
in ≥10% of all detected cells.
ALK RT-qPCR analysis
Total RNA was isolated from matching pairs of frozen
HCC tissue blocks with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocols. Each tissue was trimmed at low temperature to protect
the RNA from degradation. Prior to amplification, the purity and
concentration of the extracted RNA were assessed using gel
electrophoresis and absorbance at A260/A280. Reverse transcription
for cDNA was subsequently conducted with a cDNA synthesis kit
(Invitrogen; Thermo Fisher Scientific). cDNA was synthesized using
2.5 µg of total RNA as template and 1 mmol/l oligo(dT) primer in 25
µl of a solution that included 200 units of M-MLV reverse
transcriptase. Subsequently, 5 µl of cDNA was applied as the
template in a 20-µl reaction for RT-qPCR analysis with SYBR®
GreenER™ qPCR SuperMix (Invitrogen; Thermo Fisher Scientific) and
the ABI 7500 cycler (Applied Biosystems; Thermo Fisher Scientific)
under the following thermal cycling conditions: 95°C for 2 min, 40
cycles of 95°C for 15 sec and a final extension at 72°C for 5 min.
The remaining generated cDNA was stored at −20°C for further
RACE-coupled PCR sequencing. β-actin served as the internal
control. The primers were purchased from Shanghai GeneChem Co.,
Ltd. (Shanghai, China) and synthesized as follows: Sense,
5′-CCTGGAGCTGGTCATTACGA-3′ and antisense,
5′-TGGTTTGTGAAGGAGCCATT-3′. The melting curve of each sample was
analyzed in order to guarantee the product specification. As stated
in a previous study (26), RT-qPCR
analysis for ALK was defined as positive when the specimen
Cq value was <30.
RACE-coupled PCR sequencing
analysis
Cases with RT-qPCR positivity were selected for
RACE-coupled PCR sequencing analysis. Stored cDNA was purified
using a High Pure PCR Product Purification kit (Roche Diagnostics,
Indianapolis, IN, USA). Subsequently, reactions mixing purified
cDNA were initiated by incubation at boiling temperature followed
by incubation on ice. The following procedure was performed for
targeted capture of rearranged sequences using previously published
protocols (11). Following two
successive runs of PCR, amplification of desired cDNA segments
spanning exon 20 of the ALK gene and upstream sequences,
which may possess the transcript sequences of ALK fusion
partners, was achieved. The primers used were selected according to
the study by Zhang et al (11), and were obtained from Shanghai
GeneChem Co., Ltd. The first and second pairs of primers used were
as follows: Sense, 5′-CGCGTCCACTAGTACGGGGGGGGGG-3′ and antisense,
5′-GGCACCTCCTCGACGTCACTGATG-3′; and sense, 5′-GCGCACGGCTCCACTAGT-3′
and antisense, 5′-ACCAGGAAACAGCTATGACCGGTCTTGCCAGCAAAGCAGTAG-3′,
respectively. Following purification and labeling of the PCR
products with M13 sequencing primer, 5′-GAAACAGCTATGACC-3′, the
amplified fragments were sequenced using the Genetic Analyzer
3730×l (Applied Biosystems; Thermo Fisher Scientific). The
resulting files were aligned to the ALK reference sequence
(National Center for Biotechnology Information accession number,
NM_004304.3) in order to determine whether ALK mutations or
fusions were present.
Statistical analysis
The χ2 test was performed in order to
estimate the association between ALK protein expression and
clinicopathological features. Kaplan-Meier survival curves were
plotted with significance calculated using log-rank statistics.
Independent prognostic markers of OS and PFS were assessed using
univariate and multivariate proportional Cox models. P<0.05 was
considered to indicate a statistically significant difference. All
data analysis was performed using SPSS version 18.0 (SPSS Inc.,
Chicago, IL, USA).
Results
ALK protein is detectable using
IHC
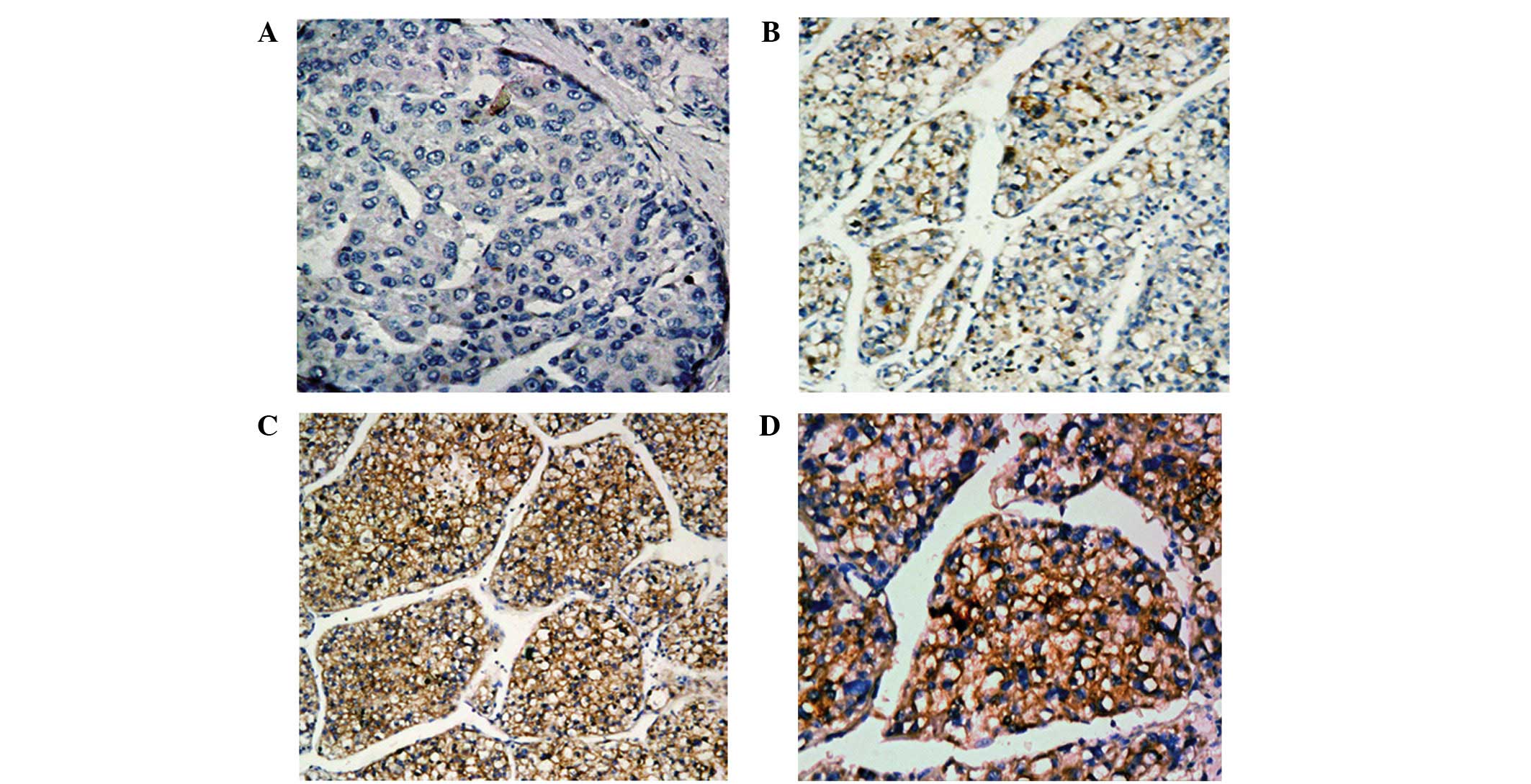
ALK protein expression was investigated using IHC
staining in all HCC biopsies (Fig.
1). In the normal non-cancerous (confirmed by histopathological
examination) biopsied samples adjacent to the tumor samples,
cytoplasmic ALK staining was absent or poor, and occurred in
very few cells (Fig. 1A). Of the 342
enrolled patients, 153 exhibited various degrees of brown
cytoplasmic staining in the majority of tumor cells (Fig. 1B–D).
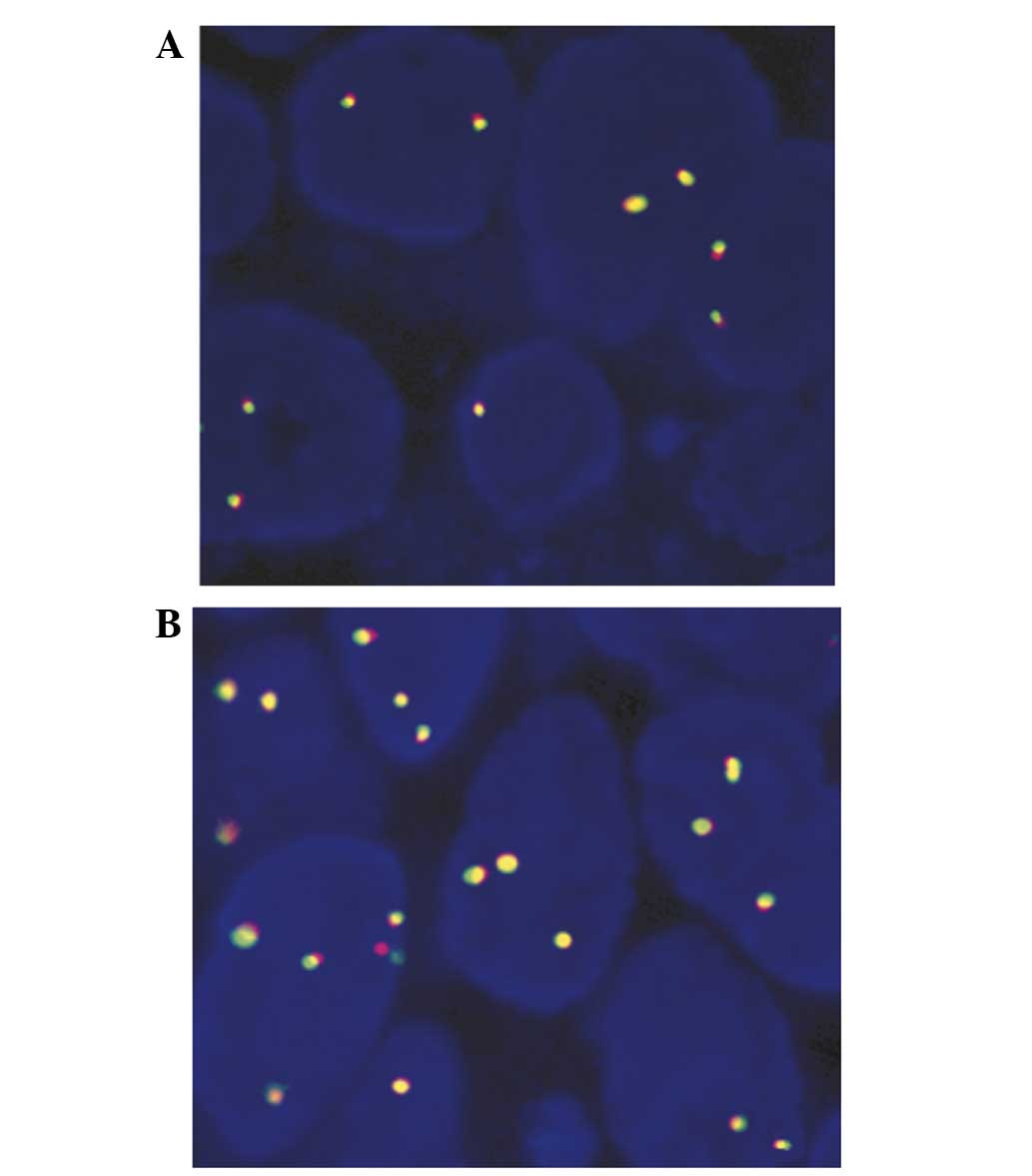
ALK status dectection via FISH
No HCC specimens demonstrated any evidence of
rearrangement or amplification in the ALK locus using a
break-apart FISH assay. However, in 112/342 images, gain of
ALK gene copy number was observed, which was regarded as
indicating FISH-positivity (Fig. 2A),
and the remaining samples were regarded to be FISH-negative
(Fig. 2B). Among the 112
FISH-positive patients, 108 were additionally positive for ALK
protein expression identified via IHC.
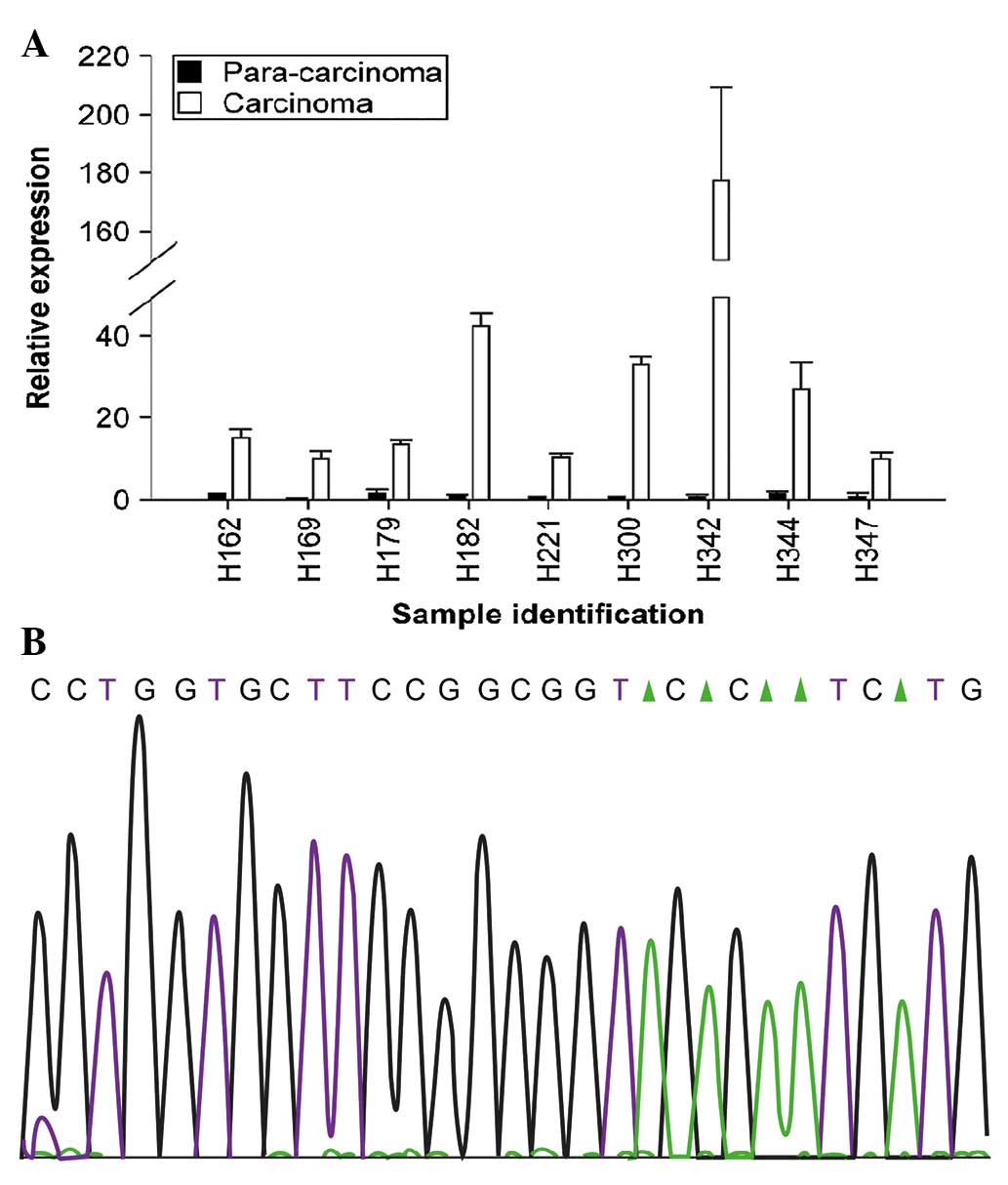
ALK status detection via RT-qPCR and
RACE-coupled PCR sequencing assays
In order to compare the levels of ALK
messenger RNA (mRNA) in 342 HCC specimens with that of adjacent
non-cancerous tissues, RT-qPCR was performed. The results of the
present study revealed that ALK mRNA was significantly
increased in 162 HCC samples. Notably, of the 162 cases exhibiting
RT-qPCR positivity, 139 were IHC-positive and 101 were additionally
FISH-positive (Table I). Fig. 3A illustrates nine representative
cases. Furthermore, ALK status was detected in 162 cases
that were RT-qPCR positive using RACE-coupled PCR sequencing.
Following alignment of the gene sequence with the ALK
reference sequence based on a previous study (11), no mutations or fusions with potential
partners were detected (Fig. 3B).
 | Table I.Concordance among IHC, FISH and
RT-qPCR results for anaplastic lymphoma kinase expression in 342
hepatocellular carcinoma patients. |
Table I.
Concordance among IHC, FISH and
RT-qPCR results for anaplastic lymphoma kinase expression in 342
hepatocellular carcinoma patients.
| RT-qPCR | FISH | IHC-positive
(n=153) | IHC-negative
(n=189) |
|---|
| Positive | Positive | 101 |
2 |
| Positive | Negative | 38 | 21 |
| Negative | Positive |
7 |
2 |
| Negative | Negative |
7 | 164 |
Certain clinicopathological features
are associated with ALK protein overexpression
The present study evaluated the association between
ALK gene expression and clinicopathological features of HCC
patients (Table II). All analyses
were performed according to IHC outcomes, as ALK protein was more
stable than DNA and RNA, which assured comparability among
histological specimens. The results of the present study indicated
that ALK protein expression was highly correlated with hepatitis C
virus (HCV) status (P<0.001) and micrometastases (P=0.011),
however, it was not significantly associated with patient age,
gender, hepatitis B surface antigen (HBsAg), α-fetoprotein (AFP),
cirrhosis, tumor size, tumor multiplicity, clinical stage, vascular
invasion, Child-Pugh classification, recurrence and lymph node
metastasis (P>0.05).
 | Table II.Correlation between
clinicopathological features and negative (n=189) and positive
(n=153) anaplastic lymphoma kinase (ALK) protein expression in
hepatocellular carcinoma patients. |
Table II.
Correlation between
clinicopathological features and negative (n=189) and positive
(n=153) anaplastic lymphoma kinase (ALK) protein expression in
hepatocellular carcinoma patients.
|
|
| ALK protein |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | n | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Age at diagnosis,
years |
|
|
|
|
|
<60 | 265 | 153
(57.7) | 112 (42.3) |
0.088 |
|
≥60 | 77 |
36 (46.8) | 41
(53.2) |
|
| Gender |
|
|
|
|
|
Male | 270 | 145
(53.7) | 126 (46.7) |
0.165 |
|
Female | 72 |
45 (62.5) | 27
(37.5) |
|
| Hepatitis B surface
antigen |
|
|
|
|
|
Negative | 81 |
45 (55.6) | 36
(44.4) |
0.952 |
|
Positive | 261 | 144
(55.2) | 117 (44.8) |
|
| Hepatitis C
virus |
|
|
|
|
|
Negative | 324 | 189
(58.3) | 135 (41.7) | <0.001 |
|
Positive | 18 |
0 (0.0) |
18 (100.0) |
|
| α-fetoprotein,
ng/ml |
|
|
|
|
|
<20 | 169 |
90 (53.3) | 79
(46.7) |
0.460 |
|
≥20 | 173 |
99 (57.2) | 74
(42.8) |
|
| Cirrhosis |
|
|
|
|
|
Absent | 155 |
90 (58.1) | 65
(41.9) |
0.343 |
|
Present | 187 |
99 (52.9) | 88
(47.1) |
|
| Tumor size, cm |
|
|
|
|
|
<5 | 189 | 108
(57.1) | 81
(42.9) |
0.437 |
| ≥5 | 153 |
81 (52.9) | 72
(47.1) |
|
| Tumor
multiplicity |
|
|
|
|
|
Single | 276 | 151
(54.7) | 125 (45.3) |
0.674 |
|
Multiple | 66 |
38 (57.6) | 28
(42.4) |
|
| Clinical stage |
|
|
|
|
|
I–II | 163 |
90 (55.2) | 73
(44.8) |
0.986 |
|
III–IV | 179 |
99 (55.3) | 80
(44.7) |
|
| Vascular
invasion |
|
|
|
|
| No | 210 | 117
(55.7) | 93
(44.3) |
0.832 |
|
Yes | 132 |
72 (54.5) | 60
(45.5) |
|
| Child-Pugh
score |
|
|
|
|
| A | 316 | 177
(56.0) | 139 (44.0) |
0.331 |
| B | 26 |
12 (46.2) | 14
(53.8) |
|
| Recurrence |
|
|
|
|
| No | 250 | 144
(57.6) | 106 (42.4) |
0.152 |
|
Yes | 92 |
45 (48.9) | 47
(51.1) |
|
| Lymph node
metastasis |
|
|
|
|
| No | 288 | 153
(53.1) | 135 (46.9) |
0.066 |
|
Yes | 54 |
36 (66.7) | 18
(33.3) |
|
|
Micrometastases |
|
|
|
|
| No | 297 | 172
(57.9) | 125 (42.1) |
0.011 |
|
Yes | 45 |
17 (37.8) | 28
(62.2) |
|
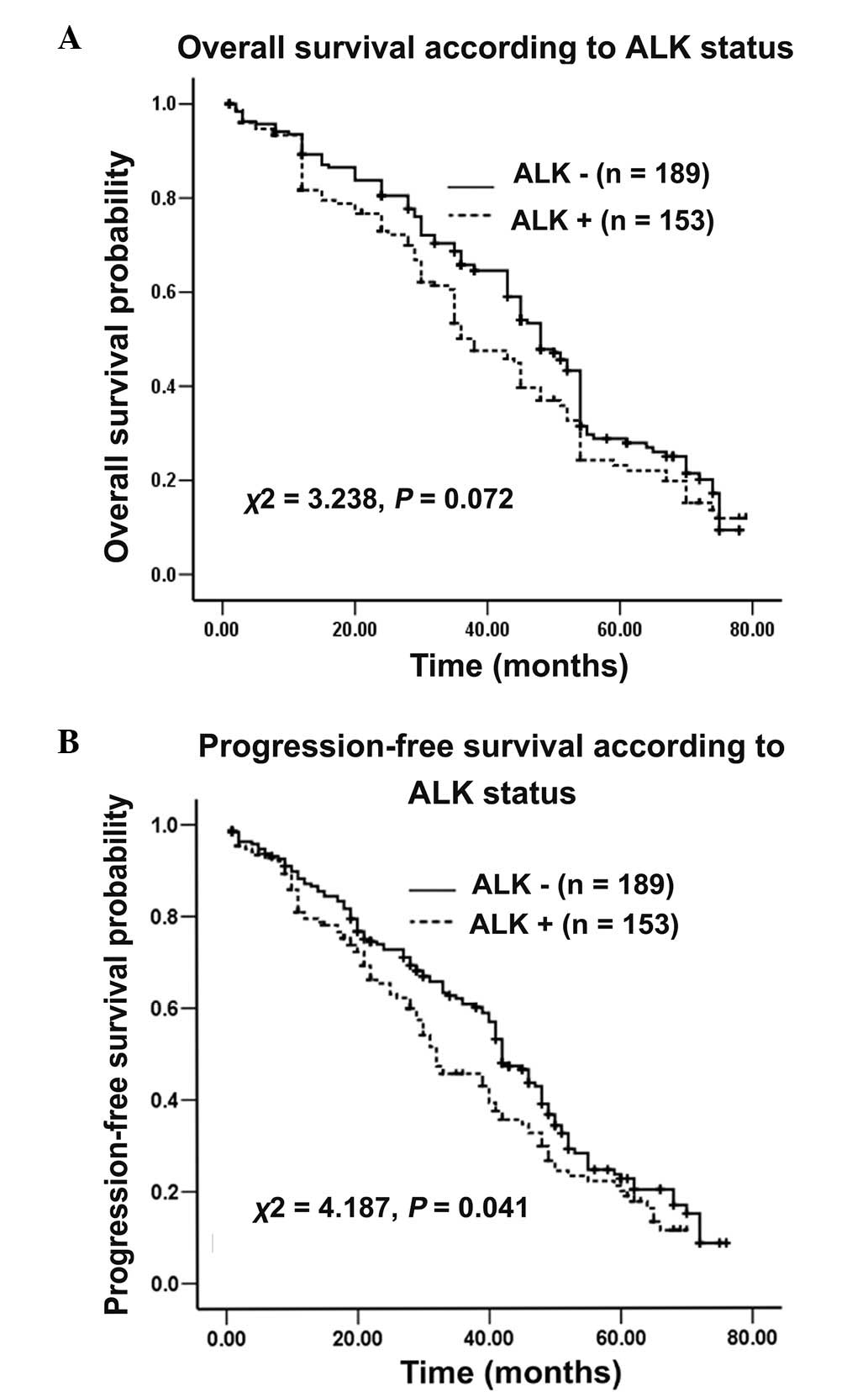
Survival analysis
By the cutoff day on June 1, 2013, 68.4% (234/342)
of the enrolled patients, all of whom exhibited recurrence or
uncontrolled HCC, had succumbed. The median follow-up duration was
36.0 months (range, 0.6–79 months), and 13 (3.8%) cases were lost
during the follow-up period. The potential effect of the ALK
gene on survival was assessed. In the total patient cohort, it was
observed that OS did not significantly differ between patients with
positive or negative ALK expression (χ2=3.238;
P=0.072; Fig. 4A. However, PFS for
patients with positive ALK expression was significantly
poorer compared with that of patients with negative ALK
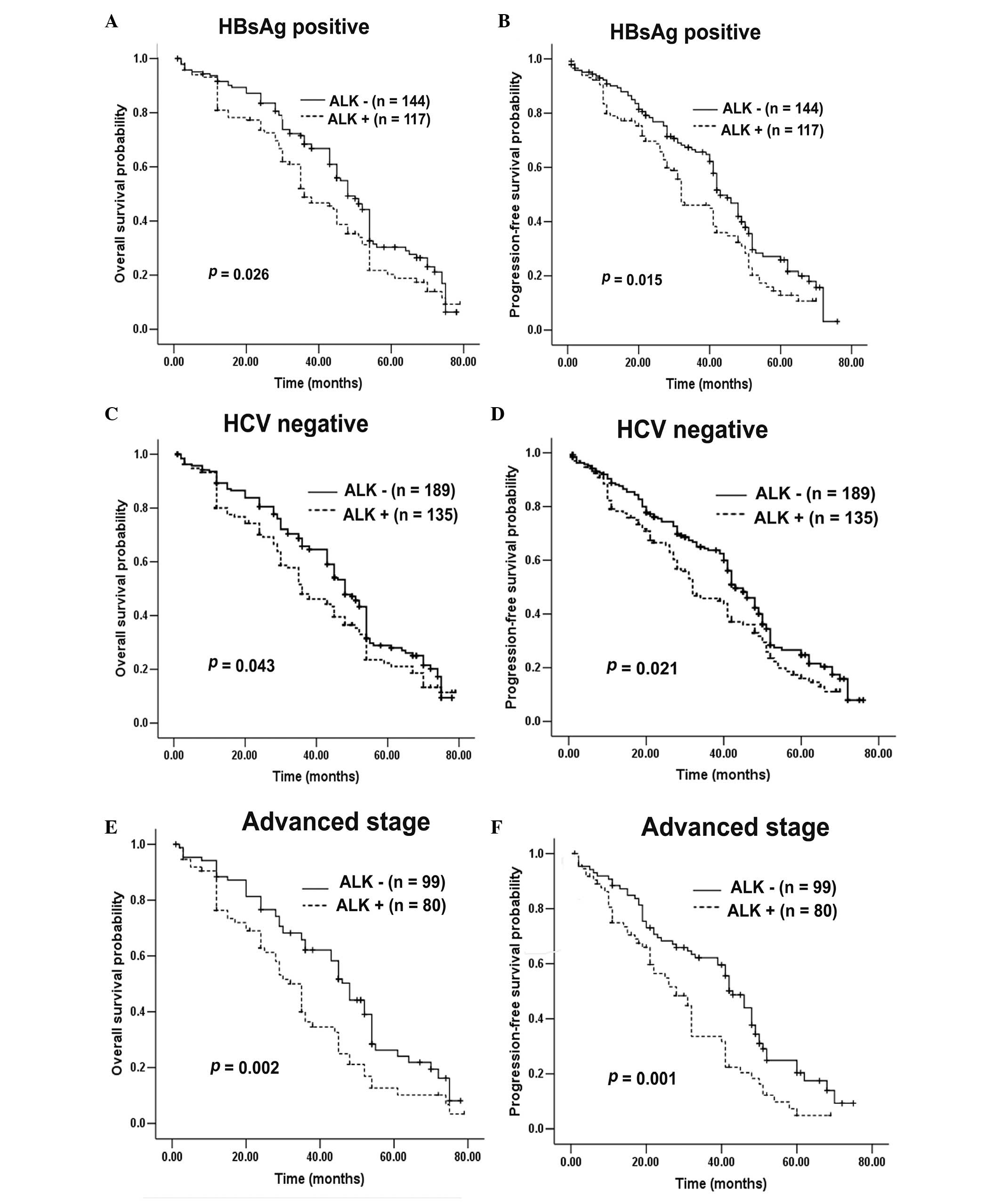
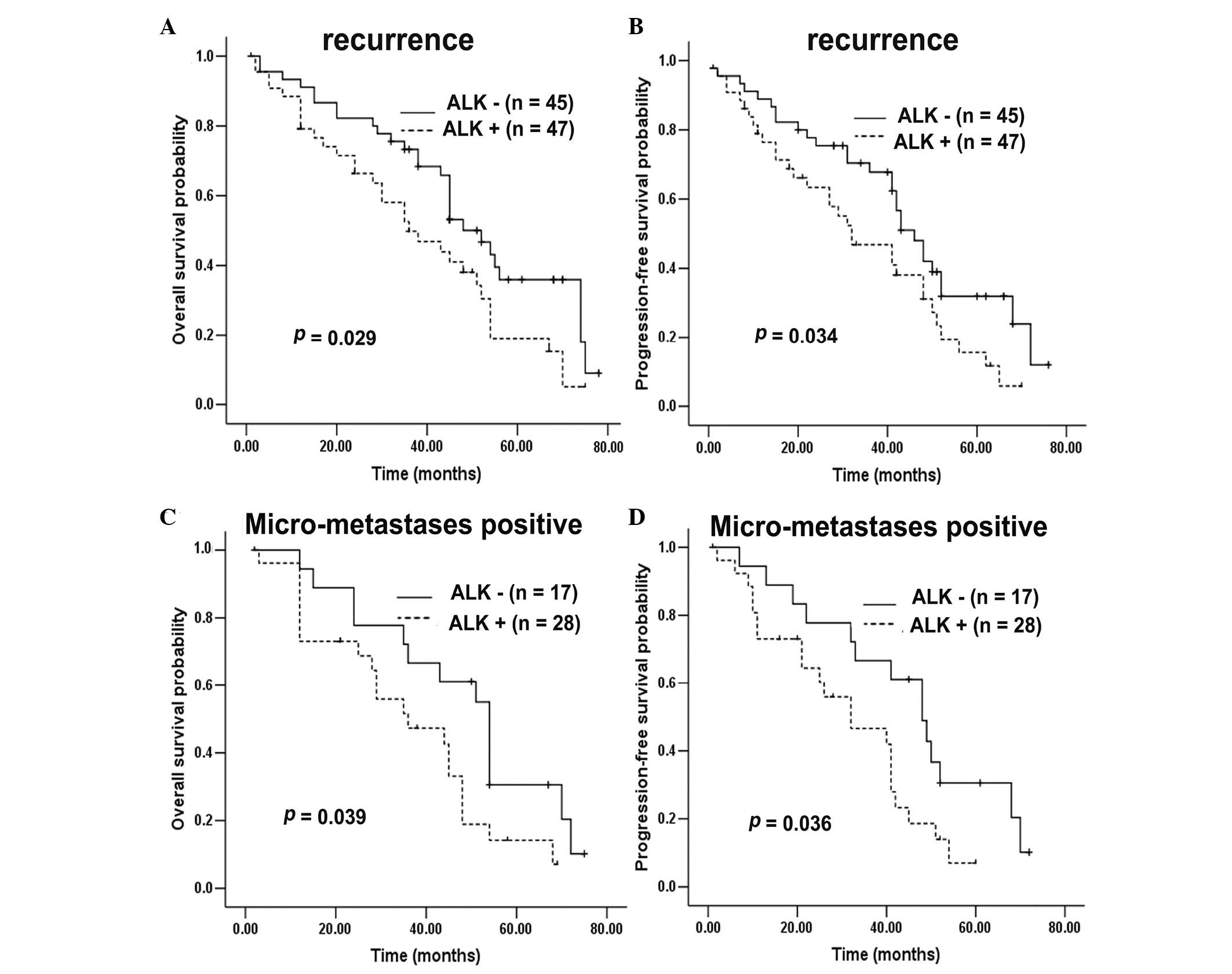
expression (χ2=4.187; P=0.041; Fig. 4B. Following additional stratification
of 342 HCC patients, OS and PFS were found to be markedly reduced
in patients exhibiting ALK expression compared with patients
without ALK expression, in subgroups that were HBsAg
positive (P=0.026 vs. 0.015; Fig.
5A–B), HCV negative (P=0.043 vs. 0.021; Fig. 5C–D), stage III–IV (P=0.002 vs. 0.001;
Fig. 5E–F), recurrence positive
(P=0.029 vs. 0.034; Fig. 6A–B) and
micrometastasis positive (P=0.039 vs. 0.036; Fig. 6C–D). However, in the HBsAg-negative
(P=0.863 vs. 0.869), HCV-positive (no comparison analysis), stage
I–II (P=0.895 vs. 0.825), recurrence-negative (P=0.375 vs. 0.267)
and micrometastasis-negative (P=0.184 vs. 0.152) subgroups,
ALK expression exerted no impact on OS or PFS. Furthermore,
ALK expression was not associated with survival stratified
by age, gender, AFP, cirrhosis, tumor size, tumor multiplicity,
vascular invasion, Child-Pugh classification and lymph node
metastasis.
In addition, Cox proportional hazards model was
applied in order to reveal the independent impacts of the following
features on OS and PFS: Age, gender, HBsAg, HCV, AFP, cirrhosis,
tumor size, tumor multiplicity, clinical stage, vascular invasion,
recurrence, lymph node metastasis, micrometastases, Child-Pugh
classification and ALK protein expression. The results of
univariate and multivariate analyses indicated that ALK protein
expression exerted a significant prognostic effect on PFS in HCC
patients (hazard ratio, 1.365; 95% confidence interval,
1.029–1.810; P=0.031; Table III),
although it was not an independent prognosticator for OS (hazard
ratio, 1.290; 95% confidence interval, 0.973–1.709; P=0.076). ALK
protein expression may be an independent risk factor for OS
(P=0.041 vs. 0.042) and PFS (P=0.029 vs. 0.033; Table IV), particularly for stage III–IV
patients.
 | Table III.Univariate and multivariate analyses
of progression-free survival in 342 hepatocellular carcinoma
patients. |
Table III.
Univariate and multivariate analyses
of progression-free survival in 342 hepatocellular carcinoma
patients.
| A, Univariate
analysis |
|
|
|
|---|
|
|
|
|
|---|
| Parameter | Variable | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Age at diagnosis,
years | <60 vs. ≥60 | 1.012
(0.747–1.372) |
0.938 |
| Gender | Male vs.
female | 0.880
(0.635–1.219) |
0.443 |
| Hepatitis B surface
antigen | Positive vs.
negative | 1.068
(0.787–1.449) |
0.673 |
| Hepatitis C
virus | Positive vs.
negative | 0.816
(0.433–1.538) |
0.529 |
| α-fetoprotein,
ng/ml | <20 vs. ≥20 | 0.932
(0.721–1.205) |
0.591 |
| Cirrhosis | Absent vs.
present | 1.026
(0.791–1.330) |
0.849 |
| Tumor size, cm | <5 vs. ≥5 | 1.143
(0.883–1.478) |
0.310 |
| Tumor
multiplicity | Single vs.
multiple | 1.050
(0.763–1.447) |
0.763 |
| Clinical stage | I–II vs.
III–IV | 1.228
(0.949–1.589) |
0.118 |
| Vascular
invasion | No vs. yes | 1.113
(0.854–1.450) |
0.430 |
| Recurrence | No vs. yes | 1.002
(0.749–1.340) |
0.990 |
| Lymph node
metastasis | No vs. yes | 1.099
(0.779–1.550) |
0.591 |
|
Micrometastases | No vs. yes | 1.397
(0.974–2.004) |
0.069 |
| Anaplastic lymphoma
kinase protein | Positive vs.
negative | 1.304
(1.005–1.691) |
0.045 |
| Child-Pugh
classification | A vs. B | 6.220
(3.764–10.278) | <0.001 |
|
| B, Multivariate
analysis |
|
| Parameter | Variable | Hazard ratio (95%
confidence interval) | P-value |
|
| Anaplastic lymphoma
kinase protein | Positive vs.
negative | 1.365
(1.029–1.810) |
0.031 |
| Child-Pugh
classification | A vs. B | 7.198
(4.261–12.159) | <0.001 |
 | Table IV.Univariate and multivariate analyses
of overall survival for 179 stage III–IV hepatocellular carcinoma
patients. |
Table IV.
Univariate and multivariate analyses
of overall survival for 179 stage III–IV hepatocellular carcinoma
patients.
| A, Univariate
analysis |
|
|
|
|---|
|
|
|
|
|---|
| Parameter | Variable | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Age at diagnosis,
years | <60 vs. ≥60 | 1.026
(0.700–1.505) |
0.894 |
| Gender | Male vs.
female | 0.958
(0.625–1.499) |
0.883 |
| Hepatitis B surface
antigen | Positive vs.
negative | 1.062
(0.708–1.593) |
0.770 |
| Hepatitis C
virus | Positive vs.
negative | 0.885
(0.389–2.014) |
0.771 |
| α-fetoprotein,
ng/ml | <20 vs. ≥20 | 0.875
(0.648–1.240) |
0.453 |
| Cirrhosis | Absent vs.
present | 1.137
(0.804–1.609) |
0.468 |
| Tumor size, cm | <5 vs. ≥5 | 1.095
(0.569–1.407) |
0.631 |
| Tumor
multiplicity | Single vs.
multiple | 1.264
(0.725–2.203) |
0.408 |
| Vascular
invasion | No vs. yes | 1.104
(0.609–1.224) |
0.410 |
| Recurrence | No vs. yes | 1.091
(0.744–1.599) |
0.656 |
| Lymph node
metastasis | No vs. yes | 1.081
(0.636–1.361) |
0.711 |
|
Micrometastases | No vs. yes | 1.230
(0.832–1.816) |
0.299 |
| Anaplastic lymphoma
kinase protein | Positive vs.
negative | 1.416
(0.999–2.006) |
0.041 |
| Child-Pugh
classification | A vs. B | 6.898
(3.593–13.240) | <0.001 |
|
| B, Multivariate
analysis |
|
| Parameter | Variable | Hazard ratio (95%
confidence interval) | P-value |
|
| Anaplastic lymphoma
kinase protein | Positive vs.
negative | 1.894
(1.023–3.507) |
0.042 |
| Child-Pugh
classification | A vs. B | 8.610
(4.246–17.786) | <0.001 |
Discussion
To the best of our knowledge, the results of the
present study demonstrated the first evidence that ALK
expression was increased at a transcriptional level, and
additionally at a translational level, in human HCC samples
compared with adjacent normal tissue samples. ALK
rearrangement was not observed in any of the examined samples. The
expression of ALK protein was significantly correlated with the
aggressiveness and prognosis of primary HCC.
HCC is a pathologically and clinically heterogeneous
neoplasm, exhibiting high malignancy and a consequent poor
prognosis due to its aggressive features (27). Despite the complexity of
hepatocarcinogenesis, the discovery of additional prognostic
predictors and therapeutic targets for HCC has attracted particular
interest. As a result, a number of genes, including epidermal
growth factor receptor, transforming growth factor β and
c-MET, have been identified as molecular targets of HCC
(28). Recently, much attention has
been paid to the ALK gene, a member of the RTK family, the
dysregulation of which is associated with abnormal development and
malignant transformation in human cancer (29). However, to the best of our knowledge,
the clinical value of ALK abnormalities in human HCC has not
previously been comprehensively evaluated.
In the present study, ALK status was
investigated in a large cohort of HCC patients. IHC, RT-qPCR and
FISH analyses revealed that increased expression of ALK protein and
mRNA, as well as ALK gene copy number gain, occurred in HCC,
with the rates of expression observed in patients being 44.7%
(153/342), 47.4% (162/342) and 32.7% (112/342), respectively.
Notably, there was concordance in the assessment of ALK
expression levels among IHC, RT-qPCR and FISH detection methods,
demonstrating that ALK gene upregulation was present at
post-transcriptional and transcriptional levels. Meanwhile, the
results of the present study revealed that RT-qPCR possessed a
higher sensitivity (90.8%) and that FISH had increased specificity
(97.9%) compared with IHC staining, which demonstrated similar
results to those of a previously published study (30). However, it is notable that ALK
overexpression or ALK gene copy number gain is not
indicative of ALK rearrangement. To the best of our
knowledge, in patients exhibiting NSCLC, copy number gain and
amplification of the ALK gene are reportedly highly
expressed, while ALK translocations are rare (13). Similar results have also been
described in esophageal carcinoma (18). In the present study, no rearrangement,
amplification or mutation of the ALK locus was identified
using FISH and RACE-coupled PCR sequencing assays. A recent
retrospective study (12) reported
that ALK gene copy number gain was common in HCC
investigated with FISH analysis, whereas ALK rearrangement
was not observed. These previous results supported the results of
the present study, which indicated that the ALK gene was
upregulated in HCC, and that this may be a potential biomarker for
HCC.
The present study additionally characterized the
association between ALK protein and the clinicopathological
features of HCC. The results of the present study demonstrated that
ALK protein overexpression possessed significant correlation with
HCV status and micrometastases. Briefly, ALK may be a
valuable indicator for the identification of subsets of HCC cases
with HCV infection and increased invasive tendency. Various
experimental model systems have proved that ALK gene
overexpression contributes to cell migration and invasion (10,31,32), which
are crucial steps in the metastatic cascade, and may lead to
distant organ involvement and tumor aggressiveness (33). Furthermore, Shao et al
(34) reported that ALK protein was
overexpressed in HCC and may be involved in the progression of HCC
tumors, although the patient cohort investigated in this previous
study was small. Similarly, the results of the present study
suggested a significant impact of ALK overexpression on the
promotion of the development and metastasis of HCC.
Activation of proto-oncogenes and inactivation of
tumor suppressors contributes to the occurrence and progression of
malignancies. Specifically, the ALK oncogene is activated by
its endogenous ligands, midkine and pleiotrophin, which serve as
mitogenic and angiogenic factors in cancer (10). Given that the activated ALK
gene induces an intricate system of downstream signaling cascades,
including phosphoinositide 3-kinase/protein kinase B and
mitogen-activated protein kinase pathways, it is possible that the
ALK gene may mediate cellular proliferation, motility and
apoptosis, and thereby possess a significant role in the promotion
of tumorigenesis and development in vitro and in vivo
(35). Recently, Hasan et al
(33) illustrated that MYC proteins
that targeted key proliferative pathways in the cancer development
process, and thus contributed to tumor growth and metastasis in
neuroblastoma, regulated ALK expression. Notably, Di Paolo et
al (36) reported that the RNA
interference-based knockdown of ALK, independent of fusion
gene status, resulted in the downregulation of proliferation and
the upregulation of apoptosis in neuroblastoma tumor cells, thereby
ultimately inhibiting tumor growth and prolonging survival in
vivo. Therefore, these previous results confirm that ALK
has a significant role in the progression and metastasis of
HCC.
In addition, ALK overexpression has been
documented as possessing an unfavorable prognosis in multiple human
cancers, including neuroblastoma (36), breast carcinoma (37) and basal cell carcinoma (38). Similarly, the present study revealed
that ALK protein was significantly correlated with poor PFS in the
entire study cohort, particularly for patients with HBsAg
positivity, HCV negativity, advanced stage tumors, recurrence or
micrometastases. Notably, there were certain differences between
the results of the present study and the previous findings of Jia
et al (12), which
demonstrated that ALK gene copy number gain predicted the
survival of patients exhibiting HBsAg negativity, however, did not
impact on the survival of patients with HBsAg positivity. The
primary reasons for the disagreement between these sets of results
may be due to the genetic backgrounds and research methods utilized
(protein versus gene copy number). The results of the current study
concluded that the prognosis of HCC patients exhibiting ALK
overexpression tended to be poorer.
Due to the retrospective nature of the present
study, a number of patients and their clinical information were
lost to follow-up, particularly those patients who were not
hospitalized following surgery, which may have led to bias in the
results. Accordingly, additional experiments in vitro and
in vivo are required to confirm the results of the present
study. In addition, it remains to be elucidated whether HCC
patients exhibiting ALK overexpression may benefit from
treatment with ALK inhibitors. Further research focusing on
these issues is required.
In conclusion, ALK may serve as a valuable
predictor of micrometastases and poor survival. Therefore,
radiological diagnosis in combination with ALK detection may
assist with the prognostic evaluation of novel HCC cases, as
optimal individualized therapeutic strategies remain to be
devised.
Acknowledgements
The present study was supported by a grant from the
Program of Science and Technology Commission Foundation of
Guangzhou City, Guangdong Province, China (grant no.
2011B031800285).
References
|
1
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing and treating hepatocellular carcinoma. CA
Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma - epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009. View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song MJ and Bae SH: Newer treatments for
advanced hepatocellular carcinoma. Korean J Intern Med. 29:149–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanaka S and Arii S: Molecular targeted
therapies in hepatocellular carcinoma. Semin Oncol. 39:486–492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crose LE and Linardic CM: Receptor
tyrosine kinases as therapeutic targets in rhabdomyosarcoma.
Sarcoma. 2011:7569822011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morris SW, Kirstein MN, Valentine MB,
Dittmer KG, Shapiro DN, Saltman DL and Look AT: Fusion of a kinase
gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's
lymphoma. Science. 263:1281–1284. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: Where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laurent C, Do C, Gascoyne RD, Lamant L,
Ysebaert L, Laurent G, Delsol G and Brousset P: Anaplastic lymphoma
kinase-positive diffuse large B-cell lymphoma: A rare
clinicopathologic entity with poor prognosis. J Clin Oncol.
27:4211–4216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Webb TR, Slavish J, George RE, Look AT,
Xue L, Jiang Q, Cui X, Rentrop WB and Morris SW: Anaplastic
lymphoma kinase: Role in cancer pathogenesis and small-molecule
inhibitor development for therapy. Expert Rev Anticancer Ther.
9:331–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Zhang S, Yang X, Yang J, Zhou Q,
Yin L, An S, Lin J, Chen S, Xie Z, et al: Fusion of EML4 and ALK is
associated with development of lung adenocarcinomas lacking EGFR
and KRAS mutations and is correlated with ALK expression. Mol
Cancer. 9:1882010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia SW, Fu S, Wang F, Shao Q, Huang HB and
Shao JY: ALK gene copy number gain and its clinical significance in
hepatocellular carcinoma. World J Gastroenterol. 20:183–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salido M, Pijuan L, Martínez-Avilés L,
Galván AB, Cañadas I, Rovira A, Zanui M, Martínez A, Longarón R,
Sole F, et al: Increased ALK gene copy number and amplification are
frequent in non-small cell lung cancer. J Thorac Oncol. 6:21–27.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shaw AT and Solomon B: Targeting
anaplastic lymphoma kinase in lung cancer. Clin Cancer Res.
17:2081–2086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ou SH, Soo RA, Kubo A, Kawaguchi T and Ahn
MJ: Will the requirement by the US FDA to simultaneously co-develop
companion diagnostics (CDx) delay the approval of receptor tyrosine
kinase inhibitors for RTK-rearranged (ROS1-, RET-, AXL-, PDGFR-α-,
NTRK1-) non-small cell lung cancer globally? Front Oncol. 4:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schoppmann SF, Streubel B and Birner P:
Amplification but not translocation of anaplastic lymphoma kinase
is a frequent event in oesophageal cancer. Eur J Cancer.
49:1876–1881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin E, Li L, Guan Y, Soriano R, Rivers CS,
Mohan S, Pandita A, Tang J and Modrusan Z: Exon array profiling
detects EML4-ALK fusion in breast, colorectal and non-small cell
lung cancers. Mol Cancer Res. 7:1466–1476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lipson D, Capelletti M, Yelensky R, Otto
G, Parker A, Jarosz M, Curran JA, Balasubramanian S, Bloom T,
Brennan KW, et al: Identification of new ALK and RET gene fusions
from colorectal and lung cancer biopsies. Nat Med. 18:382–384.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Debelenko LV, Raimondi SC, Daw N,
Shivakumar BR, Huang D, Nelson M and Bridge JA: Renal cell
carcinoma with novel VCL-ALK fusion: New representative of
ALK-associated tumor spectrum. Mod Pathol. 24:430–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu ZY, Yuan SX, Yang Y, Zhou WP and Jiang
H: Pleomorphic adenoma gene 1 mediates the role of karyopherin
alpha 2 and has prognostic significance in hepatocellular
carcinoma. J Exp Clin Cancer Res. 33:612014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vauthey JN, Lauwers GY, Esnaola NF, Do KA,
Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A,
et al: Simplified staging for hepatocellular carcinoma. J Clin
Oncol. 20:1527–1536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Zhang X, Bai H, Zhao J, Zhuo M, An
T, Duan J, Yang L, Wu M, Wang S, et al: EML4-ALK rearrangement and
its clinical significance in Chinese patients with advanced
non-small cell lung cancer. Oncology. 83:248–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pietrantonio F, Maggi C, Di Bartolomeo M,
Facciorusso MG, Perrone F, Testi A, Iacovelli R, Miceli R, Bossi I,
Leone G, et al: Gain of ALK gene copy number may predict lack of
benefit from anti-EGFR treatment in patients with advanced
colorectal cancer and RAS-RAF-PI3KCA wild-type status. PLoS One.
9:e921472014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YG, Jin ML, Li L, Zhao HY, Zeng X,
Jiang L, Wei P, Diao XL, Li X, Cao Q and Tian XX: Evaluation of ALK
rearrangement in Chinese non-small cell lung cancer using FISH,
immunohistochemistry and real-time quantitative RT-PCR on
paraffin-embedded tissues. PLoS One. 8:e648212013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiraha H, Yamamoto K and Namba M: Human
hepatocyte carcinogenesis (review). Int J Oncol. 42:1133–1138.
2013.PubMed/NCBI
|
|
28
|
Shen YC, Lin ZZ, Hsu CH, Hsu C, Shao YY
and Cheng AL: Clinical trials in hepatocellular carcinoma: An
update. Liver Cancer. 2:345–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calero R, Morchon E, Johnsen JI and
Serrano R: Sunitinib suppress neuroblastoma growth through
degradation of MYCN and inhibition of angiogenesis. PLoS One.
9:e956282014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu YC, Chang IC, Wang CL, Chen TD, Chen
YT, Liu HP, Chu Y, Chiu YT, Wu TH, Chou LH, et al: Comparison of
IHC, FISH and RT-PCR methods for detection of ALK rearrangements in
312 non-small cell lung cancer patients in Taiwan. PLoS One.
8:e708392013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
George REI, Sanda T, Hanna M, Fröhling S,
Luther W 2nd, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, et al:
Activating mutations in ALK provide a therapeutic target in
neuroblastoma. Nature. 455:975–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duijkers FA, Gaal J, Meijerink JP,
Admiraal P, Pieters R, de Krijger RR and van Noesel MM: High
anaplastic lymphoma kinase immunohistochemical staining in
neuroblastoma and ganglioneuroblastoma is an independent predictor
of poor outcome. Am J Pathol. 180:1223–1231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hasan MK, Nafady A, Takatori A, Kishida S,
Ohira M, Suenaga Y, Hossain S, Akter J, Ogura A, Nakamura Y, et al:
ALK is a MYCN target gene and regulates cell migration and invasion
in neuroblastoma. Sci Rep. 3:34502013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao CK, Su ZL, Feng ZY, Rao HL and Tang
LY: Significance of ALK gene expression in neoplasms and normal
tissues. Ai Zheng. 21:58–62. 2002.(In Chinese). PubMed/NCBI
|
|
35
|
Kinoshita Y, Tajiri T, Ieiri S, Nagata K,
Taguchi T, Suita S, Yamazaki K, Yoshino I, Maehara Y, Kohashi K, et
al: A case of an inflammatory myofibroblastic tumor in the lung
which expressed TPM3-ALK gene fusion. Pediatr Surg Int. 23:595–599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Paolo D, Ambrogio C, Pastorino F,
Brignole C, Martinengo C, Carosio R, Loi M, Pagnan G, Emionite L,
Cilli M, et al: Selective therapeutic targeting of the anaplastic
lymphoma kinase with liposomal siRNA induces apoptosis and inhibits
angiogenesis in neuroblastoma. Mol Ther. 19:2201–2212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krishnamurthy S, Woodward W, Yang W,
Reuben JM, Tepperberg J, Ogura D, Niwa S, Huo L, Gong Y, El-Zein R,
et al: Status of the anaplastic lymphoma kinase (ALK) gene in
inflammatory breast carcinoma. Springerplus. 2:4092013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ning H, Mitsui H, Wang CQ, Suárez-Fariñas
M, Gonzalez J, Shah KR, Chen J, Coats I, Felsen D, Carucci JA and
Krueger JG: Identification of anaplastic lymphoma kinase as a
potential therapeutic target in basal cell carcinoma. Oncotarget.
4:2237–2248. 2013. View Article : Google Scholar : PubMed/NCBI
|