Introduction
In 1863, Virchow first described myxoma as a tumor
anatomically resembling the umbilical cord (1). Myxomas (from the Greek word ‘muxa’
meaning mucus) are rare, benign connective tissue tumors arising
from stellate mesenchymal cells (2),
comprising entities such as fibromyxoma, cardiac myxoma and
intramuscular myxoma (IM).
IM is an uncommon variant of the disease that
typically presents between the fourth and seventh decades, with a
slight female predilection (3). The
majority of IMs present as slow-growing, painless masses within the
thigh muscles and lower limb girdle (4,5). By
contrast, IMs are rarely found in the head and neck region
(4,5).
Histopathologically, the lesions are usually
recognized by their paucicellularity and minimal vascularity.
Similar to other myxomas, IMs consist of fibroblasts and an
abundant myxoid stroma (2), primarily
composed of glycosaminoglycans and fibrous structural proteins
(6). However, cases of IM displaying
hypercellularity and abundant vascularity have been reported, often
incorrectly leading towards a diagnosis of myxoid sarcoma (7). IMs are characterized by small stellate
or spindle cells without features of atypia, mitosis and necrosis
(8). Tumor cells possess small,
hyperchromatic nuclei and inconspicuous cytoplasm.
Immunohistochemically, they generally stain positively for vimentin
and cluster of differentiation 34 (9). Mutations activating Gs (α) have been
suggested to show correlation with this disease process (10).
Imaging modalities, including magnetic resonance
imaging (MRI), computed tomography (CT) and ultrasonography, are
useful for diagnosis, but the definitive diagnosis is
histopathological. IMs display low signal intensity on T1-weighted
MRI images and high intensity on T2-weighted images, with
peripheral or patchy enhancement following gadolinium injection
(3). CT scan evaluation typically
reveals a hypodense mass in comparison to adjacent musculature,
without contrast enhancement (3).
Consistent with CT and MRI, ultrasonography reveals a hypoechoic
lesion with a partial or complete capsule (3).
Other conditions that should be considered in the
differential diagnosis include aggressive angiomyxoma, myxoid
neurofibroma, low-grade fibromyxoid sarcoma, myxoid liposarcoma,
low grade myxofibrosarcoma, cellular myxoma, juxta-articular myxoma
and nodular fasciitis (11). IMs are
located entirely inside the skeletal muscle, in contrast to myxoid
liposarcomas, which are intermuscular.
Histopathologically, the absence of vascularity
decreases the likelihood of sarcoma, and S-100 protein negativity
excludes myxoid neurofibromas and low-grade malignant peripheral
nerve sheath tumors (11). Once the
diagnosis of IM is confirmed through biopsy, the treatment of
choice is surgical excision (5,7).
The current study reports a new case of IM involving
the levator scapulae and scalene muscles, and presents a systematic
review of head and neck IMs, with a summary of the clinical and
demographic parameters of all reported cases in the head and neck
region.
Case report
Presentation
A 45-year-old, otherwise healthy, male presented to
the Medical University of South Carolina (Charleston, USA) in March
2013 with a painless mass in the posterior of the neck that had
been noticed by the patient 2 months earlier. The patient exhibited
no sensory impairment, numbness or weakness of the right
extremities. Physical examination revealed a deep, fixed,
non-tender mass, with ill-defined borders.
Diagnosis
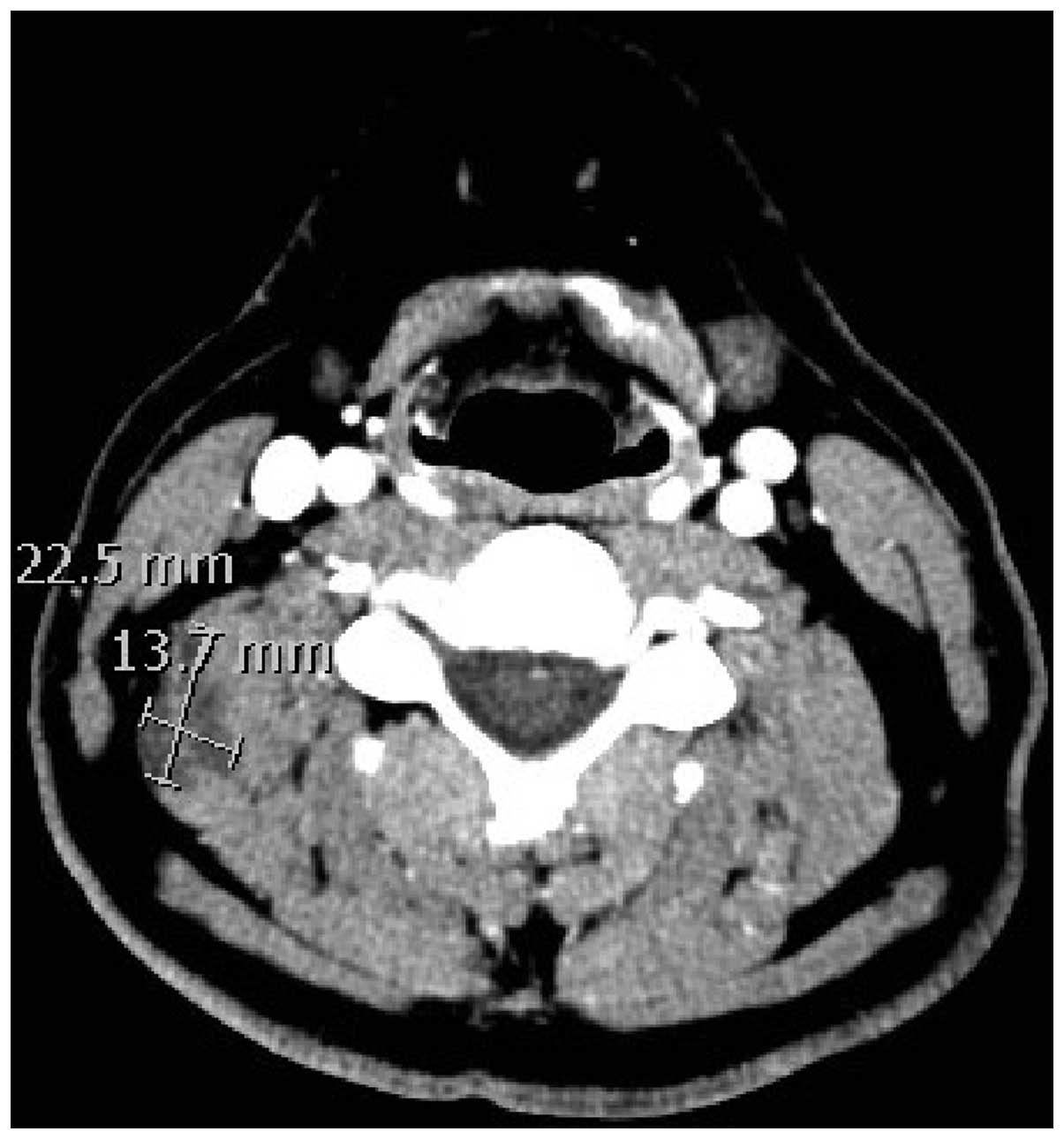
Ultrasound imaging showed an ill-defined, hypoechoic
irregularity of a deep muscle of the right posterior neck, which
corresponded to a hypodense lesion within the levator scapulae
muscle on a contrast CT scan (Fig.
1). There was no significant internal flow on Doppler imaging
and no lymphadenopathy. MRI revealed a 2.7×2.5×1.4-cm mass within
the right superficial paraspinal musculature, likely involving the
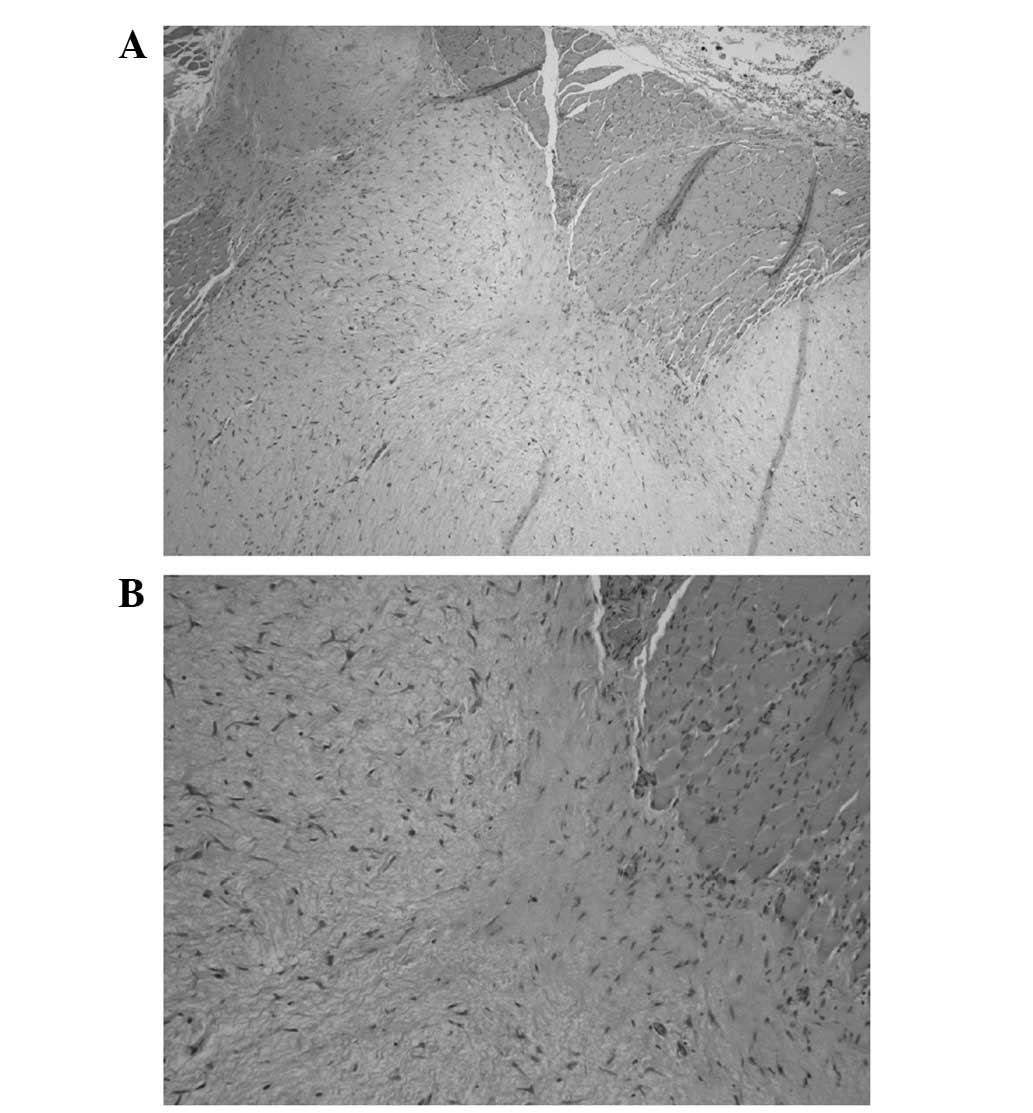
levator scapulae and scalene muscles. Pathological examination of a
core needle biopsy showed spindle cells with a bland appearance, in
a hypovascular, myxoid stroma, confirming the diagnosis of IM
(Fig. 2).
Surgical excision
Dissection was performed down to the
sternocleidomastoid muscle, which was mobilized along the posterior
border. The spinal accessory nerve was identified and preserved.
Electromyography was used to monitor the brachial plexus and the
spinal accessory nerve. Erb's point was identified and the greater
auricular nerve was preserved. Level 5 dissection of the lymph
nodes was undertaken, preserving cranial nerve XI, to provide
access to the tumor. The mass was palpated deep to these lymph
nodes, and was located within the levator scapulae muscle,
extending medially to involve the posterior and middle scalene
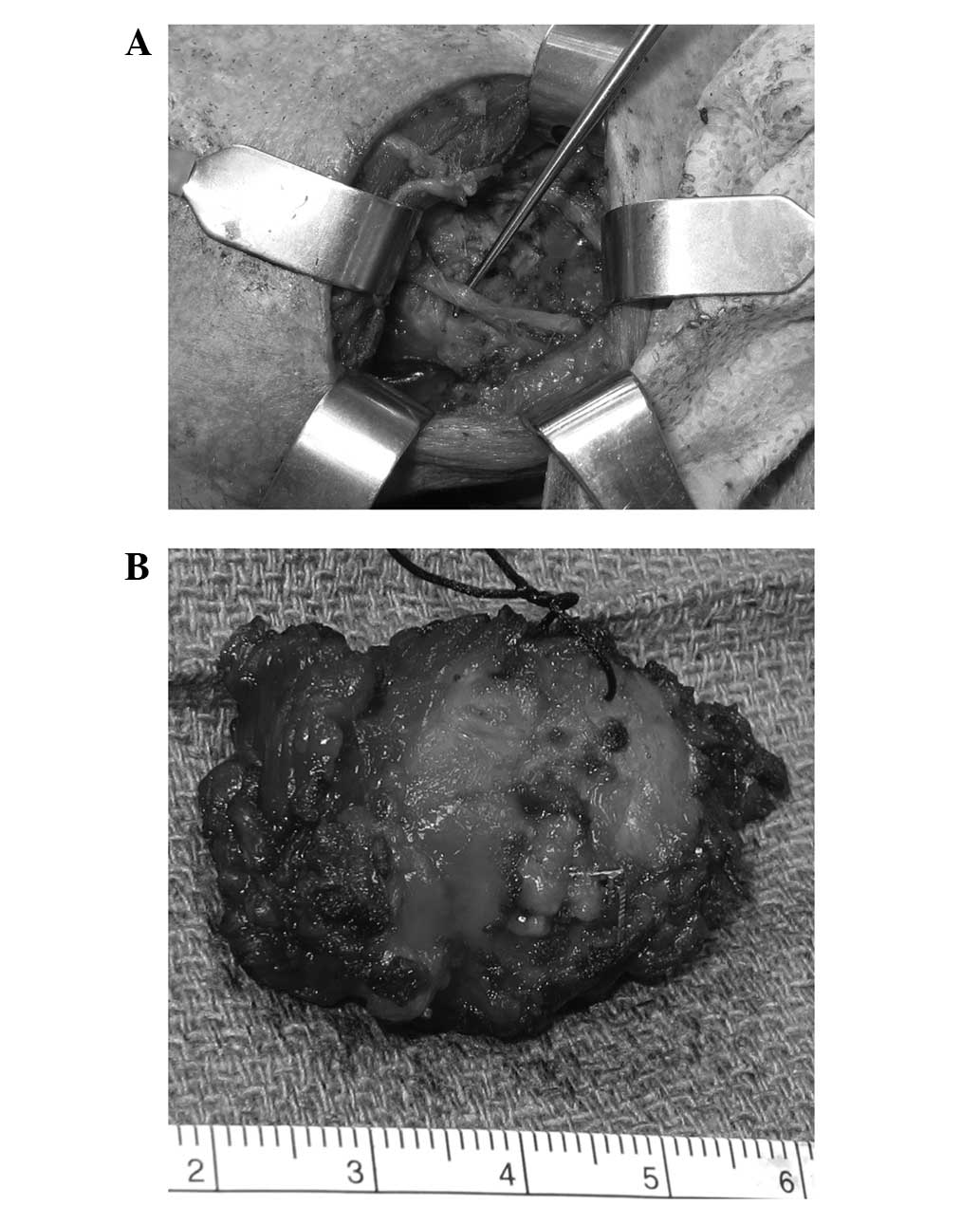
muscles. Dissection was performed around the tumor, taking an ~1-cm
cuff of muscle circumferentially around the tumor. The lesion was
fully resected (Fig. 3) with
tumor-free borders. Following 30 months of follow-up, no further
treatment was needed and the tumor did not recur.
Literature review
Methods
Pubmed search
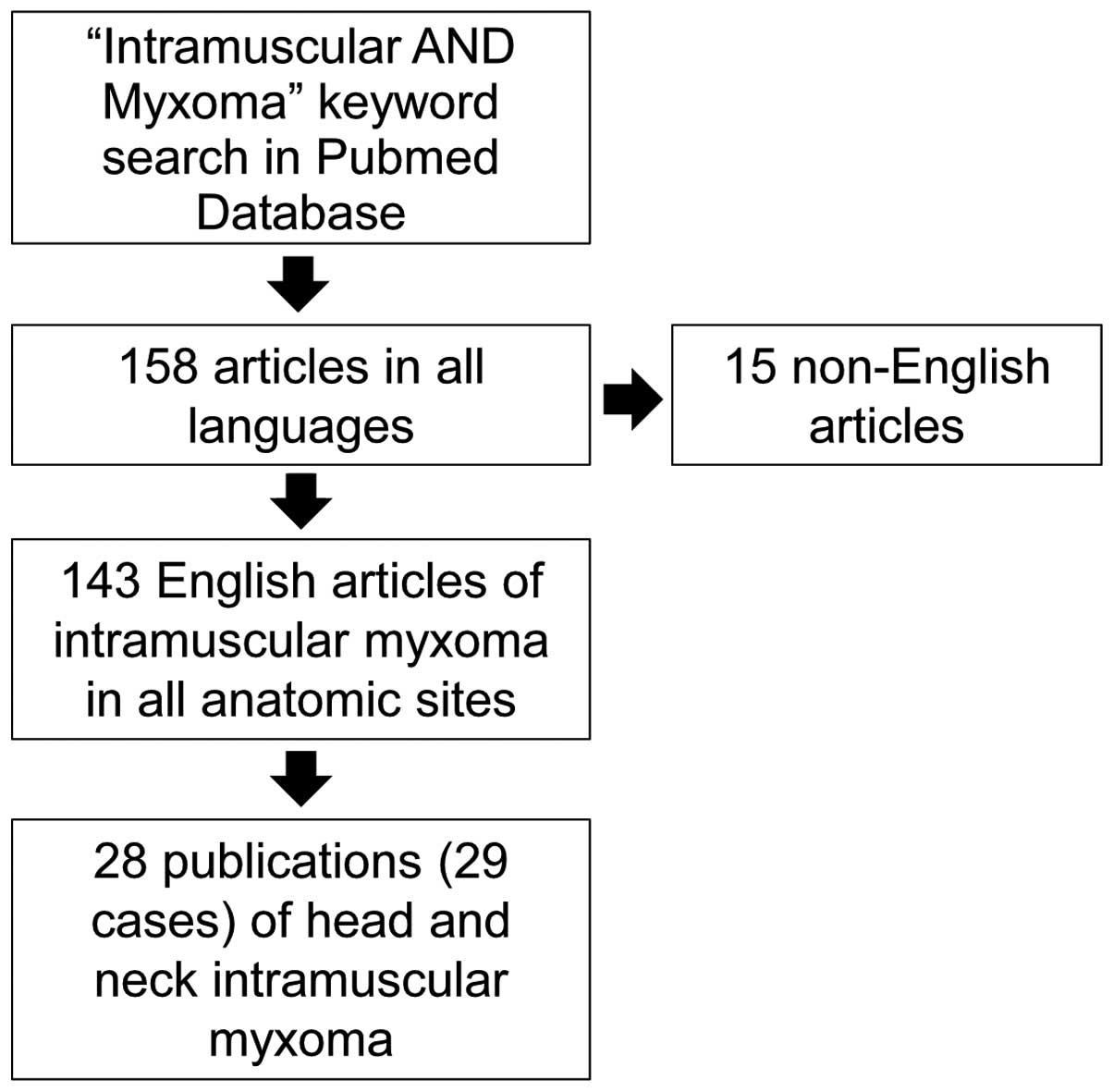
A comprehensive literature review of the literature
was performed by searching the Pubmed-National Center for
Biotechnology Information database, using the keyword search
‘intramuscular AND myxoma’. The search yielded 158 studies
published prior to December 2014, 15 of which were excluded, as
they were in a language other than English, leaving 143 studies. Of
these, 28 included cases in the head and neck region, and were
included in the present literature review. The inclusion criteria
encompassed all studies with IM cases of the head and neck region
that were published prior to December 2014. The exclusion criteria
were as follows: i) Reports published in a non-English language;
and ii) cases that had been already published in another study
(i.e., duplicated cases). This yielded 28 studies (12–39), with
29 cases, in addition to the currently presented case. Fig. 4 outlines the case selection
method.
Statistical analysis
Using Excel software (Microsoft Corporation,
Redmond, WA, USA), two-tailed Student's t-test for independent
samples was performed to compare the ages of the two genders.
Values are reported as mean ± standard deviation (SD). P<0.05
was used to indicate a statistically significant difference.
Results
A total of 28 studies were included in this review,
constituting 29 cases of IM in the head and neck region, in
addition to the currently presented case (n=30; Table I).
 | Table I.Summary of the head and neck
intramuscular myxomas reported in the literature. |
Table I.
Summary of the head and neck
intramuscular myxomas reported in the literature.
| Reference no. | Gender | Age, years | Ethnicity | Anatomical
location | Treatment | FU, years | Recurrence
status | Size on PE, cm | Size on CT/MRI,
cm | Size after excision,
cm |
|---|
| (12) | F | 57 | NA | Paraspinal
muscles | Surgical
excision | NA | NA | NA | 2 | NA |
| (13) | F | 63 | NA | Parasipnal
muscles | Surgical
excision | 1.5 | No recurrence | NA | 27×16×38 | NA |
| (14) | M | 74 | NA | Hyoglossus | Surgical
excision | 3 | No recurrence | 8 | 8 | NA |
| (15) | M | 51 | NA | Temporalis | Surgical
excision | 0.5 | No recurrence | >5 | NA | 6.5×4×3 |
| (26) | F | 70 | NA | SCM | Surgical
excision | 5 | No recurrence | 2 | 2×1.2×1.6 | NA |
| (27) | F | 45 | Asian | Trapezius | Surgical
excision | 1 | No recurrence | 2.5×3 | 4.1×2.8×4.9 | 3.5×2.5×2 |
| (28) | M | 52 | NA | Nasal vestibule
(mimetic muscle) | Surgical
excision | 1 | No recurrence | NA | 2×1.3 | 2.5 |
| (19) | F | 64 | NA | Paraspinal
muscles | Surgical
excision | 1 | No recurrence | 12 | 15 | NA |
| (20) | M | 74 | NA | Masseter | Surgical
excision | 2 | No recurrence | 2×3 | NA | NA |
| (21) | F | 2 | NA | Trapezius and
paraspinal muscles | Surgical
excision | 2 | No recurrence | 4×5 | 4.1×2.8×4.9 | NA |
| (22) | M | 22 | NA | Scalene and
SCM | Surgical
excision | 4 | No recurrence | 6×4 | 7×3 | 7×4×3 |
| (23) | F | 43 | B | Temporalis | Surgical
excision | 1.5 | No recurrence | 3.5×2.5 | NA | 3.5×2×2.6 |
| (24) | F | 5 | NA | Deep to
trapezius | Surgical
excision | 1 | No recurrence | 4 | 4 | NA |
| (25) | F | 60 | NA | Posterior scapular
muscles | No intervention
(monitor only) | 1 | No change in size
by MRI | Four deep
nodules | NA | NA |
| (25) | M | 56 | NA | Right cheek | Surgical
excision | 4 | No recurrence | NA | 3 | NA |
| (26) | M | 62 | NA | Temporalis | Surgical
excision | 1 | No recurrence | 5×4 | NA | NA |
| (27) | M | 46 | NA | Orbicularis
oris | Surgical
excision | 2 | Recurrence in 5
months, then no recurrence | 3 | NA | NA |
| (28) | F | 60 | NA | Tongue | Surgical
excision | NA | NA | 2 | NA | NA |
| (29) | F | 69 | B | Levator
scapula | Surgical
excision | 1 | No recurrence | 4×3 | 4 | NA |
| (30) | F | 43 | NA | Masseter | Surgical
excision | 5 | No recurrence | NA | NA | 2×1 |
| (31) | F | 16 | NA | Intermediary
tendons of the digastric muscles, bilaterally | Surgical
excision | 1 | No recurrence | 6×1.5 | NA | 1.5 each |
| (32) | M | 51 | NA | Posterior neck
(recurrent after previous excision) | Surgical
excision | 16 | No recurrence | NA | NA | NA |
| (33) | F | 79 | NA | Masseter | Surgical
excision | NA | NA | 1 | NA | NA |
| (34) | F | 62 | W | Posterior neck | Surgical
excision | 10 | No recurrence | NA | NA | 3 |
| (35) | F | 46 | NA | Lateral neck | Surgical
excision | 2 | No recurrence | NA | NA | NA |
| (36) | F | 42 | NA | Forehead | Surgical
excision | 12 | No recurrence | NA | NA | NA |
| (37) | M | 44 | NA | Geniohyoid | Surgical
excision | 1.5 | No recurrence | 2 | NA | NA |
| (38) | M | 15 | W | Cheek | Surgical
excision | 5 | No recurrence | NA | NA | 2×1.5 |
| (39) | M | 74 | NA | Cheek muscles | Surgical
excision | NA | NA | NA | NA | NA |
| Present study | M | 45 | W | Levator scapulae
and scalene | Surgical
excision | 1 | No recurrence | NA | 2.7×2.5×1.4 | 4 |
The cases consisted of 43.3% males (n=13) and 56.7%
females (n=17), with an age range of 2–79 years and a mean age
(mean ± SD) of 49.7±20.4 years (males, 51.2±18.2 years; females,
48.6±22.4 years; P=0.73). The most common head and neck site was
the paraspinal muscles, followed by the trapezius, masseter, cheek
and temporal muscles (n=3 each).
The size of the mass on physical examination was
available for 17 cases, with a length range of 2–12 cm and a mean
length of 4.4±2.7 cm. Of all the cases, 96.7% (29 of 30) underwent
surgery as the treatment of choice, with a recurrence rate of 3.3%
(n=1). One case was monitored only and no change in size was
observed upon MRI at 1 year post-diagnosis. The mean follow-up time
for all patients was 3.3±3.8 years.
Discussion
IM is a benign tumor that commonly affects the
skeletal muscles of the thigh (4). IM
of the neck paraspinal muscles is extremely rare. Although
non-invasive and non-metastatic, local impingement of adjacent
muscles, nerves or arteries could result in significant functional
impairments.
IM could present as part of Mazbraud's syndrome, a
rare disease displaying one or more IMs with fibrous dysplasia in
one or more bones (40). Therefore,
patients presenting with IMs should be examined for bone lesions.
The number of reported cases of this syndrome in 2004 was 55
(41).
IM is the most common form of myxoma after
myocardial myxoma. In addition to IM, soft-tissue myxomas include
juxta-articular myxoma, superficial angiomyxoma, aggressive
angiomyxoma and nerve sheath myxoma (42). The incidence of IM is ~1 per million
individuals (4,43). In descending order, IMs most commonly
arise in the thighs, shoulders, buttocks and upper arms. Other
organs reported in the literature include the hands, face, tongue
and abdominal muscles.
We recommend imaging of deep neck masses, and when
surgical resection is performed, consideration of the proximity to
the phrenic nerve and brachial plexus is important.
In summary, the present study reports a case of IM
of the paraspinal muscles in a 45-year-old man. Following
radiographic imaging with ultrasound, CT scan and MRI, a core
needle biopsy confirmed the diagnosis. Surgical excision was
performed and follow-up for 30 months demonstrated no recurrence.
IMs should be considered in the differential diagnosis of deep neck
masses. Surgical excision has shown to be curative in the vast
majority of cases, with minimal recurrence rates.
References
|
1
|
Virchow R: Cellular pathology. As based
upon physiological and pathological histology. Lecture
XVI-Atheromatous affection of arteries. 1858. Nutr Rev. 47:23–25.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stout AP: Myxoma, the tumor of primitive
mesenchyme. Ann Surg. 127:706–719. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphey MD, McRae GA, Fanburg-Smith JC,
Temple HT, Levine AM and Aboulafia AJ: Imaging of soft-tissue
myxoma with emphasis on CT and MR and comparison of radiologic and
pathologic findings. Radiology. 225:215–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enzinger FM: Intramuscular myxoma; A
review and follow-up study of 34 cases. Am J Clin Pathol.
43:104–113. 1965.PubMed/NCBI
|
|
5
|
Miettinen M, Höckerstedt K, Reitamo J and
Tötterman S: Intramuscular myxoma-a clinicopathological study of
twenty-three cases. Am J Clin Pathol. 84:265–272. 1985.PubMed/NCBI
|
|
6
|
van Graadt Roggen JF, Hogendoorn PC and
Fletcher CD: Myxoid tumours of soft tissue. Histopathology.
35:291–312. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nielsen GP, O'Connell JX and Rosenberg AE:
Intramuscular myxoma: A clinicopathologic study of 51 cases with
emphasis on hypercellular and hypervascular variants. Am J Surg
Pathol. 22:1222–1227. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wakely PE Jr, Bos GD and Mayerson J: The
cytopathology of soft tissue mxyomas: Ganglia, juxta-articular
myxoid lesions and intramuscular myxoma. Am J Clin Pathol.
123:858–865. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weiss SW and Goldblum JR: Enzinger and
Weiss's Soft Tissue Tumors. Mosby (St. Louis, MO, USA). (4th).
2001.
|
|
10
|
Okamoto S, Hisaoka M, Ushijima M, Nakahara
S, Toyoshima S and Hashimoto H: Activating Gs (alpha) mutation in
intramuscular myxomas with and without fibrous dysplasia of bone.
Virchows Arch. 437:133–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen PW: Myxoma is not a single entity: A
review of the concept of myxoma. 4:99–123. 2000.
|
|
12
|
Tataryn Z, Tracy J, Tsang C, et al:
Intramuscular myxoma of the cervical paraspinal musculature: Case
report and review of the literature. Am J Otolaryngol. 36:273–276.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manoharan SR, Shaw AB, Arnold CA and
Farhadi HF: Infiltrative intramuscular myxoma of the cervical
spine: A case report. Spine J. 15:e1–e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li G, Jiang W, Li W and Li J:
Intramuscular myxoma of the hyoglossus muscle: A case report and
literature review. Oncol Lett. 7:1679–1682. 2014.PubMed/NCBI
|
|
15
|
Higashida T: Radiological characteristics
and management of intramuscular myxoma of the temporal muscle: Case
report. Neurol Med Chir (Tokyo). 54:1022–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalsi JS, Pring M, Hughes C and Fasanmade
A: Presentation of intramuscular myxoma as an unusual neck lump. J
Oral Maxillofac Surg. 71:e210–e214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Wang J and Shi Z: Intramuscular
myxoma of trapezius in an adult woman. Am Surg. 78:E135–E136.
2012.PubMed/NCBI
|
|
18
|
Patsiaoura K, Anagnostou E and Benis N:
Intramuscular myxoma of the nasal vestibule. Auris Nasus Larynx.
37:100–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falavigna A, Righesso O, Volquind D and
Teles AR: Intramuscular myxoma of the cervical paraspinal muscle.
Eur Spine J. 18(Suppl 2): 245–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Papadogeorgakis N, Petsinis V, Nikitakis
N, Goutzanis L and Alexandridis C: Intramuscular myxoma of the
masseter muscle. A case report. Oral Maxillofac Surg. 13:37–40.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishoo E: Intramuscular myxoma presenting
as a rare posterior neck mass in a young child: Case report and
literature review. Arch Otolaryngol Head Neck Surg. 133:398–401.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozawa H, Fujii M, Tomita T and Ogawa K:
Intramuscular myxoma of scalene muscle: A case report. Auris Nasus
Larynx. 31:319–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robin C, Bastidas JA and Boguslaw B: Case
report: Myxoma of the temporalis muscle. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 97:620–624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crankson SJ, Al Namshan M, Al Mane K and
Bamefleh H: Intramuscular myxoma: A rare neck mass in a child.
Pediatr Radio. 32:120–122. 2002. View Article : Google Scholar
|
|
25
|
van Roggen JF, McMenamin ME and Fletcher
CD: Cellular myxoma of soft tissue: A clinicopathological study of
38 cases confirming indolent clinical behaviour. Histopathology.
39:287–297. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Serrat A, Verrier A, Espeso A and Martin
J: Intramuscular myxoma of the temporalis muscle. J Oral Maxillofac
Surg. 56:1206–1208. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orlandi A, Bianchi L, Marino B, Spagnoli
LG and Nini G: Intramuscular myxoma of the face: An unusual
localization. A clinicopathological study. Dermatol Surg.
21:251–254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mockli GC, Ljung BM and Goldman RL: Fine
needle aspiration of intramuscular myxoma of the tongue. A case
report. Acta Cytol. 37:226–228. 1993.PubMed/NCBI
|
|
29
|
Shugar JM, Som PM, Meyers RJ and Schaeffer
BT: Intramuscular head and neck myxoma: Report of a case and review
of the literature. Laryngoscope. 97:105–107. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hashimoto H, Tsuneyoshi M, Daimaru Y,
Enjoji M and Shinohara N: Intramuscular myxoma. A
clinicopathologic, immunohistochemical and electron microscopic
study. Cancer. 58:740–747. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishijima W, Tokita N, Watanabe I and
Takooda S: Intramuscular myxoma of the neck. Arch Otolaryngol.
111:699–701. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wood WJ Jr: Intramuscular myxoma: Report
of two cases and review of the literature. Ariz Med. 42:417–419.
1985.PubMed/NCBI
|
|
33
|
Bedrosian SA, Goldman RL and Pearl MJ:
Intramuscular myxoma of the masseter. J Oral Maxillofac Surg.
42:684–686. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feldman PS: A comparative study including
ultrastructure of intramuscular myxoma and myxoid liposarcoma.
Cancer. 43:512–525. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Canalis RF, Smith GA and Konrad HR:
Myxomas of the head and neck. Arch Otolaryngol. 102:300–305. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kindblom LG, Stener B and Angervall L:
Intramuscular myxoma. Cancer. 34:1737–1744. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosin RD: Intramuscular myxomas. Br J
Surg. 60:122–124. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dutz W and Stout AP: The myxoma in
childhood. Cancer. 14:629–635. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Louvel R: Benign myxoma of the cheek; case
report. Prensa Med Argent. 44:3083–3084. 1957.(In Spanish).
PubMed/NCBI
|
|
40
|
Mazabraud A, Semat P and Roze R: Apropos
of the association of fibromyxomas of the soft tissues with fibrous
dysplasia of the bones. Presse Med. 75:2223–2228. 1967.(In French).
PubMed/NCBI
|
|
41
|
Kabukcuoglu F, Kabukcuoglu Y, Yilmaz B,
Erdem Y and Evren I: Mazabraud's syndrome: Intramuscular myxoma
associated with fibrous dysplasia. Pathol Oncol Res. 10:121–123.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dormand EL, Prabhu-Desai A, Rice AJ and
Rosin RD: Not all pain in the left iliac fossa is diverticular
disease: A case study of a psoas myxoma and review. Surgeon.
4:239–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Silver WP, Harrelson JM and Scully SP:
Intramuscular myxoma: A clinicopathologic study of 17 patients.
Clin Orthop Relat Res. 191–197. 2002. View Article : Google Scholar : PubMed/NCBI
|