Introduction
Diffuse large B-cell lymphoma (DLBCL), the most
common type of non-Hodgkin lymphoma, is a heterogeneous group of
tumors with an aggressive clinical course (1). The application of R-CHOP as the
first-line treatment regimen has led to complete remission for
75–80% of patients (2). However, ~20%
of patients are not sensitive to this immunochemotherapy, and these
patients then relapse following the first-line regimen and tend to
respond poorly to additional chemotherapy lines. Studies suggest
that abnormalities in signaling pathways including NF-YB, SFPQ,
MYBL2, BIM/APAF-1, AEG-1, BCL-2, XIAP, DR4 and ABCA1 are involved
in primary or acquired chemoresistance and poor prognosis (3–9).
Therefore, the identification of chemoresistance-related biomarkers
may achieve a better prediction of chemotherapy efficacy for DLBCL
patients, and could be used for the diagnosis of poor outcome
cases, providing an advanced treatment strategy.
microRNAs (miRNAs) are small, non-coding RNAs that
regulate gene expression at the post-transcriptional level through
sequence complementation with target mRNAs to repress transcription
or induce mRNA degradation (10).
Previous studies have determined a link between the expression of
miRNAs and cancer pathogenesis, and miRNAs have revealed themselves
to be significant biomarkers for tumor diagnosis, invasion,
metastasis and evaluation of prognosis in certain types of tumors
(11–14). However, chemoresistance-related miRNA
expression profiles in DLBCL are reported less often. In this
study, we aim to identify a series of serum miRNAs that may be
involved in drug resistance in a cohort of 56 DLBCL patients
treated with R-CHOP, then focus on monitoring the changes in these
chemoresistance-related miRNAs dynamically. The correlation between
serum and tumor tissues of miRNA expression levels was also
assessed at the start of the study. Our results may help to
elucidate potential chemoresistance-related miRNAs for DLBCL so as
to provide guidance for a rational therapeutic schedule for
clinical use.
Materials and methods
Patients
A total of 56 DLBCL patients undergoing treatment in
Shandong Cancer Hospital, China, between May 2010 and June 2011
were recruited in this study. A summary of the DLBCL patient
details is provided in Table I. All
patients had a histologically confirmed diagnosis of DLBCL
according to the 2008 World Health Organization classification. All
cases demonstrated positive staining for CD20. Patients who had
localized extranodal invasion only, central nervous system
involvement, previous immunosuppressive treatments, or who were
associated with HIV or HBV infections were excluded.
Formalin-fixed, paraffin-embedded (FFPE) tissues and blood samples
were collected before patients were treated. In addition, blood
samples from twenty age-and gender-matched healthy subjects were
also collected. The study was approved by the Medical Ethics
Committee of Shandong Cancer Hospital for Clinical Research, and
was conducted in accordance with the Declaration of Helsinki.
Written informed consent for use of biomaterials was obtained from
all patients and donors.
 | Table I.Clinical characteristics of 56
patients with diffuse large B-cell lymphoma. |
Table I.
Clinical characteristics of 56
patients with diffuse large B-cell lymphoma.
| Clinical
characteristics | Number (%) |
|---|
| Age (range) | 23–74 |
| Median age | 54.7 |
|
<55 | 26 (46) |
|
≥55 | 30 (54) |
| Gender |
|
|
Male | 32 (57) |
|
Female | 24 (43) |
| B symptoms |
|
|
Yes | 15 (29) |
| No | 41 (71) |
| Extranodal site
involvement |
|
|
Yes | 38 (68) |
| No | 18 (32) |
| Ann Arbor
stage |
|
| I | 6 (10) |
| II | 13 (24) |
|
III | 21 (38) |
| IV | 16 (28) |
| International
prognostic index |
|
|
0–2 | 16 (28) |
| ≥3 | 40 (72) |
| Disease
progression |
|
|
Yes | 29 (52) |
| No | 27 (48) |
| Chemotherapy
response |
|
| Drug
sensitivity | 35 (62) |
| Drug
resistance | 21 (38) |
Treatment and follow-up
All patients received R-CHOP chemotherapy as the
first-line treatment and this was administered for 6–8 cycles in
most cases. The R-CHOP regimen consisted of rituximab 375
mg/m2 on day 0, cyclophosphamide 600 mg/m2 on
day 1, vincristine 1.4 mg/m2 on day 1, epirubicin 60
mg/m2 on day 1 and prednisolone 60 mg/m2 on
days 1–5. If patients were defined as having no response or
progression during the treatment, or relapsing after achievement of
complete remission or unconfirmed complete remission within 3
months of the end of treatment, it was considered that treatment
failure and chemotherapy resistance had occurred. Such patients
went on to receive second-line regimens. Responses were evaluated
according to the Revised International Workshop criteria (15).
All follow-up data were updated in October 2014.
Patients who succumbed for other reasons (treatment toxicity
unrelated to the disease, for example) or who had insufficient data
available were excluded, leaving 56 patients in the final analysis.
Tumor response was re-evaluated after every two cycles (after
cycles 2, 4, 6 and 8) of chemotherapy. Then periodic examination
was performed every 3 months for the first year and every 6 months
for the next years. Blood counts, a blood biochemical examination,
an electrocardiogram, thoracic and abdominal computed tomography CT
scans and a bone marrow biopsy were performed every time. Five
milliliters of peripheral blood was obtained at every periodic
examination to monitor serum miRNA levels dynamically. The blood
samples were allowed to stand for ~1 h at room temperature. The
samples were centrifuged at 2,000 × g for 15 min, then centrifuged
again at 12,000 × g for 10 min at 4°C. The resultant serum was
aliquoted into Eppendorf tubes (Eppendorf, Hamburg, Germany) and
stored at −80°C until further use.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total miRNA was extracted from the FFPE using the
RecoverAll™ Total Nucleic Acid isolation kit (Ambion, Austin, TX,
USA), and from the peripheral blood using a miRcute miRNA isolation
kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer's instructions. Total miRNA quality and quantity were
assessed using the NanoDrop 1000 (Thermo Scientific, Wilmington,
DE, USA). Then miRNA was reverse transcribed using a miRcute miRNA
First-Strand cDNA synthesis kit (Tiangen), and qPCR was performed
using a miRcute miRNA qPCR detection kit (Tiangen). The forward
primers for amplification are shown in Table II and the common reverse primers were
provided with the kit. Twenty microliters of the reaction product
was incubated at 94°C for 2 min, then 40 cycles at 94°C for 20 sec
and 60°C for 35 sec were performed with ABI PRISM 7000HT (Applied
Biosystems, Foster City, CA, USA). Each sample was run in
triplicate. The miRNA expression value was expressed relative to
that of U6 and the 2−ΔCT method was used in the analysis
of PCR data [ΔCt = mean Ct (miRNA of interest) - mean Ct (U6)].
 | Table II.Forward primer sequences. |
Table II.
Forward primer sequences.
| Name | Sequences
(5′-3′) |
|---|
| miR-155 |
TTAATGCTAATCGTGATAGCCTG |
| miR-200c |
TGGCAATAATACTGCCGGGTAAG |
| miR-29c |
CCGGTACTAGCACCATTTGAAATCG |
| miR-130a |
TTCGGCAGTGCAATGTTAAGC |
| miR-145 |
ATCCGCGTCCAGTTTTCCCAGGA |
| miR-451 |
CGTTAAACCGTTACCATTACTTCG |
| miR-125b |
GGGCACTCCCTGAGCCCTAAC |
| miR-21 |
CGCATCCGGTAGCTTATCAGACTGA |
| U6 |
CGGCTTCGGCAGCACATATAAC |
Statistical analysis
The statistical differences in mean values were
tested using the Mann-Whitney U test. Pearson's correlation
analysis was carried out to assess the correlation of miRNAs
expression levels between the serum samples and FFPE tissue
samples. Receiver operating characteristic (ROC) curves were
constructed and the area under the ROC curve (AUC) was calculated
to evaluate the specificity and sensitivity of the diagnostic and
predictive values of miRNA levels. Overall survival (OS) was
calculated as the time from diagnosis to the date of mortality or
last contact. The OS of different groups of DLBCL patients was
calculated using the Kaplan-Meier method and compared by the
log-rank test. The percentage of patients alive at the median
follow-up time and 95% confidence intervals were noted. All tests
were two-sided, and P≤0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed with GraphPad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Characteristics of enrolled
patients
The baseline characteristics of the 56 patients are
summarized in Table I. The median
follow-up time for all patients was 37 months (range, 2–50 months).
Among these patients, 29 patients (52%) experienced disease
progression during or after treatment with R-CHOP, of which 5
progressed during the treatment and 24 progressed after the
treatment. Twenty-seven patients (48%) succumbed due to disease
progression. The chemotherapy response of the 56 patients was as
follows: 21 patients experienced drug resistance to R-CHOP, of
which 7 had primary resistance and 14 had secondary resistance. In
addition, 35 patients remained sensitive to chemotherapy from
beginning to end.
Correlation of miRNA expression levels
in serum and FFPE tissue
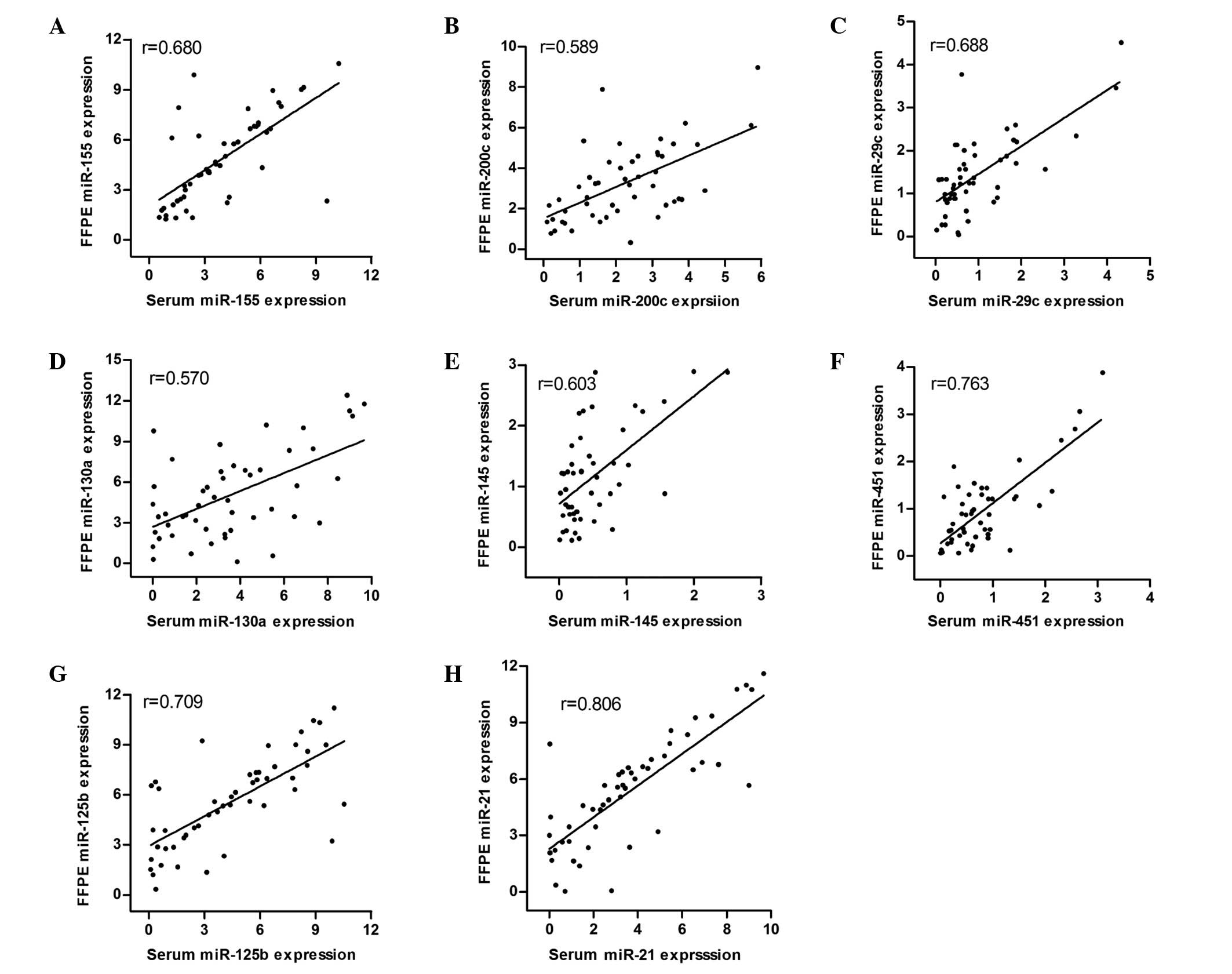
To elucidate the correlation of miRNA expression
levels between serum and FFPE tissue, we analyzed the expression
levels of eight miRNAs in the 56 DLBCL patients prior to treatment.
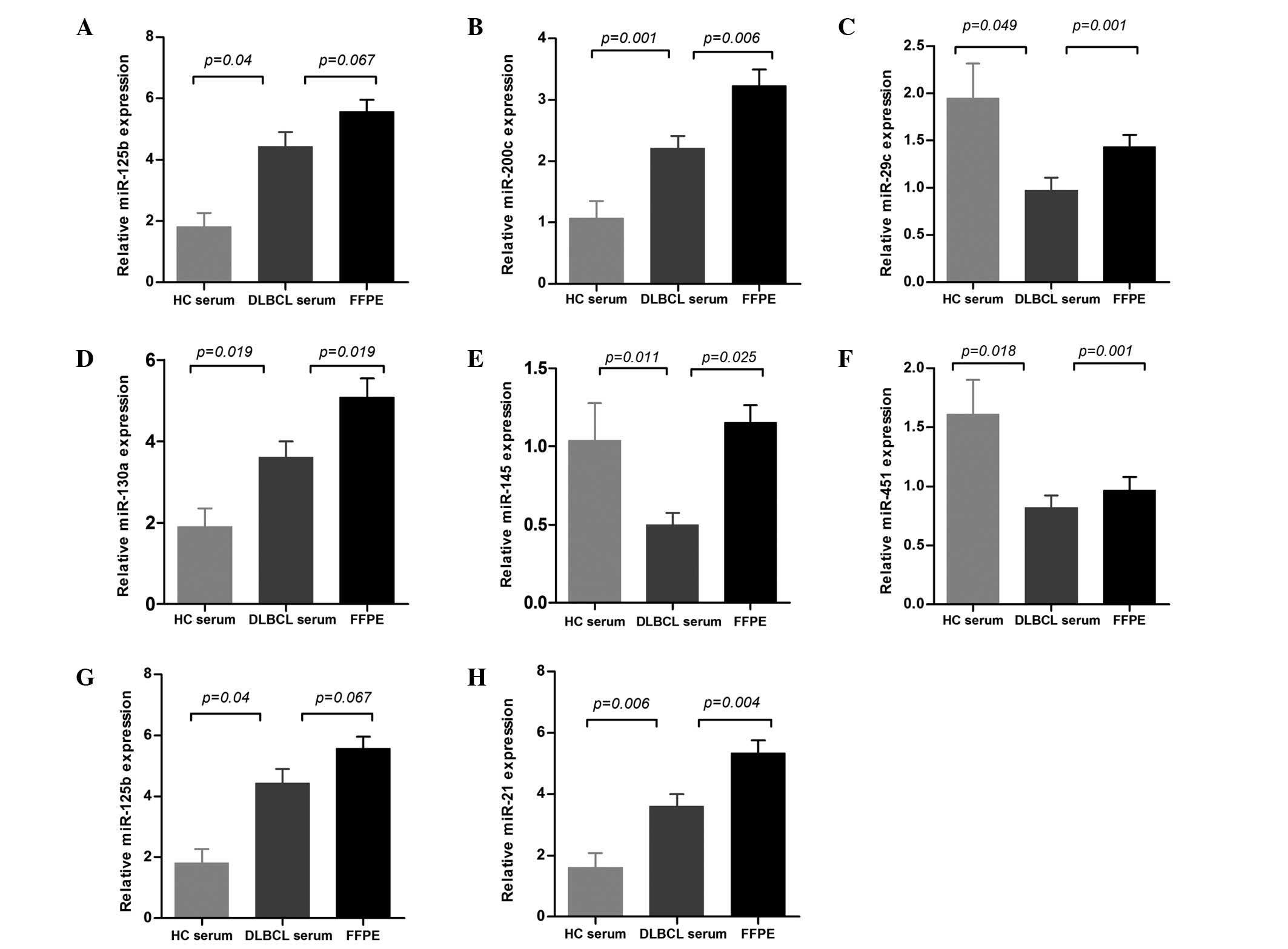
The expression levels of miR-155, miR-200c, miR-29c, miR-130a,
miR-145, miR-451 and miR-21 (P=0.035, P=0.006, P=0.001, P=0.019,
P=0.025, P=0.001 and P=0.004, respectively) were significantly
higher in FFPE than those in serum. The expression of miR-125b was
also higher in FFPE, although this was not significant (P=0.067;
Fig. 1). Furthermore, Pearson's
correlation analysis revealed that the serum miR-155, miR-200c,
miR-29c, miR-130a, miR-145, miR-451, miR-125b and miR-21 expression
levels were significantly associated with their expression levels
in FFPE tissues. (r=0.68, r=0.589, r=0.688, r=0.57, r=0.603,
r=0.763, r=0.709 and r=0.806, respectively; Fig. 2).
Dysregulated expression profiles of
eight miRNAs in DLBCL serum
The expression of eight miRNAs was compared in the
serum of the 56 DLBCL patients and 20 healthy controls, and all of
the miRNAs were observed to be diversely dysregulated in DLBCL
serum. The levels of miR-155, miR-200c, miR-130a, miR-125b and
miR-21 were significantly upregulated (P=0.004, P=0.001, P=0.019,
P=0.04 and P=0.006, respectively), whereas the levels of miR-29c,
miR-451 and miR-145 were downregulated (P=0.049, P=0.011 and
P=0.018, respectively) when compared with healthy controls
(Fig. 1).
Expression status of eight miRNAs
associated with chemoresistance
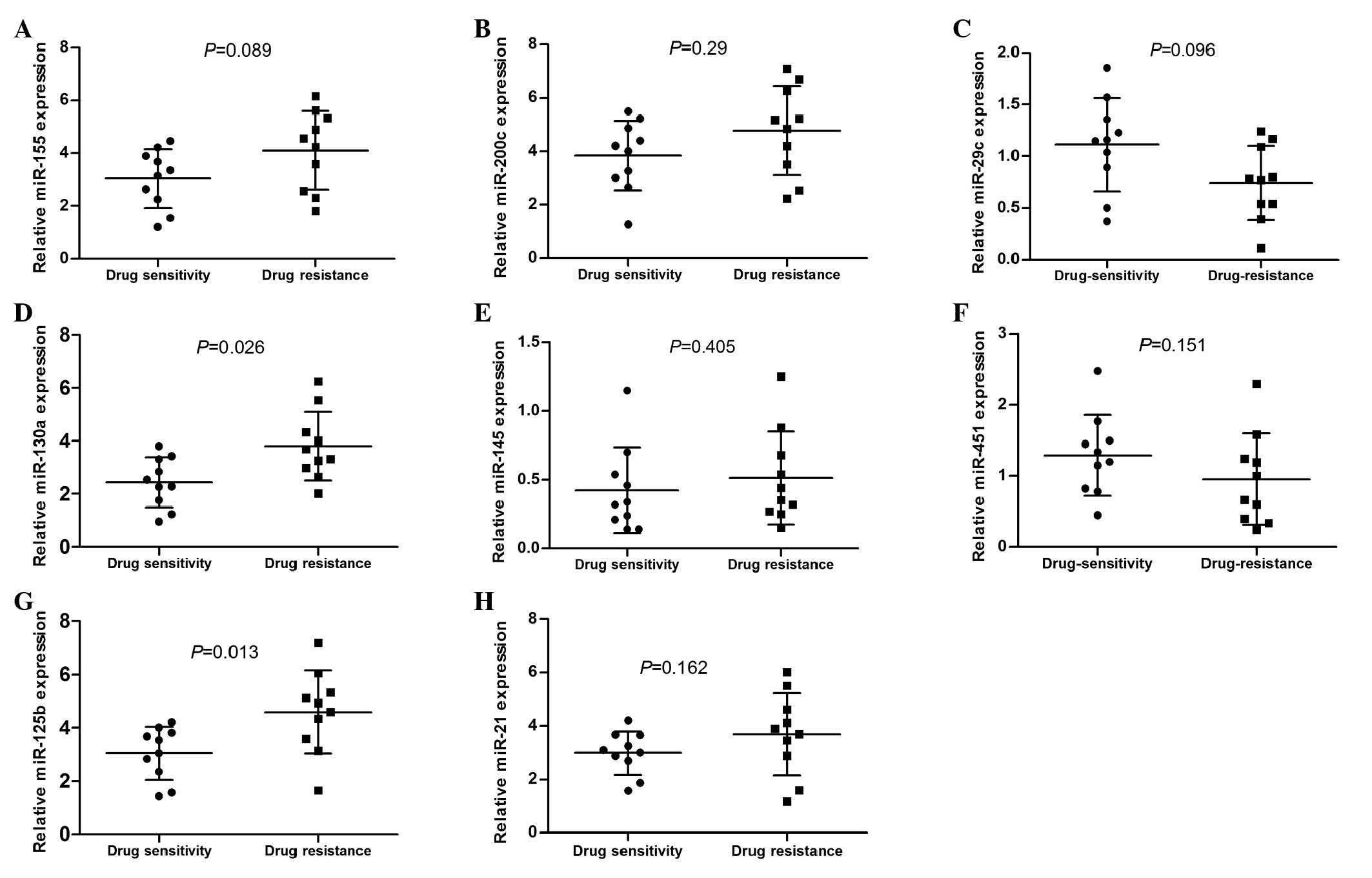
A total of 20 DLBCL patients were enrolled in the
preliminary experiment, of which 10 were chemotherapy-resistant
cases and 10 were chemotherapy-sensitive cases. There were no
significant differences between the two groups in terms of gender,
age, international prognostic index (IPI) and stage. We
investigated the pretreatment concentrations of serum miR-155,
miR-200c, miR-29c, miR-130a, miR-145, miR-451, miR-125b and miR-21
expression levels, which have been reported in certain other tumor
types to be associated with chemotherapy resistance (Table III) (9,14,16–29). We
observed that only the expression of miR-130a and miR-125b was
correlated significantly with the response to chemotherapy.
miR-130a and miR-125b were upregulated in the drug-resistant group
compared with the chemotherapy-sensitive group (P=0.026 and
P=0.013, respectively; Fig. 3).
 | Table III.Eight selected miRNAs involved in
chemoresistance. |
Table III.
Eight selected miRNAs involved in
chemoresistance.
| miRNA | Expression | Targets | Drug
resistance | Tissue type | References |
|---|
| miR-125b | Up | E2F3 | 5-FU | Breast cancer | Wang et al
(16) |
|
| Up | ABCC4 | Multidrug | Hepatocellular
carcinoma | Borel et al
(9) |
| miR-451 | Down | ABCB1 | Irinotecan | Colon cancer | Bitarte et
al (17) |
| miR-29c | Down | Bcl-2 Mcl-1 | Cisplatin | Nasopharyngeal
carcinoma | Zhang et al
(18) |
| miR-200c | Up | AKT | Cisplatin | Esophageal
cancer | Hamano et al
(19) |
|
| Down | TrkB Bmi1 | Doxorubicin | Breast cancer | Kopp et al
(20) |
|
| Down | Bmi1 | Cisplatin | Melanoma | Liu et al
(21) |
|
| Down |
| Paclitaxel | Ovarian cancer | Cittelly et
al (14) |
| miR-155 | Up | FOXO3a | Doxorubicin | Breast cancer | Kong et al
(22) |
| miR-145 | Down | Oct4 Sox2 | TMZ | Glioblastoma | Yang et al
(23) |
| miR-21 | Up | STAT3 | 5-FU | Glioblastoma | Ren et al
(24) |
|
| Up | PTEN | CHOP | Diffuse large
B-cell lymphoma | Bai et al
(25) |
| miR-130a | Down | XIAP | Cisplatin | Ovarian cancer | Zhang et al
(26) |
|
| Down | Met | Gefitinib | Lung cancer | Zhou et al
(27) |
|
| Up |
MDR1/P-glycoprotein | Cisplatin | Ovarian cancer | Yang et al
(28) |
|
| Up | RUNX3 Wnt | Cisplatin | Hepatocellular
carcinoma | Xu et al
(29) |
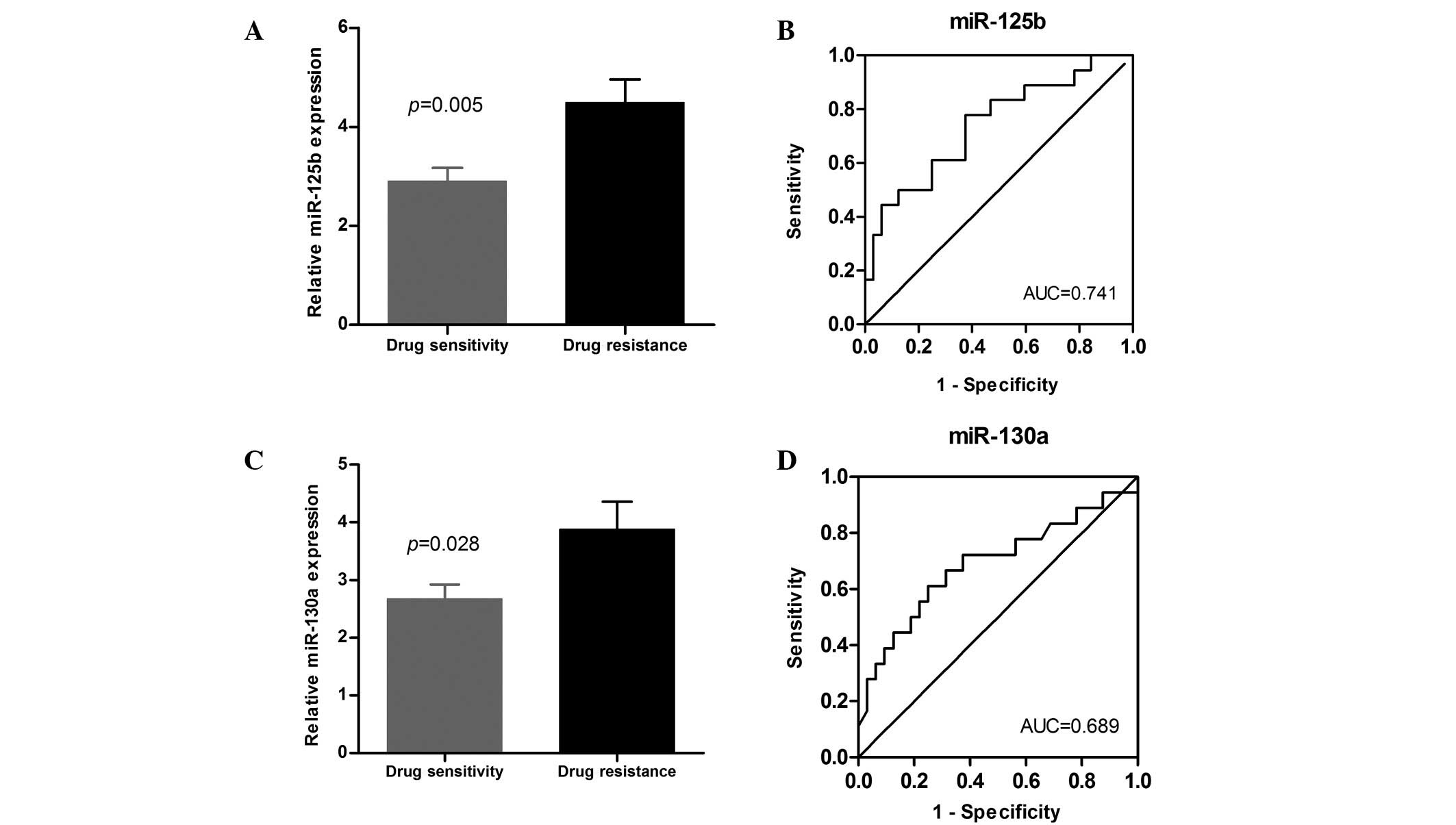
To further verify the discriminating power of
miR-130a and miR-125b identified in the preliminary marker
selection stage, the levels of the two miRNAs were measured in a
cohort of 56 DLBCL patients comprising 21 drug-resistant cases and
35 drug-sensitive cases, which also included the 20 cases from the
preliminary experiment. In line with the results of the preliminary
evaluation of 20 patients, miR-130a and miR-125b were significantly
elevated in the serum of the chemoresistant cases (P=0.028 and
P=0.005, respectively; Fig. 4A and
C). To verify the sensitivity and specificity of miR-130a and
miR-125b, we used the the ROC curves to demonstrate the relative
separation of the chemoresistance and chemosensitivity groups with
the AUCs. These were identified to be 0.689 for miR-130a (95% CI,
0.524–0.854; P=0.028), and 0.741 for miR-125b (95% CI, 0.595–0.887;
P=0.005; Fig. 4B and D). At the
cut-off value of 3.275/3.07 for miR-130a and miR-125b, the
sensitivity was 61.1/77.8% and the specificity was 75.0/62.5%,
respectively.
miR-130a and miR-125b are involved in
recurrence, progression and chemoresistance of DLBCL
To directly test the correlation between miR-130a
and miR-125b expression and patients' response to chemotherapy, the
levels of the two high-risk miRNAs were dynamically analyzed.
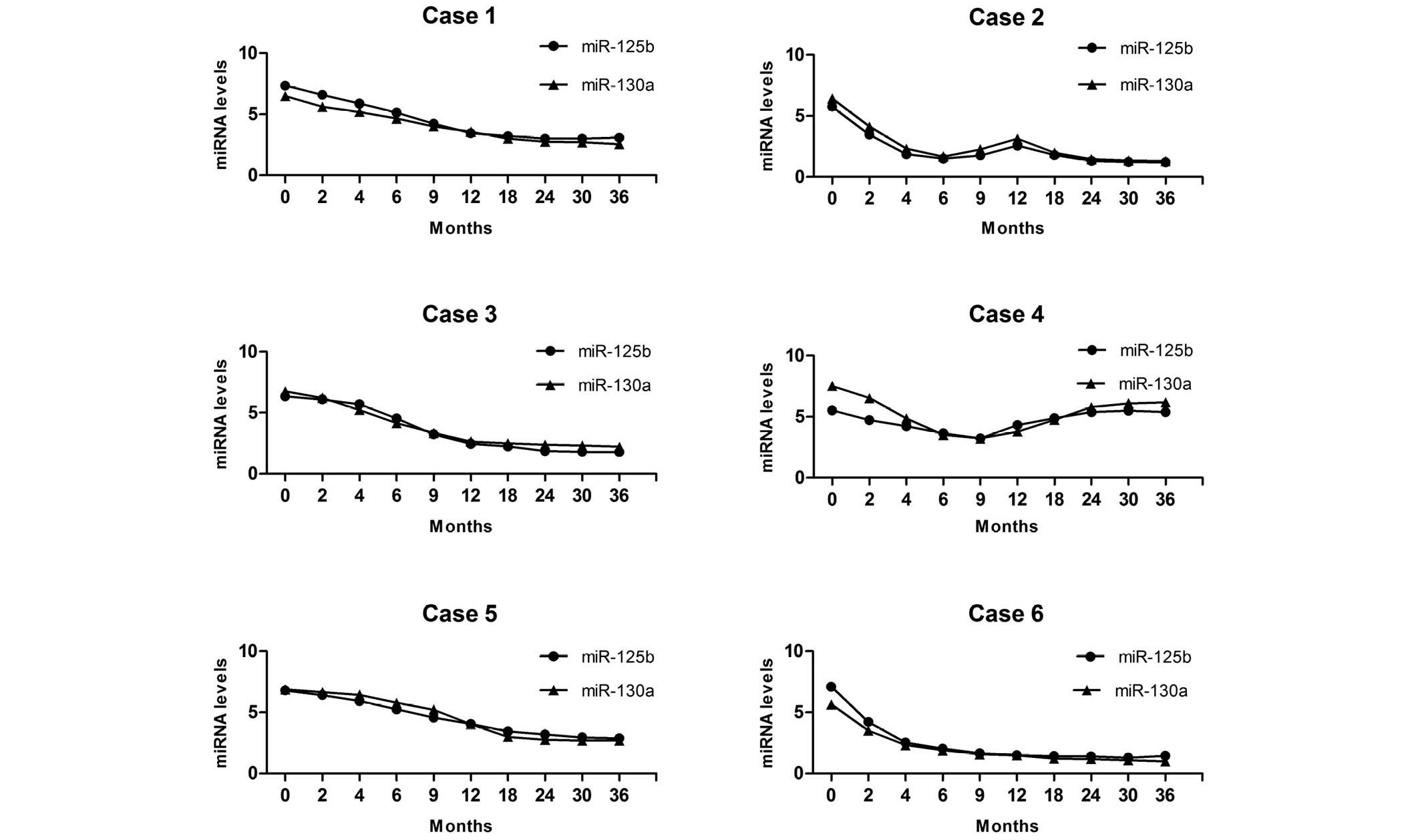
Fig. 5 reveals the miR-130a and
miR-125b continuous-time analysis profiles for six DLBCL patients.
In case 1 and case 6, where no recurrence or progression was
observed during the three-year follow-up, serum miR-130a and
miR-125b expression decreased significantly at 2 months after
R-CHOP chemotherapy and returned to normal levels at 12 months
after the treatments. Case 2 relapsed following a long-term
remission, then achieved remission again with the R-CHOP
chemotherapy. In this case, the serum miR-130a and miR-125b
expression decreased after the first treatment, but increased at 12
months, then decreased again. In case 3, R-CHOP was switched for
the second-line regimen at 3 months due to primary refractory
disease when using the first-line therapy. In this case the serum
miR-130a and miR-125b expression was not significantly
downregulated even following the change in the therapeutic
schedule. Case 4, who relapsed at 18 months, received R-CHOP and
the second-line regimen for salvage treatment, but failure occurred
due to chemoresistance. In this case the serum miR-130a and
miR-125b expression was re-elevated following the first treatment.
Case 5 was administered the second-line regimen following a partial
response, and in this case the serum miR-130a and miR-125b did not
significantly decrease for 6 months.
Based on our observations, serum miR-130a and
miR-125b changes always occur ahead of the clinical diagnosis of
recurrence or progression. Patients who maintained miR-130a and
miR-125b overexpression had a significantly increased probability
of chemoresistance compared with those with none or only one
high-regulated miRNA. In addition, miR-125b and miR-130a levels
always remained high before treatment, then returned to normal
levels gradually following R-CHOP treatment in drug-resistant
cases. In contrast, in primary/secondary resistant cases, these
miRNAs did not decrease significantly or increase again after
remaining at normal levels for some time. Moreover, re-elevated
levels of miR-125b and miR-130a were also observed in patients with
recurrence or progression.
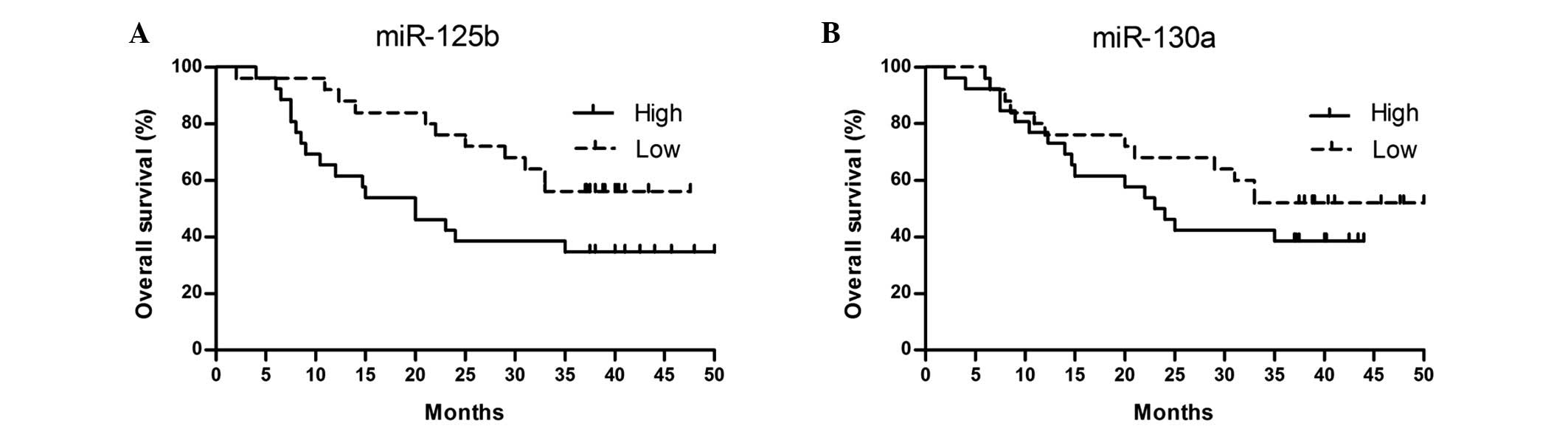
High levels of miR-130a and miR-125b
are associated with poor prognosis
To calculate the potential prognostic impact of
miR-130a and miR-125b in DLBCL, the patients were divided into two
groups according to the median value of their expression levels of
the two miRNAs. Using Kaplan-Meier survival analysis, we observed
that DLBCL patients with high miR-125b but not miR-130a relative
expression had a significantly shorter OS compared with those with
a low expression. The three-year OS rates of DLBCL patients was 44%
(95% CI, 30.25–41.72) in the high miR-125b group and 65% (95% CI,
19.08–33.31) in the low miR-125b group (log-rank test, P=0.048).
The Kaplan-Meier curves according to miR-125b and miR-130a
expression are shown in Fig. 6.
Furthermore, we used multivariate Cox regression
analysis to examine the effect of various parameters (including
miR-125b levels as well as age, gender, B symptoms, Ann Arbor stage
and IPI) that may affect prognosis. We observed that the
upregulated miR-130a expression levels and high IPI scores were
independent indicators for poor outcome (Table IV).
 | Table IV.Multivariate Cox regression analysis
of various parameters involved in overall survival in diffuse large
B-cell lymphoma. |
Table IV.
Multivariate Cox regression analysis
of various parameters involved in overall survival in diffuse large
B-cell lymphoma.
| Factor | Cox regression
coefficient | Hazard ratio (95%
CI) | P-value |
|---|
| miR-125b | 0.399 | 1.491
(1.073–2.072) | 0.017 |
| miR-130a | 0.207 | 1.230
(0.938–1.624) | 0.135 |
| IPI status | 0.742 | 2.099
(1.068–4.126) | 0.031 |
Discussion
A number of investigators have attempted to identify
different miRNAs to be used for diagnosis or to identify cancer
patients with the poorest prognosis in order to design a more
appropriate therapeutic strategy for them (30). Understandably, due to the ease and the
non-invasive method of obtaining them, circulating miRNAs are
becoming more attractive biomarkers compared with miRNAs isolated
from tumor tissue, particularly in certain late-stage cancers. In
addition, studies have demonstrated that miRNAs are stable in serum
(30). Further studies have
identified a significant correlation between serum and tumor
tissues in certain solid tumors (31,32).
However, for DLBCL, as a hematological malignant disease, no
evidence for the clinical application of serum miRNA has been
demonstrated up to the present day. In this study, we confirmed
that miRNA expression levels in serum were significantly associated
with their levels in FFPE tissues in DLBCL patients before
treatment. We therefore propose that serum miRNAs levels might
reflect tumor cell growth of DLBCL, although serum from a larger
cohort of DLBCL patients should be tested.
Next, we demonstrated the overexpression of miR-155,
miR-200c, miR-130a, miR-125b and miR-21 and underexpression of
miR-29c, miR-451 and miR-145 in DLBCL serum. Notably, deregulation
profiling of most of these miRNAs in DLBCL has been reported in a
previous study (12), with the
exception of miR-130a and miR-451. In this study, we first
demonstrated the deregulated expression of circulating miR-130a and
miR-451 in DLBCL. According to the results of other studies,
miR-451 is a significant anti-oncomiRNA in colorectal carcinoma,
and promoted PI3k/AKT and LKB1/AMPK activation by repressing the
mitogen-activated protein kinase (MAPK) signaling pathway, and
caused an decreased in self-renewal, tumorigenicity and vascular
endothelial cell proliferation (33,34). In
accordance with the above results, we also detected reduced miR-451
expression, which confirmed the function of miR-451 as a oncogene
in DLBCL for the first time. miR-130a has been observed to play a
critical role in various types of cancer. He et al (35) observed that miRNA-130a was upregulated
in cervical cancer, which directly targeted Dicer mRNA, enhancing
cellular growth, migration and invasion. In contrast, Li et
al (36) detected that miR-130a
was significantly downregulated in hepatocellular carcinoma. In the
present study, we observed low miR-130a expression in DLBCL. The
contradiction may be attributed to the small cohort of clinical
experiments or the complex cell pathology and physiology in the
different tumors.
Chemoresistance as a multifactorial progress has
posed a critical issue in managing or preventing tumor progression.
Particularly for DLBCL, the choice of a first-line treatment
achieving complete remission without drug resistance is crucial. If
the R-CHOP induction chemotherapy fails, then the prognosis remains
poor even if another therapeutic schedule is used.
A number of previous studies have revealed that
miRNAs are involved in the progress of drug resistance in certain
types of cancer (16–29). These miRNAs might play a crucial role
in drug resistance by targeting their specific genes. In this
study, RT-qPCR was used to identify and quantitate the expression
levels of a series of miRNAs (namely miR-155, miR-200c, miR-29c,
miR-130a, miR-145, miR-451, miR-125b and miR-21) to evaluate their
low or high sensitivity to R-CHOP chemotherapy. Two miRNAs with
differential expression were identified: the expression of miR-130a
and miR-125b was not only notably upregulated in the drug resistant
groups treated with R-CHOP, but also demonstrated a strong
separation between the drug-resistance group and drug-sensitivity
group. miR-125b is an essential oncomiR, restraining the tumor
necrosis factor necrosis factor, alpha-induced protein 3 (TNFAIP3)
to enhance the nuclear factor kappa-light-chain-enhancer of
activated B-cells (NF-κB) signaling pathway, and is significantly
overexpressed in DLBCL (37). Davoudi
et al (38) reported that the
NF-κB pathway cross-talks with the PI3k/AKT pathway to promote
anti-apoptosis and multidrug resistance, and was correlated with
the expression of the MDR1 gene. When targeting the NF-κB pathway
by suppressing MDR1 gene expression, these authors observed
significantly increasing apoptotic stimuli and decreasing drug
resistance. In our study we also observed upregulated miR-125b, so
it was speculated that NF-κB, as one of the most altered pathways
in DLBCL (39), may mediate the
miR-125b-induced chemoresistance occurring in DLBCL patients
treated with the R-CHOP regimen. Zhang et al (26) reported that low miR-130a expression
led to a significant upregulation in the XIAP mRNA levels, which
further resulted in the development of ovarian cancer cell
resistance to cisplatin. However, Yang et al (28) provided the adverse conclusion that low
miR-130a restrains MDR1 mRNA/P-glycoprotein, which plays a
repressive role in the ABC superfamily drug transporters and the
drug resistance pathways of PI3k/AKT/PTEN/mTOR. Similarly,
upregulated miR-130a expression was also detected in the
drug-resistant group in the present study. miR-130a expression
profiling in DLBCL has not previously been reported in the
literature, and the apparently contradictory roles of miR-130a as
oncomiRNA or anti-oncomiRNA in different tumors may be due to
different circumstances or tissues. In addition, Bai et al
(25) reported that the upregulation
of miR-21 decreased the sensitivity of DLBCL cell lines to the CHOP
regimen; however, these results were not reproduced in our study,
so further research is required to confirm this finding.
Our further research focused on monitoring the
changes of circulating miR-125b and miR-130a levels dynamically.
According to our findings, patients with upregulated miR-130a and
miR-125b had a significant probability of recurrence, progression
and chemoresistance, whereas patients with downregulated miR-130a
and miR-125b were inclined to achieve complete remission and
chemosensitivity. In addition, we observed that the changes in
miRNA expression occurred prior to clinical manifestations and
image diagnosis. Therefore we deduced that miR-130a and miR-125b
may constitute new biomarkers for the determination of treatment
response and prognosis in DLBCL; in particular, assessment of the
two high-risk miRNAs simultaneously would be effective. More
notably, patients with miR-130a and miR-125b overexpression who
were resistant to R-CHOP were more inclined to be refractory to
other chemotherapy regimens; that is, miR-130a and miR-125b may be
associated with multidrug resistance. However, further research is
required to prove this theory. To our knowledge, only one similar
investigation relating to colorectal cancer has been published
which elaborates the implications of dynamically monitoring the
levels of miRNAs for disease progression and prognosis (40), and there are no other studies
concerned with DLBCL. Thereby, our study is the first to
demonstrate that dynamic monitoring of the levels of circulating
miR-125b and miR-130a could reflect the therapeutic response and
disease status of DLBCL patients, which may have considerable
significance for DLBCL management.
Since the introduction of rituximab and
hematopoietic stem cell transplantation in the treatment of DLBCL,
a relatively effective therapeutic model has been established.
Thereby, strategies including intensive immunochemotherapy have
improved the outcome for a number of such patients. However, there
is a certain risk that due to the inability to accurately
differentiate DLBCL, such patients are not receiving the necessary
intensive treatment. In addition, other patients at high risk who
are not diagnosed in a timely manner miss out on being considered
for immediate treatment. The IPI is one of the crucial prognostic
indicators and is based on patient characteristics including age,
lactate dehydrogenase levels and extranodal invasion status.
Although IPI retains a predictive role in DLBCL patients treated
with R-CHOP, to our knowledge, the application of IPI in the
rituximab era has not been established. Sehn et al (41) stated that IPI was no longer capable of
differentiating four risk groups in the rituximab era, and
advocated a revised IPI (R-CHOP). Even so, the high-risk group
still could not be discriminated accurately. For this reason,
additional prognostic factors are required to determine a favorable
or poor outcome in DLBCL patients so that appropriate therapeutic
strategies may be selected. In the present study, we explored the
feasibility of serum miR-125b and miR-130a, which are treatment
response-associated miRNAs, as outcome predictors for DLBCL
patients treated with R-CHOP. We observed that a low expression of
miR-125b was associated with a long OS; furthermore, serum miR-125b
signatures used as the prognostic indicator were independent of the
IPI score. Our results suggest that high serum miR-125b levels are
associated with a poor prognosis, and that serum miR-125b, a
simple, accurate, non-invasive biomarker which may be easily
measured in clinical practice, might be a significant prognostic
marker in the rituximab era.
In summary, our data demonstrated for the first time
that the expression of miR-125b and miR-130a has potential as a
chemoresistance-related indicator to evaluate the risk of
chemoresistance in DLBCL patients. In addition, it was also
demonstrated for the first time that dynamically monitoring the
levels of circulating miR-125b and miR-130a could reflect
therapeutic response and disease status in DLBCL patients. Notably,
miR-125b but not miR-130a was identified as an independent poor
prognostic factor. The correlation between miRNA expression levels
in serum and in FFPE tissues in DLBCL was also considered in our
study. Our results may help to provide a new potential biomarker
and therapeutic target for DLBCL. However, additional studies are
required to elucidate the molecular mechanisms of miR-125b and
miR-130a in chemoresistance and disease progression, and to
characterize the expression of these miRNAs in a large cohort of
DLBCL patients.
References
|
1
|
De Paepe P and De Wolf-Peeters C: Diffuse
large B cell lymphoma: a heterogeneous group of non-Hodgkin
lymphomas comprising several distinct clinicopathological entities.
Leukemia. 21:37–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coiffier B, Thieblemont C, Van Den Neste
E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M,
Sebban C, et al: Long-term outcome of patients in the LNH-98.5
trial, the first randomized study comparing rituximab-CHOP to
standard CHOP chemotherapy in DLBCL patients: a study by the groupe
d'Etudes des lymphomes de l'adulte. Blood. 116:2040–2045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CF, He X, Arslan AD, Mo YY, Reinhold
WC, Pommier Y and Beck WT: Novel regulation of nuclear factor-YB by
miR-485-3p affects the expression of DNA topoisomerase IIα and drug
responsiveness. Mol Pharmacol. 79:735–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurokawa K, Tanahashi T, Iima T, Yamamoto
Y, Akaike Y, Nishida K, Masuda K, Kuwano Y, Murakami Y, Fukushima M
and Rokutan K: Role of miR-19b and its target mRNAs in
5-fluorouracil resistance in colon cancer cells. J Gastroenterol.
47:883–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garofalo M, Romano G, Di Leva G, Nuovo G,
Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, et al:
EGFR and MET receptor tyrosine kinase-altered microRNA expression
induces tumorigenesis and gefitinib resistance in lung cancers. Nat
Med. 18:74–82. 2011.PubMed/NCBI
|
|
6
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J,
Jiang B, Shu Y and Liu P: miR-200bc/429 cluster modulates multidrug
resistance of human cancer cell lines by targeting BCL2 and XIAP.
Cancer Chemother Pharmacol. 69:723–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Razumilava N, Bronk SF, Smoot RL, Fingas
CD, Werneburg NW, Roberts LR and Mott JL: miR-25 targets
TNF-related apoptosis inducing ligand (TRAIL) death receptor-4 and
promotes apoptosis resistance in cholangiocarcinoma. Hepatology.
55:465–475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borel F, Han R, Visser A, Petry H, van
Deventer SJ, Jansen PL and Konstantinova P: Réseau Centre de
Ressources Biologiques Foie (French Liver Biobanks Network),
France: Adenosine triphosphate-binding cassette transporter genes
up-regulation in untreated hepatocellular carcinoma is mediated by
cellular microRNAs. Hepatology. 55:821–832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan X, Wang R and Wang ZX: The potential
role of miR-451 in cancer diagnosis, prognosis and therapy. Mol
Cancer Ther. 12:1153–1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tagawa H, Ikeda S and Sawada K: Role of
microRNA in the pathogenesis of malignant lymphoma. Cancer Sci.
104:801–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Y, Song X, Du H, Luo C, Wang X, Yang
X, Wang Y and Wu X: Down-regulation of miR-29c in human bladder
cancer and the inhibition of proliferation in T24 cell via PI3K-AKT
pathway. Med Oncol. 31:652014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cittelly DM, Dimitrova I, Howe EN,
Cochrane DR, Jean A, Spoelstra NS, Post MD, Lu X, Broaddus RR,
Spillman MA and Richer JK: Restoration of miR-200c to ovarian
cancer reduces tumor burden and increases sensitivity to
paclitaxel. Mol Cancer Ther. 11:2556–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Tan G, Dong L, Cheng L, Li K, Wang
Z and Luo H: Circulating MiR-125b as a marker predicting
chemoresistance in breast cancer. PLoS One. 7:e342102012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bitarte N, Bandres E, Boni V, Zarate R,
Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM,
Fortes P and Garcia-Foncillas J: MicroRNA-451 is involved in the
self-renewal, tumorigenicity and chemoresistance of colorectal
cancer stem cells. Stem Cells. 29:1661–1671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang JX, Qian D, Wang FW, Liao DZ, Wei
JH, Tong ZT, Fu J, Huang XX, Liao YJ, Deng HX, et al: MicroRNA-29c
enhances the sensitivities of human nasopharyngeal carcinoma to
cisplatin-based chemotherapy and radiotherapy. Cancer Lett.
329:91–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamano R, Miyata H, Yamasaki M, Kurokawa
Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M and
Doki Y: Overexpression of miR-200c induces chemoresistance in
esophageal cancers mediated through activation of the AKT signaling
pathway. Clin Cancer Res. 17:3029–3038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kopp F, Oak PS, Wagner E and Roidl A:
miR-200c sensitizes breast cancer cells to doxorubicin treatment by
decreasing TrkB and Bmi1 expression. PLoS One. 7:e504692012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Tetzlaff MT, Cui R and Xu X:
miR-200c inhibits melanoma progression and drug resistance through
down-regulation of BMI-1. Am J Pathol. 181:1823–1835. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kong W, He L, Coppola M, Guo J, Esposito
NN, Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth and chemosensitivity by targeting FOXO3a in breast cancer. J
Biol Chem. 285:17869–17879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang YP, Chien Y, Chiou GY, Cherng JY,
Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, et al:
Inhibition of cancer stem cell-like properties and reduced
chemoradioresistance of glioblastoma using microRNA145 with
cationic polyurethane-short branch PEI. Biomaterials. 33:1462–1476.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren Y, Kang CS, Yuan XB, Zhou X, Xu P, Han
L, Wang GX, Jia Z, Zhong Y, Yu S, et al: Co-delivery of as-miR-21
and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell
growth in vitro. J Biomater Sci Polym Ed. 21:303–314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai H, Wei J, Deng C, Yang X, Wang C and
Xu R: MicroRNA-21 regulates the sensitivity of diffuse large B-cell
lymphoma cells to the CHOP chemotherapy regimen. Int J Hematol.
97:223–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Huang L, Zhao Y and Tan W:
Downregulation of miR-130a contributes to cisplatin resistance in
ovarian cancer cells by targeting X-linked inhibitor of apoptosis
(XIAP) directly. Acta Biochim Biophys Sin (Shanghai). 45:995–1001.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou YM, Liu J and Sun W: MiR-130a
overcomes gefitinib resistance by targeting met in non-small cell
lung cancer cell lines. Asian Pac J Cancer Prev. 15:1391–1396.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Li N, Wang H, Jia X, Wang X and
Luo J: Altered microRNA expression in cisplatin-resistant ovarian
cancer cells and upregulation of miR-130a associated with
MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep.
28:592–600. 2012.PubMed/NCBI
|
|
29
|
Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q
and Zhang J: Upregulated miR-130a increases drug resistance by
regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell.
Biochem Biophys Res Commun. 425:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: a new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
LaConti JJ, Shivapurkar N, Preet A,
Deslattes Mays A, Peran I, Kim SE, Marshall JL, Riegel AT and
Wellstein A: Tissue and serum microRNAs in the Kras(G12D)
transgenic animal model and in patients with pancreatic cancer.
PLoS One. 6:e206872011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahn R, Heukamp LC, Rogenhofer S, von
Ruecker A, Müller SC and Ellinger J: Circulating microRNAs (miRNA)
in serum of patients with prostate cancer. Urology. 77:1265.e9–e16.
2011. View Article : Google Scholar
|
|
33
|
Li HY, Zhang Y, Cai JH and Bian HL:
MicroRNA-451 inhibits growth of human colorectal carcinoma cells
via down-regulation of Pi3k/Akt pathway. Asian Pac J Cancer Prev.
14:3631–3634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen H, Untiveros GM, McKee LA, Perez J,
Li J, Antin PB and Konhilas JP: Micro-RNA-195 and −451 regulate the
LKB1/AMPK signaling axis by targeting MO25. PLoS One. 7:e415742012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He L, Wang HY, Zhang L, Huang L, Li JD,
Xiong Y, Zhang MY, Jia WH, Yun JP, Luo RZ and Zheng M: Prognostic
significance of low DICER expression regulated by miR-130a in
cervical cancer. Cell Death Dis. 5:e12052014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li B, Huang P, Qiu J, Liao Y, Hong J and
Yuan Y: MicroRNA-130a is down-regulated in hepatocellular carcinoma
and associates with poor prognosis. Med Oncol. 31:2302014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SW, Ramasamy K, Bouamar H, Lin AP,
Jiang D and Aguiar RC: MicroRNAs miR-125a and miR-125b
constitutively activate the NF-κB pathway by targeting the tumor
necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl
Acad Sci USA. 109:7865–7870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davoudi Z, Akbarzadeh A, Rahmatiyamchi M,
Movassaghpour AA, Alipour M, Nejati-Koshki K, Sadeghi Z,
Dariushnejad H and Zarghami N: Molecular target therapy of AKT and
NF-kB signaling pathways and multidrug resistance by specific cell
penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer
Prev. 15:4353–4358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Compagno M, Lim WK, Grunn A, Nandula SV,
Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano
A, et al: Mutations of multiple genes cause deregulation of
NF-kappaB in diffuse large B-cell lymphoma. Nature. 459:717–721.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, Wang W, Zhang Y, Chen Y and Hu T:
Predicting distant metastasis and chemoresistance using plasma
miRNAs. Med Oncol. 31:7992014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised international prognostic index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|