Introduction
Metanephric adenoma (MA) is a rare and frequently
benign tumor that accounts for 0.2–0.7% of adult renal epithelial
neoplasms (1,2). MA is observed predominantly in women,
with a 2:1 female to male ratio (3).
Currently, <200 cases have been reported in the literature,
often through case reports. The clinical presentation of MA is
similar to malignant renal masses; MA possesses two distinct renal
lesions, which share several morphological and immunohistochemical
features with solid variants of papillary renal cell carcinomas
(2). Consequently, this may lead to
potential misdiagnosis and inadequate treatment. The present study
reports the case of a 54-year-old female that presented with MA
associated with polycythemia.
Case Report
A 54-year-old female patient presented to the
Affiliated Hospital of Guizhou Medical University (Guiyang, China),
with complaints of intermittent right flank pain and anterior
abdominal pain that occurred over a 2-year period and sporadic
gross hematuria that occurred over 3 months in March 2013. The
patient had no other symptoms. Physical examination revealed no
significant conclusions. Urinalysis revealed hematuria in the urine
culture and a routine blood examination exhibited a hematocrit
(Hct) volume of 61%, a hemoglobin volume of 174 g/l, and a red
blood cell count of 6.2×1012cells/l. Ultrasonography and
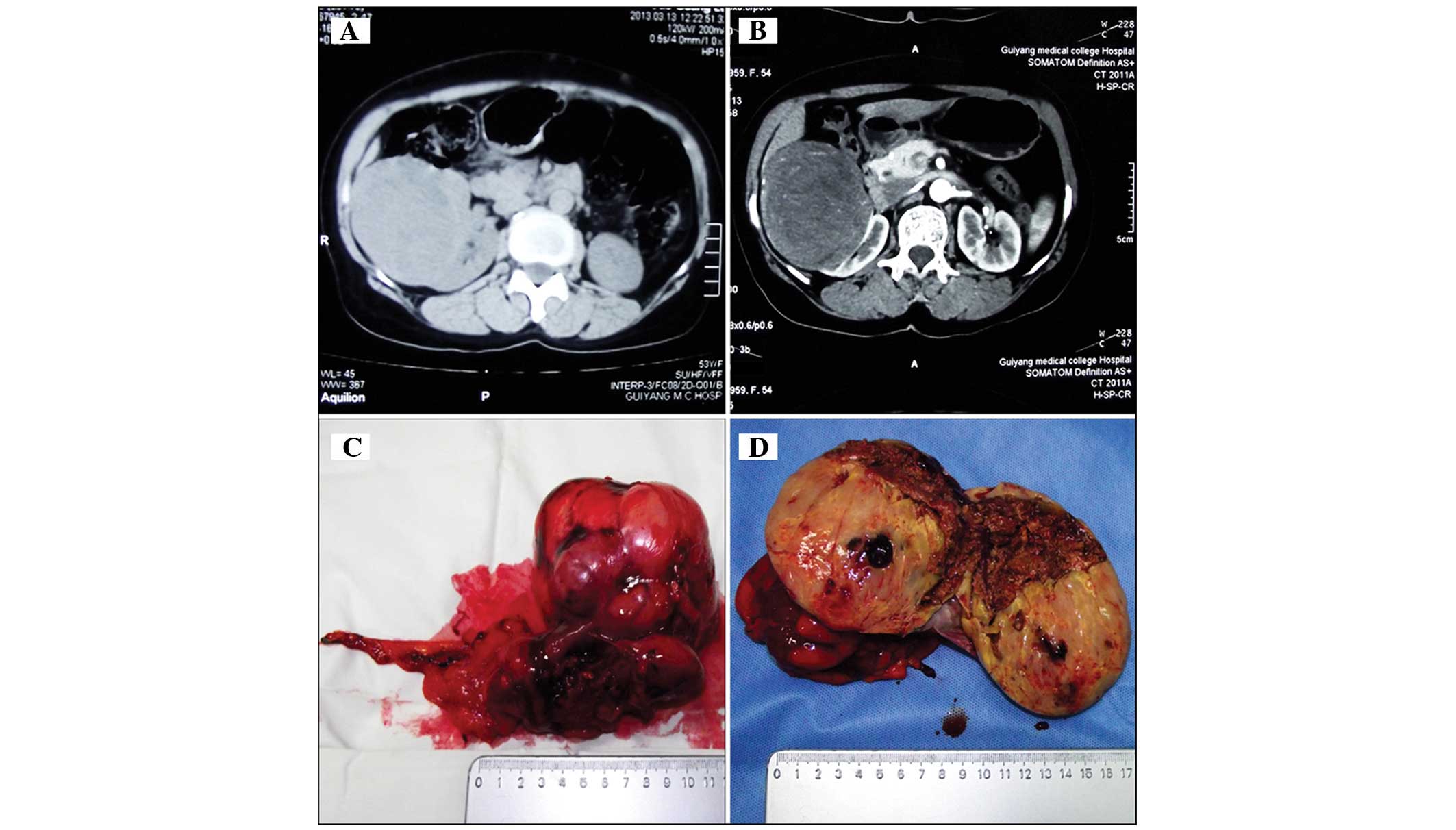
computerized tomography (CT) imaging revealed a neoplasm lesion
localized in the right kidney. No lymphadenopathy was detected
(Fig. 1A and B). Following discussion
with the patient and the patient's family, a traditional open
surgical treatment was proposed. Subsequent to extensive discussion
with urologists of the Affiliated Hospital of Guizhou Medical
University an open approach radical nephrectomy was performed.
Macroscopically, the tumor consisted of renal tissue measuring
8.0×6.0×5.0 cm in size, and was a well-circumscribed, soft,
white-gray mass with a cut surface that was focally friable and
accompanied by necrosis (Fig. 1C and
D).
A hematoxylin and eosin staining kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) was applied to
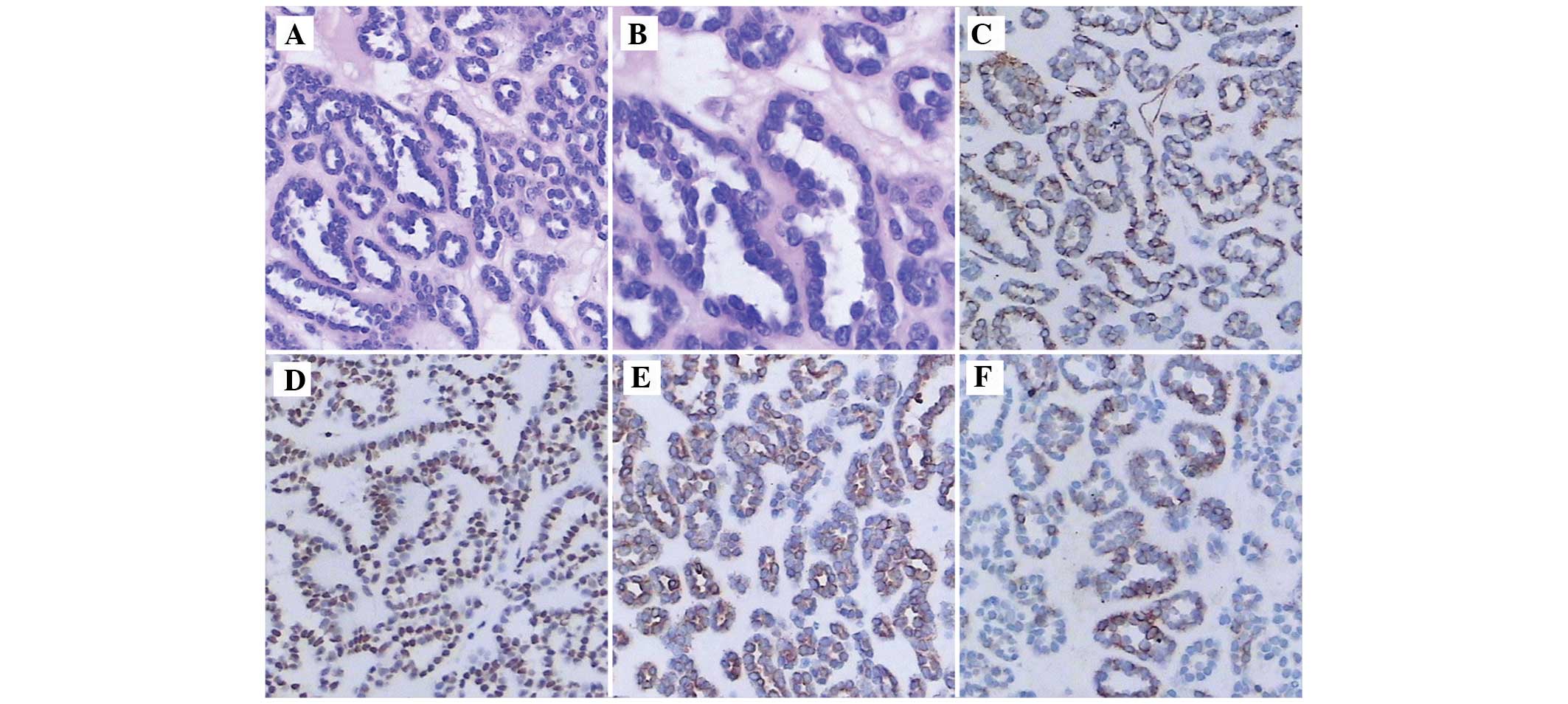
the resected specimen. Microscopic examination (IMT-2; Olympus
Corporation, Tokyo, Japan) revealed that the cellular mass was
composed of hyperchromatic cells with scant cytoplasm, tightly
packed tubules and glomeruloid-like structures (Fig. 2A and B). The tumor cells were stained
with antibodies against S-100 protein (polyclonal rabbit
anti-human; 1:100; cat. no. BA0120, Vimentin (polyclonal rabbit
anti-human; 1:100; cat. no. PB0378), common acute lymphoblastic
leukemia antigen (CD10; monclonal mouse anti-human; 1:200; cat. no.
BM3410), cytokeratin (CK; polyclonal mouse anti-human; 1:100; cat.
no. BA4051), α-methylacyl-coenzyme-A racemase (AMACR; monoclonal
mouse anti-human; 1:200; cat. no. BM1712), CK7 (monoclonal mouse
anti-human; 1:200; cat. no. BM1618), and Wilms' tumor antigen (WT1;
monoclonal mouse anti-human; 1:200; cat. no. MK3212) (all purchased
from Boster Inc., Wuhan, China). The tumor cells expressed
Vimentin, WT1, CK and CK7 (Fig.
2C–F); however, the cells did not express S-100 protein, AMACR
or CD10 (data not shown).
The duration of the follow-up was 20 months. Every 6
months, the patient received a routine blood examination and a
renal ultrasound. The patient was alive at 20 months, with no
clinical complaints. At the first 6-month follow-up the routine
blood examination demonstrated a Hct volume of 46%, a hemoglobin
volume of 152 g/l, and a red blood cell count of
5.6×1012cells/l. At the second 6-month follow-up the
renal ultrasound revealed there was no metastasis.
Written informed consent was obtained from the
patient prior to publication of the case report.
Discussion
MA is characterized as a mass consisting of spindle
cells associated with epithelial cells (2). In 1988, Mostofi et al (4) described MA as a distinct nosologic
entity among renal neoplasms, with tubular-like epithelium cells.
MA is a rare renal epithelial neoplasm and has a peak age of
occurrence in the fifth or sixth decade of life. It is closely
associated with other metanephric neoplasms, including pure stromal
lesions and metanephric adenofiromas (3,5).
Clinically, MA may present with hematuria, flank pain, hypertension
or abdominal mass. In total, 12% of patients present with
polycythemia vera in addition to MA, which is a higher percentage
compared with other renal neoplasms (3,6,7).
MA appears as a well-defined, round, solid, soft
mass varying between 0.3 and 15.0 cm in size (3,8).
Histologically, MA exhibits uniform small cells with a high
nuclear-to-cytoplasmic ratio with an acinar arrangement, without
mitosis or embryonic appearance and with a tubular, glomeruloid or
papillary structure that is distributed in small round acini, and
MA is phenotypically similar to nephroblastomas (2,3).
Immunohistochemically, MA expresses CK7 and WT1 and does not
express epithelial membrane antigen or AMACR (2,9). To verify
the diagnosis of MA, ultrasonography or radiological imaging may be
required; the tumors appear hyperechoic with enhanced
through-transmission with ultrasonography, while with unenhanced CT
the tumor increases attenuation relative to the adjacent parenchyma
(10). However, it remains
challenging to differentiate MA from renal cell carcinoma solely by
imaging.
Since MA is frequently a benign tumor, it is
important to quickly distinguish this lesion from other renal
neoplasms, which presents a challenge as MA and renal neoplasms
clinically present in an identical manner. Partial or radical
nephrectomy is the mainstay of treatment for MA; however, radical
nephrectomy may lead to the onset and/or progression of chronic
kidney disease, leading to dialysis or transplant treatment.
Therefore, open, laparoscopic, robotic or hand assisted partial
nephrectomy, or thermoablative procedures are recommended,
according to the American Urological Association Guidelines
(11). In addition, Conzo et
al performed radiofrequency-assisted partial nephrectomy on a
patient with MA that led to an excellent hemostasis and a rapid
conservative resection, with low morbidity (9).
Overall, MA is an extremely rare benign tumor.
Radical nephrectomy, cryoablation or radiofrequency may be used to
treat MA. MA cannot be easily distinguished from other malignant
neoplasms using imaging alone; however, MA is clearly recognized by
microscopy. When diagnosis is challenging, a selective panel of
immunostains, including WT1, EMA and AMACR, may be a useful
tool.
Acknowledgements
The present study was supported by the Science and
Technology Fund Project of Guizhou Province [grant no.
QKHJZ(2013)2051].
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
MA
|
metanephric adenoma
|
References
|
1
|
Amin MB, Amin MB, Tamboli P, Javidan J,
Stricker H, de-Peralta Venturina M, Deshpande A and Menon M:
Prognostic impact of histologic subtyping of adult renal epithelial
neoplasms: An experience of 405 cases. Am J Surg Pathol.
26:281–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantoan Padilha M, Billis A, Allende D,
Zhou M and Magi-Galluzzi C: Metanephric adenoma and solid variant
of papillary renal cell carcinoma: Common and distinctive features.
Histopathology. 62:941–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis CJ Jr, Barton JH, Sesterhenn IA and
Mostofi FK: Metanephric adenoma. Clinicopathological study of fifty
patients. Am J Surg Pathol. 19:1101–1114. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mostofi FK, Sesterhenn IA and Davis CJ:
Benign tumors of the kidney. Prog Clin Biol Res. 269:329–346.
1988.PubMed/NCBI
|
|
5
|
Patel RD, Frederick L, Kohler T and
Schwartz B: A case of a metanephric adenoma of the kidney
surgically treated with robot-assisted laparoscopic partial
nephrectomy. Case Rep Urol. 2013:7038592013.PubMed/NCBI
|
|
6
|
Raman SP, Hruban RH and Fishman EK: Beyond
renal cell carcinoma: Rare and unusual renal masses. Abdom Imaging.
37:873–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bastide C, Rambeaud JJ, Bach AM and Russo
P: Metanephric adenoma of the kidney: Clinical and radiological
study of nine cases. Bju Int. 103:1544–1548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones EC, Pins M, Dickersin GR and Young
RH: Metanephric adenoma of the kidney. A clinicopathological,
immunohistochemical, flow cytometric, cytogenetic, and electron
microscopic study of seven cases. Am J Surg Pathol. 19:615–626.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conzo G, Sciascia V, Palazzo A, Stanzione
F, Della Pietra C, Insabato L, Natella V, Radice L and Santini L:
Radiofrequency-assisted partial nephrectomy for metanephric
adenoma: A case report and literature review. Surg Innov. 20:55–58.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fielding JR, Visweswaran A, Silverman SG,
Granter SR and Renshaw AA: CT and ultrasound features of
metanephric adenoma in adults with pathologic correlation. J Comput
Assist Tomogr. 23:441–444. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campbell SC, Novick AC, Belldegrun A, et
al: Guideline for management of the clinical T1 renal mass. J Urol.
182:1271–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|