Introduction
Endometrial cancer (EC) is the most common
gynecological malignancy, with nearly 50,000 new cases diagnosed
each year in the USA (1). Although
the majority of EC patients present with early-stage, curable
disease, a significant subset present with advanced-stage or
develop recurrent disease that is associated with a less favorable
outcome (1). Currently, the prognosis
and survival of patients with EC have been largely indicated by the
information obtained during surgery (2). A significant number of clinically
early-stage cases have extrauterine disease within the pelvic and
paraaortic lymph nodes, which are the most common sites for
metastasis. The risk of pelvic lymph node metastasis for clinical
stage I EC has been associated with tumor grade and the depth of
myometrial invasion (2). A
Gynecologic Oncology Group Study reported that patients with poorly
differentiated cancers or who have cancer invading the outer half
of the myometrium have a >10% risk of pelvic lymph node
metastasis (2). However, in that
study, 22% of clinical stage I cancers had extrauterine disease.
Although many clinicians believe that nodal dissections must be
reserved for those with sufficient risk of nodal metastasis
(2–5),
the risk level of nodal disease that warrants this procedure is
unclear. The major risks associated with nodal dissection include
increased operative time, potential for blood loss associated with
vascular injury, genitofemoral nerve injury, lymphocyst formation
and lymphedema (3,6–8).
Selective surgical staging allows the identification
of patients with lymph node metastasis and avoids the morbidity of
routine lymphadenectomy. Although a number of preoperative and
intraoperative risk factors for nodal metastasis have been
identified, they have a low positive predictive value in clinical
practice. Preoperative radiological testing appears to identify
patients with a low rather than high risk of nodal metastasis.
Furthermore, ultrasound (9), computed
tomography (10) and magnetic
resonance imaging (11) have been
found to be suboptimal in identifying patients with pelvic or
paraaortic nodal metastases. The identification of an accurate test
to predict lymph node metastasis in patients with EC would have
substantial clinical application. The present study sought to
identify the molecular and clinicopathological markers determining
lymph node metastasis. These markers will be the basis for a
predictive model for lymph node metastasis in EC.
Materials and methods
Databases used for the study
The Cancer Genome Atlas (TCGA) database of the
National Cancer Institute (Bethesda, MD, USA) was used for the
current study (http://cancergenome.nih.gov/). Data were collected in
August 2012. All cases of EC with information regarding lymph node
status were selected for inclusion. TCGA gene expression, protein
expression and clinicopathological data for EC patients were
downloaded. The clinicopathological parameters included tumor
grade, depth of myometrial invasion and lymph node status. TCGA has
collected >373 EC samples with gene expression (mRNA) data as
well as clinical information (12).
Statistical analysis
Analysis of gene expression was performed using
Biometric Research Branch (BRB) ArrayTools (Version 2.13.2 for ×64
systems), an integrated package for visualization and statistical
analysis that utilizes Excel (Microsoft, Redmond, WA) as front-end,
and tools developed in the R statistical system. BRB-ArrayTools
were developed by Dr Richard Simon and the BRB-ArrayTools
development team (http://linus.nci.nih.gov/BRB-ArrayTools/). Additional
analyses were performed using the R statistical software
environment for statistical computing and graphics (http://www.r-project.org/), including Bioconductor, an
open source software for bioinformatics (http://bioconductor.org/).
Two strategies were used to design a predictor for
lymph node metastasis in EC patients: A gene expression signature
to predict lymph node status and significantly independent
molecular, clinical and pathological variables predicting lymph
node status.
Gene expression signature
To construct a gene signature profile that would
classify patients into lymph node positive and lymph node negative
groups, the Class Prediction Tool of BRB-ArrayTools was used. This
tool optimizes the significance level threshold used for gene
selection. Genes that were differentially expressed between the
classes at a univariate significance level of <0.001 were
included in the predictor. A number of methods are included in the
tool to evaluate the predictor or gene signature. To assess how
accurately the groups are predicted by this multivariate class
predictor (internal validation), a cross-validated
misclassification rate is computed, usually in the form of the
leave-one-out cross-validation method (13).
Independent variables predicting lymph
node status
A univariate two-tailed t-test analysis was
performed using lymph node status and gene expression, protein
expression and clinicopathological parameters. The nominal
significance level of each univariate test was P<0.001. For the
gene expression comparison, 10,000 random permutations were
performed to determine the probability of ascertaining significant
differential expression by chance parameters at the P<0.001
level. Significantly associated parameters were subjected to a
multivariable analysis to determine independently associated
molecular markers.
Subsequently, independent molecular markers were
uploaded to GeneGo MetaCore™ for pathway analysis (http://www.genego.com/metacore.php; Thomson
Reuters, New York, NY) to identify biological processes that may
participate in lymph node invasion in EC. Pathways with P<0.05
were considered significant, based on the GeneGo MetaCore™
statistical test for significance.
Results
Database analysis results
Using publicly available data for EC collected in
TCGA database, 262 patients with lymph node status and clinical
information were identified. Of these, 203 patients had available
information on genomic expression data and lymph node status and
165 patients had available information on protein data and lymph
node status. The 203 patients with available genomic expression
data were divided according to their lymph node status, with 165
patients having negative lymph nodes and 38 having positive lymph
nodes. These groups were similar with regard to age, menopausal
status, peritoneal washing status and body mass index (Table I). Furthermore, of the 165 patients
with available protein expression data, 139 were identified to have
negative lymph nodes whilst 26 had positive lymph nodes. These
groups were also similar with regard to age, menopausal status,
peritoneal washing status, overall survival and body mass index
(Table II).
 | Table I.Characteristics of patients with
available genetic expression data (n=203). |
Table I.
Characteristics of patients with
available genetic expression data (n=203).
| Characteristics | Lymph node positive
(n=38) | Lymph node negative
(n=165) | P-value |
|---|
| Age, years
(mean) | 62.6 | 62.7 | 0.98 |
| Menopausal status,
% |
|
| 0.73 |
|
Premenopausal | 8.8 | 7.3 |
|
|
Postmenopausal | 91.2 | 92.7 |
|
| Surgical approach,
% |
|
| 0.08 |
|
Laparotomy | 81.6 | 67.3 |
|
| Minimally
invasive | 18.4 | 32.7 |
|
| Body mass index
(mean) | 33.6 | 32.1 | 0.29 |
| Depth of invasion, %
(mean) | 65.0 | 37.6 | <0.01 |
| Grade, % |
|
| <0.01 |
| 1 | 5.3 | 27.9 |
|
| 2 | 7.9 | 33.9 |
|
| 3 | 86.8 | 38.2 |
|
| Peritoneal washings,
% |
|
| 1.00 |
|
Positive | 12.5 | 12.8 |
|
|
Negative | 87.5 | 87.2 |
|
 | Table II.Characteristics of patients with
available protein expression (n=165). |
Table II.
Characteristics of patients with
available protein expression (n=165).
| Characteristics | Lymph node positive
(n=26) | Lymph node negative
(n=139) | P-value |
|---|
| Age, years
(mean) | 62.3 | 62.8 | 0.89 |
| Menopausal status,
% |
|
| 0.67 |
|
Premenopausal | 8.0 | 6.5 |
|
|
Postmenopausal | 92.0 | 93.5 |
|
| Surgical approach,
% |
|
| 0.02 |
|
Laparotomy | 92.4 | 67.4 |
|
| Minimally
invasive |
7.6 | 32.6 |
|
| Body mass index
(mean) | 31.9 | 33.1 |
0.45 |
| Depth of invasion,
% (mean) | 68.6 | 37.2 | <0.01 |
| Grade, % |
|
| <0.01 |
| 1 |
7.7 | 32.4 |
|
| 2 | 11.5 | 36.7 |
|
| 3 | 80.8 | 30.9 |
|
| Peritoneal
washings, % |
|
|
0.73 |
|
Positive | 15.0 | 13.3 |
|
|
Negative | 85.0 | 86.7 |
|
| Stage, n |
|
| <0.01 |
| 1 | 0 | 113 |
|
| 2 | 0 | 11 |
|
| 3 | 22 | 11 |
|
| 4 |
4 |
4 |
|
| Overall survival
time (days) | 764 | 928 |
0.34 |
| Histology, n |
|
| <0.01 |
|
Serous | 9 | 13 |
|
|
Non-serous | 17 | 126 |
|
Gene expression signature
The class prediction tool identified a gene
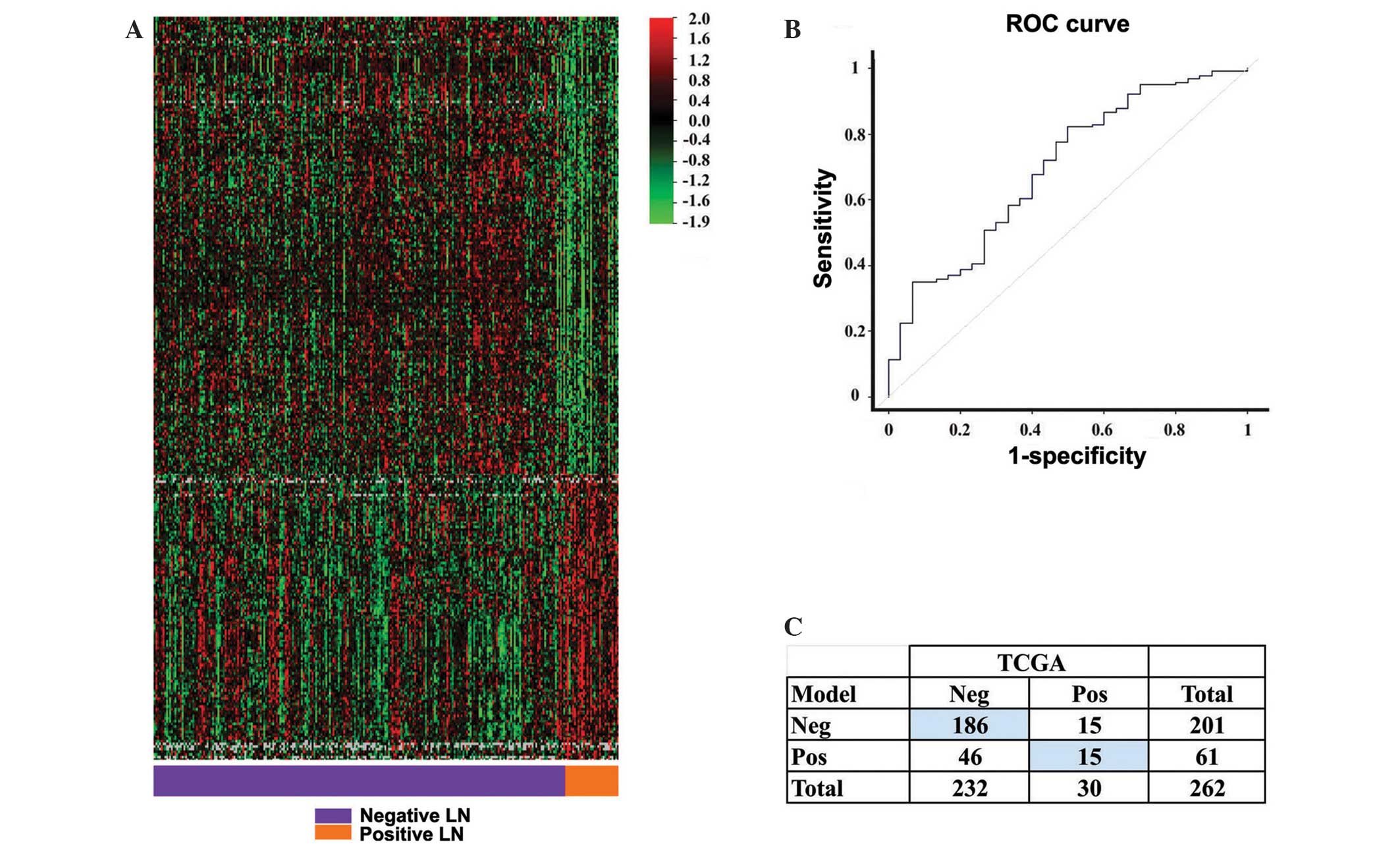
expression signature composed of 295 genes (Fig. 1A). This signature or model correctly
predicted lymph node status in the current patient group at a rate
of 77%, with P=0.04 (internal validation with the compound
covariate predictor). The model was more accurate in predicting
negative lymph node status, with a sensitivity of 80% and a
specificity of 50%. The κ coefficient was 0.20, indicating fair
agreement between the model and the surgical result (Fig. 1C). The performance of the model,
measured by the area under the receiver operating characteristic
(ROC) curve, was 70% (Fig. 1B).
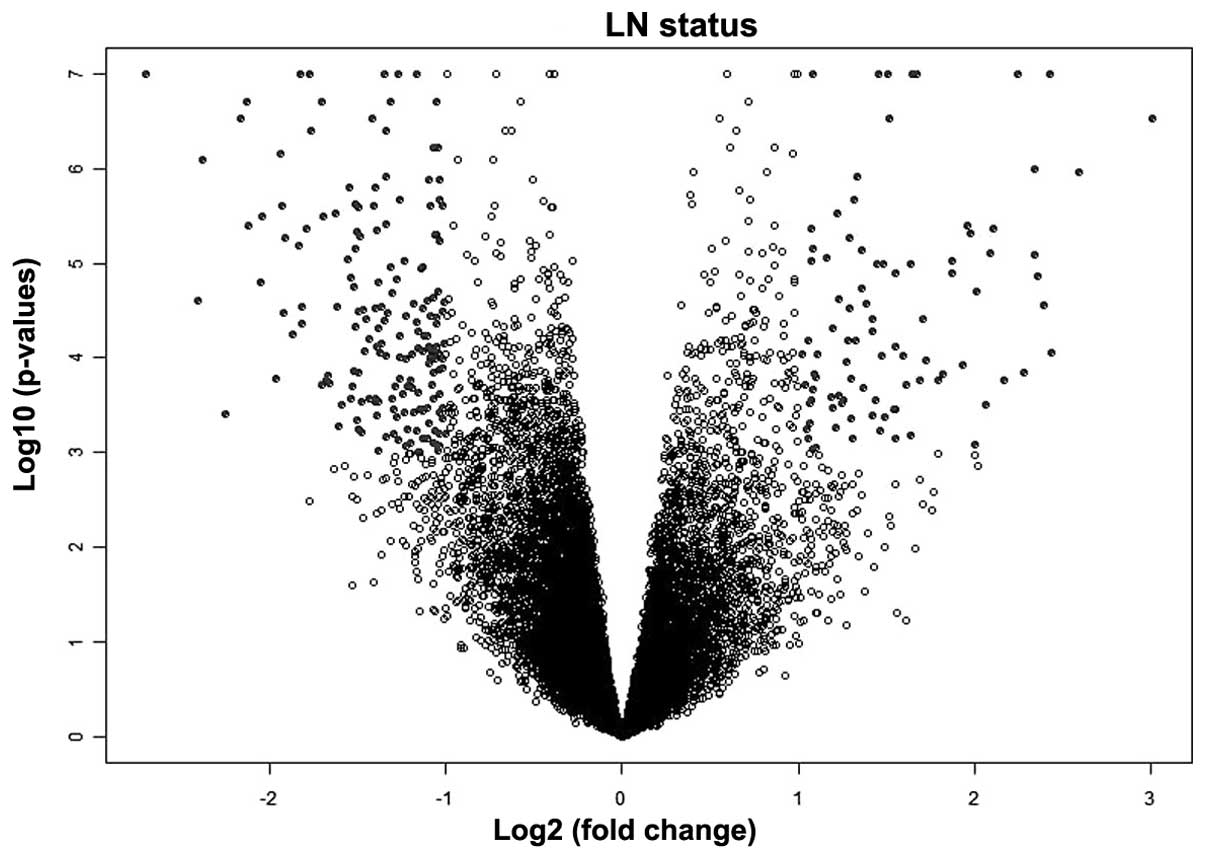
Independent variables predicting lymph node status.
The univariate analysis test using lymph node status and EC gene
expression and protein expression data identified 268 unique genes
(P≤0.001; Fig. 2) and 19 unique
proteins (P<0.05; Table III) as
associated with lymph node metastasis. When the clinicopathological
parameters were evaluated with respect to lymph node status, only
tumor grade (P=0.0003) and depth of myometrial invasion
(P=8.15×10−6; Table III)
were determined to be significantly associated with lymph node
metastasis.
 | Table III.Univariate analysis of protein and
clinicopathological data. |
Table III.
Univariate analysis of protein and
clinicopathological data.
|
Characteristics | P-value |
|---|
| Invasion | <0.01 |
| Grade | <0.01 |
| BCL-2-M-V |
0.03 |
| Dvl3-R-V | <0.01 |
| eEF2K-R-V |
0.04 |
| EGFR-R-C |
0.03 |
|
EGFR_pY1068-R-V |
0.01 |
|
EGFR_pY1173-R-C | <0.01 |
|
FOXO3a_pS318_S321-R-C | <0.01 |
|
HER3_pY1298-R-C |
0.02 |
| Notch1-R-V |
0.01 |
| p21-R-C |
0.02 |
|
p70S6K_pT389-R-V |
0.02 |
| PDK1_pS241-R-V |
0.01 |
| PR-R-V |
0.04 |
| Shc_pY317-R-NA | <0.01 |
| Smad4-M-V |
0.02 |
| Src_pY527-R-V |
0.03 |
| YB-1-R-V |
0.02 |
Based on these findings, the unique genes, proteins
and clinicopathological parameters determined to be significantly
associated with lymph node metastasis were subjected to a
multivariable analysis. This identified 10 genes [arylsulfatase I
(ARSI); ring finger protein 183 (RNF183);
delta/notch-like EGF repeat containing (DNER); dual
specificity phosphatase 9 (DUSP9); testis expressed 19
(TEX19); ribosomal protein S6 kinase, 90kDa, polypeptide 6
(RPS6KA6); fibrillin 3 (FBN3); mucin 6 (MUC6);
gamma-aminobutyric acid A receptor, theta (GABRQ); and
FLJ16779) and 4 proteins [elongation factor 2 kinase (EF2K);
epidermal growth factor receptor (EGFR); phosphoinositide-dependent
kinase 1 (PDK1); and Y box binding protein (YB)] that were
independently associated with lymph node metastasis (Table IV). Interestingly, depth of
myometrial invasion was the only clinicopathological parameter to
be independently associated with lymph node metastasis. The genes
determined to be independently associated with lymph node
metastasis (n=10) were uploaded into GeneGo MetaCore™ pathway
analysis, which identified 3 molecular signaling pathways
associated with lymph node metastasis (P<0.0001). These
molecular signaling pathways included the expression of EGFR
(P=4.23×10−5, 2/23 pathway objects), Bcl2 antagonist of
cell death (BAD; P=1.44×10−4, 2/42 pathway
objects), and phosphatase and tensin homolog (PTEN;
P=1.73×10−4, 2/46 pathway objects) pathways.
 | Table IV.Statistically significant results
from multivariate analysis of genes, proteins, and clinical
variables. |
Table IV.
Statistically significant results
from multivariate analysis of genes, proteins, and clinical
variables.
|
Characteristics | Standard error | t-value | P-value |
|---|
| Clinicopathological
variables |
|
|
|
|
Invasion |
6.12×10−4 |
5.115 | <0.01 |
| Differentially
expressed genes |
|
|
|
|
ARSI |
2.39×10−4 | −2.802 |
0.01 |
|
RNF183 |
2.41×10−4 | −4.644 | <0.01 |
|
DNER |
2.37×10−4 |
3.321 | <0.01 |
|
DUSP9 |
2.47×10−4 | −2.934 | <0.01 |
|
TEX19 |
2.62×10−4 | −3.171 | <0.01 |
|
RPS6KA6 |
2.84×10−4 | −3.036 | <0.01 |
|
FBN3 |
2.52×10−4 | −2.970 | <0.01 |
|
MUC6 |
2.15×10−4 | −2.846 |
0.01 |
|
GABRQ |
3.30×10−4 |
3.477 | <0.01 |
|
FLJ16779 |
2.54×10−4 | −2.791 |
0.01 |
| Independently
associated proteins |
|
|
|
|
EF2K |
3.42×10−4 |
3.705 | <0.01 |
|
EGFR |
3.38×10−4 |
3.330 | <0.01 |
|
PDK1 |
2.93×10−4 |
2.557 |
0.01 |
| YB |
3.51×10−4 | −2.399 |
0.02 |
Discussion
The precise molecular events that occur during the
development, invasion and formation of metastasis in EC are largely
uncharacterized and remain poorly understood (14). In the present analysis, an in
silico genome-wide approach was employed to define the
molecular underpinnings of lymph node metastasis in EC. Using TCGA
database, a number of genes, proteins, and molecular signaling
pathways associated with EC lymph node metastasis were identified,
and these classifiers were included with the clinicopathological
parameters to be able to generate a predictive test for lymph node
metastasis.
The objective of TCGA is the collection and
processing of biospecimens that may be used for cancer diagnosis
and analysis. The biospecimens collected from these cancers meet a
stringent set of quality criteria, enabling extracted DNA and RNA
to be used for advanced genomic analysis and sequencing
technologies. The present analysis included a highly stringent
level of statistical testing for associations between
clinicopathological parameters, gene and protein expression levels
and lymph node metastasis in EC. This stringent statistical
methodological approach accounts for a potential bias in genomic
datasets and ensures that generated P-values may be interpreted as
significant at a relative, as well as an absolute, level.
Ten genes were determined to be independently
associated with lymph node metastasis in EC: ARSI, RNF183, DNER,
DUSP9, TEX19, RPS6KA6, FBN3, MUC6, GABRQ and FLJ16779.
Notably, the ring finger RNF183 gene has been previously
reported to be differentially expressed in EC (15). In addition, the DNER gene was
found to regulate glioblastoma-derived neurosphere cell
differentiation and tumor progression (16). RPS6KA6, a putative tumor
suppressor gene expressed in normal endometrial tissue, was
demonstrated to be silenced via hypermethylation in EC cell lines;
however, its role as a suppressor in EC remains uncertain (17). MUC6 gene expression was
documented in pancreatic carcinomas and cholangiocarcinomas and
focally in endocervical adenocarcinomas (18).
The multivariate analysis identified 4 proteins
independently associated with lymph node metastasis in EC: EF2K,
EGFR, PDK1, and YB. EGFR is the prototypic member of the ErbB/HER
receptor tyrosine kinase family and binds to multiple ligands,
including epidermal growth factor, transforming growth factor α and
amphiregulin. EGFR is crucial in cellular functions implicated in
cancer development (19) and has been
revealed to be expressed in a large percentage of endometrial
tumors (20).
PDK1 is a key regulator of the AGC protein kinase
family, which includes the proto-oncogene AKT/protein kinase B
implicated in a number of malignancies, including breast cancer.
YB-1 appears to play a critical role in cell proliferation and
growth, DNA replication, cell cycle and drug resistance, as well as
malignancy. Furthermore, YB-1 is overexpressed in
cisplatin-resistant cancer cell lines (13,21).
The current data demonstrated an association between
lymph node metastasis and a number of gene expression pathways in
EC: EGFR, BAD, and PTEN. EGFR has been
shown to be a principal growth-promoting pathway in EC cells
(22). The BAD pathway
influences EC cell sensitivity to cisplatin, likely via modulation
of the phosphorylation status of the BAD protein (23). PTEN is the most commonly
mutated gene identified in endometrial carcinoma. This mutation is
considered to be an early event in endometrial carcinogenesis
(24).
The clinical heterogeneity of EC is likely a
reflection of an underlying molecular heterogeneity. As such, the
mechanisms by which EC cells metastasize are likely to be equally
diverse. In the present study, a genome-wide strategy was adopted
to characterize some of the molecular signaling pathways and
cellular processes that are associated with lymph node metastasis
in EC. Such findings may have substantial implications for future
clinical treatment of patients with this disease. Empirical
treatment based on one-size-fits-all could be replaced with a more
tailored therapy that matches the right patient with the right
treatment plan based on their own molecular fingerprint. The next
phase of this study will be to evaluate and validate the ability of
a signature containing independent molecular and
clinicopathological parameters to predict lymph node metastasis in
patients with EC.
Acknowledgements
The authors would like to thank the Jacquie Liggett
Hearing the Ovarian Cancer Whisper for their fellowship grant
support and Rasa Hamilton (Moffitt Cancer Center) for editorial
assistance.
References
|
1
|
Siegel R, Maj Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creasman WT, Morrow CP, Bundy BN, Homesley
HD, Graham JE and Heller PB: Surgical pathologic spread patterns of
endometrial cancer. A Gynecologic Oncology Group Study. Cancer.
60:2035–2041. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morrow CP, Bundy BN, Kurman RJ, Creasman
WT, Heller P, Homesley HD and Graham JE: Relationship between
surgical-pathological risk factors and outcome in clinical stage I
and II carcinoma of the endometrium: A gynecologic oncology group
study. Gynecol Oncol. 40:55–65. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faught W, Krepart GV, Lotocki R and
Heywood M: Should selective paraaortic lymphadenectomy be part of
surgical staging for endometrial cancer? Gynecol Oncol. 55:51–55.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YB and Niloff JM: Endometrial
carcinoma: Analysis of recurrence in patients treated with a
strategy minimizing lymph node sampling and radiation therapy.
Obstet Gynecol. 82:175–180. 1993.PubMed/NCBI
|
|
6
|
Orr JW Jr, Holimon JL and Orr PF: Stage I
corpus cancer: Is teletherapy necessary? Am J Obstet Gynecol.
176:777–788; discussion 788–779. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Homesley HD, Kadar N, Barrett RJ and Lentz
SS: Selective pelvic and periaortic lymphadenectomy does not
increase morbidity in surgical staging of endometrial carcinoma. Am
J Obstet Gynecol. 167:1225–1230. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abu-Rustum NR, Alektiar K, Iasonos A, Lev
G, Sonoda Y, Aghajanian C, Chi DS and Barakat RR: The incidence of
symptomatic lower-extremity lymphedema following treatment of
uterine corpus malignancies: A 12-year experience at memorial
Sloan-Kettering cancer center. Gynecol Oncol. 103:714–718. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng WF, Chen CA, Lee CN, Chen TM, Huang
KT, Hsieh CY and Hsieh FJ: Preoperative ultrasound study in
predicting lymph node metastasis for endometrial cancer patients.
Gynecol Oncol. 71:424–427. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
La Fianza A, Di Maggio EM, Preda L, Coscia
D, Tateo S and Campani R: Clinical usefulness of CT in the
treatment of stage I endometrial carcinoma. Radiol Med. 93:567–571.
1997.PubMed/NCBI
|
|
11
|
Frei KA, Kinkel K, Bonél HM, Lu Y,
Zaloudek C and Hricak H: Prediction of deep myometrial invasion in
patients with endometrial cancer: Clinical utility of
contrast-enhanced MR imaging-a meta-analysis and Bayesian analysis.
Radiology. 216:444–449. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cancer Genome Atlas Research Network.
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohga T, Koike K, Ono M, Makino Y, Itagaki
Y, Tanimoto M, Kuwano M and Kohno K: Role of the human Y
box-binding protein YB-1 in cellular sensitivity to the
DNA-damaging agents cisplatin, mitomycin C and ultraviolet light.
Cancer Res. 56:4224–4228. 1996.PubMed/NCBI
|
|
14
|
Abal M, Llauradó M, Doll A, Monge M, Colas
E, González M, Rigau M, Alazzouzi H, Demajo S, Castellví J, et al:
Molecular determinants of invasion in endometrial cancer. Clin
Transl Oncol. 9:272–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colas E, Perez C, Cabrera S, Pedrola N,
Monge M, Castellvi J, Eyzaguirre F, Gregorio J, Ruiz A, Llaurado M,
et al: Molecular markers of endometrial carcinoma detected in
uterine aspirates. Int J Cancer. 129:2435–2444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun P, Xia S, Lal B, Eberhart CG,
Quinones-Hinojosa A, Maciaczyk J, Matsui W, Dimeco F, Piccirillo
SM, Vescovi AL and Laterra J: DNER, an epigenetically modulated
gene, regulates glioblastoma-derived neurosphere cell
differentiation and tumor propagation. Stem Cells. 27:1473–1486.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dewdney SB, Rimel BJ, Thaker PH, Thompson
DM Jr, Schmidt A, Huettner P, Mutch DG, Gao F and Goodfellow PJ:
Aberrant methylation of the X-linked ribosomal S6 kinase RPS6KA6
(RSK4) in endometrial cancers. Clin Cancer Res. 17:2120–2129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartman AE, Buisine MP, Aubert JP, Niehans
GA, Toribara NW, Kim YS, Kelly EJ, Crabtree JE and Ho SB: The MUC6
secretory mucin gene is expressed in a wide variety of epithelial
tissues. J Pathol. 186:398–405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herbst RS: Review of epidermal growth
factor receptor biology. Int J Radiat Oncol Biol Phys. 59:21–26.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konecny GE, Santos L, Winterhoff B, Hatmal
M, Keeney GL, Mariani A, Jones M, Neuper C, Thomas B, Muderspach L
and Riehle D: HER2 gene amplification and EGFR expression in a
large cohort of surgically staged patients with nonendometrioid
(type II) endometrial cancer. Br J Cancer. 100:89–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ise T, Nagatani G, Imamura T, Kato K,
Takano H, Nomoto M, Izumi H, Ohmori H, Okamoto T, Ohga T, et al:
Transcription factor Y-box binding protein 1 binds preferentially
to cisplatin-modified DNA and interacts with proliferating cell
nuclear antigen. Cancer Res. 59:342–346. 1999.PubMed/NCBI
|
|
22
|
Albitar L, Pickett G, Morgan M, Wilken JA,
Maihle NJ and Leslie KK: EGFR isoforms and gene regulation in human
endometrial cancer cells. Mol Cancer. 9:1662010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chon HS, Marchion DC, Xiong Y, Chen N,
Bicaku E, Stickles XB, Bou Zgheib N, Judson PL, Hakam A,
Gonzalez-Bosquet J, et al: The BCL2 antagonist of cell death
pathway influences endometrial cancer cell sensitivity to
cisplatin. Gynecol Oncol. 124:119–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tashiro H, Blazes MS, Wu R, Cho KR, Bose
S, Wang SI, Li J, Parsons R and Ellenson LH: Mutations in PTEN are
frequent in endometrial carcinoma but rare in other common
gynecological malignancies. Cancer Res. 57:3935–3940.
1997.PubMed/NCBI
|