Introduction
Helicobacter pylori (H. pylori)
infection-associated gastric cancer (GC) is a common digestive
tract malignancy that is associated with high rates of mortality
and serious health effects (1,2).
Metastasis is a significant stage in GC development, and the
majority of patients succumb to H. pylori
infection-associated GC due to metastasis. (2,3).
Consequently, investigation into the mechanisms underlying GC
metastasis has become a key area of GC research. Invasion and
metastasis of GC tumors are thought to be the most lethal and
prominent features associated with disease recurrence (4). However, the mechanisms underlying the
involvement of H. pylori in the invasion, metastasis and
recurrence of H. pylori infection-associated GC remain to be
elucidated. Previous studies have suggested that the
epithelial-mesenchymal transition (EMT) is critical for the
invasion and metastasis of malignant tumors (5). EMT is associated with normal tissue
development, organogenesis, tissue remodeling and wound healing
(6). By contrast, aberrant EMT
reactivation contributes to the initiation of numerous human
pathologies, particularly those associated with certain types of
solid tumor invasion and metastasis (4), including that exhibited by GC cells
(7). Gaining an understanding of
these mechanisms may aid the therapeutic control of EMT, in order
to promote tissue regeneration, treat fibrosis and prevent cancer
invasion and metastasis.
Mesenchymal stem cells (MSCs) are multipotent adult
stem cells, which have been observed in multiple types of tissue
(8,9).
MSCs have been reported to exhibit tropism for inflammation and
cancer sites (10–14). In addition, H. pylori-induced
epithelial responses may contribute to directing the homing of MSCs
into the gastric mucosa (15,16). As a major component of the H.
pylori infection-associated GC microenvironment, MSCs may be
critical for malignant tumor invasion and metastasis; however, the
role of H. pylori-infected MSCs remains to be elucidated and
is subject to controversy. In the present study, a human umbilical
cord MSC (hucMSC)/H. pylori co-culture model was developed.
The effects of H. pylori-infected hucMSCs on SGC-7901 GC
cell migration were evaluated in vitro using a Transwell
migration assay. During infection, MSC cytokine expression was
evaluated using Luminex/ELISA, and the abilities of certain
identified cytokines to induce GC cell migration were individually
evaluated in vitro. Finally, the significance of the nuclear
factor-κB (NF-κB) signaling pathway in cytokine secretion was
evaluated.
The results of the present study may enhance
understanding of the significance of MSCs in H. pylori
infection-associated GC and offer therapeutic benefits by
inhibiting malignant processes involved in the promotion of
cancer.
Materials and methods
Cell culture and H. pylori strain
growth conditions
The SCG-7901 human gastric cancer cell line was
purchased from the Institute of Biochemistry and Cell Biology at
the Chinese Academy of Sciences (Shanghai, China). Fresh umbilical
cords were collected from healthy puerperal mothers after written
informed consent was obtained, and MSCs were isolated from these
human umbilical cord tissues and characterized as described by Qiao
et al (17). Pregnant women
with pre-eclampsia, sexually transmitted diseases or hepatitis were
excluded from the study. HucMSCs at passage 3 were selected for use
in the present study. SGC-7901 cells and hucMSCs were cultured in
Invitrogen low-glucose Dulbecco's modified Eagle's medium (L-DMEM;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.). All
cells were incubated at 37°C in a humidified cell culture incubator
in an atmosphere of 5% CO2. The 11673 H. pylori
strain was provided by Dr Weng-Rong Xu (Jiangsu University,
Zhenjiang, China). The H. pylori strain was grown in
trypticase soy agar (QingDao Hope Bio-technology Co., Ltd.,
Qingdao, China) supplemented with 5% sheep blood (QingDao Hope
Bio-technology Co., Ltd.) and incubated at 37°C under microaerobic
conditions. For the co-culture experiments, the H. pylori
strain was grown for 48 h, resuspended in L-DMEM with 10% FBS and
adjusted to optical density 600 nm=1 [corresponding to
1×108 colony-forming units (CFU)/ml] prior to infection.
All experimental protocols were approved by the Ethics Committee of
Bengbu Medical College, Bengbu, China.
Co-culture of hucMSCs with H.
pylori
A hucMSCs/H. pylori co-culture model was
designed as previously described (18). Briefly, hucMSC cells were trypsinized
(Trypsin; Amresco LLC, Solon, OH, USA), resuspended in L-DMEM with
10% FBS and seeded into a culture flask. Colonies of H.
pylori (48 h) were collected and bacterial cells were added to
the monolayer at a multiplicity of infection (MOI) of 100
bacteria/cell. Cultures were maintained in a 5% CO2
humidified atmosphere at 37°C for 24 h. The supernatants were
collected and centrifuged at 800 × g for 10 min at 4°C, and were
subsequently filtered through a 0.22-µm membrane (EMD Millipore,
Billerica, MA, USA) and stored at −80°C until use. Following the
collection of supernatants, PBS was replaced twice in order to
remove floating hucMSCs, debris and H. pylori. The infected
hucMSCs were then harvested and subjected to the following
experiments. As a control, uninfected hucMSCs were processed in a
similar manner, but in the absence of bacteria.
Transwell migration assay
The migration assay was adapted from a previously
described protocol (19). Briefly,
migration was measured in cell culture inserts with 8.0-µm pore
filters (Corning Inc., Corning, NY, USA). SGC-7901 cells
(5×104 cells/200 µl) suspended in serum-free medium
(Invitrogen; Thermo Fisher Scientific, Inc.) were loaded into the
upper compartment of a Transwell chamber and 600 µl 10% FBS-L-DMEM
containing hucMSCs (5×104 cells/well) in the presence or
absence of H. pylori (MOI, 100:1) was added to the lower
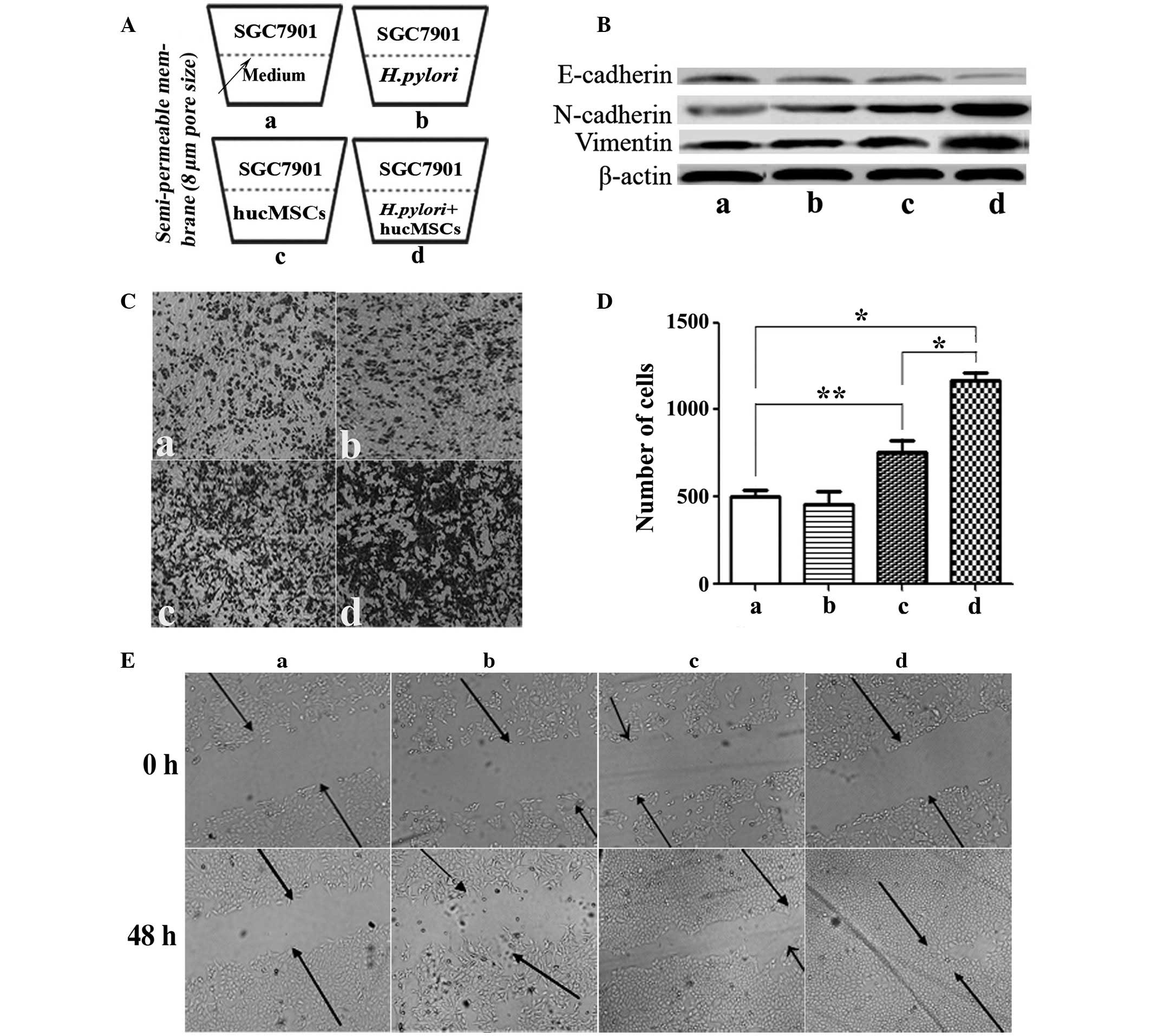
well of the Transwell chamber (Fig.
1A). Following incubation for 8 h, SGC-7901 cells which
remained at the bottom of the polycarbonate membrane were removed
using cotton swabs. Cells which migrated to the lower surface of
the membrane were fixed with 4% paraformaldehyde (AR-0211; DingGuo
Biotechnology Co., Ltd, Shanghai, China) for 30 min. The migrated
cells were subsequently stained with crystal violet (C3886;
Sigma-Aldrich, Shanghai, China) for 15 min and counted in 10 random
fields under a microscope (TE300; Nikon Corporation, Tokyo, Japan).
Each assay was repeated three times.
Luminex assay/ELISA
The concentrations of interleukin (IL)-8, IL-6,
granulocyte macrophage colony-stimulating factor (GM-CSF),
platelet-derived growth factor-B (PDGF-B), monocyte chemoattractant
protein-1 (MCP-1), vascular endothelial growth factor (VEGF),
epidermal growth factor (EGF), tumor necrosis factor-α (TNF-α),
IL-10, IL-1β, IL-15, IL-2 and IL-17 in the supernatants of H.
pylori-infected hucMSCs were evaluated by Luminex assay
(Cytokine and Chemokine Magnetic Bead Panel kit; #HCYTOMAG −60K;
Merck Millipore, Darmstadt, Germany) or human IL-6 ELISA Kit
(DKW12-1060-096), human IL-8 ELISA Kit (DKW12-1080-096) or human
PDGF-BB ELISA Kit (GWB-SKR056; all Dakewe Biotech Co., Ltd.,
Shenzhen, China), according to the manufacturer's instructions. The
Luminex assay detected the expression of cytokines in the
supernatants from hucMSCs and H. pylori-infected hucMSCs.
All procedures were processed according to the manufacturer's
instructions. The ELISA assay was subsequently used to evaluate
IL-6, IL-8 and PDGF-B levels, as these were observed to be the most
highly expressed cytokines in the supernatants of hucMSCs and H.
pylori-infected hucMSCs. The signals were detected and analyzed
by using the Luminex 200 system (Merck Millipore).
Western blot analysis
Western blot analysis was adapted as previously
described (19). Cells were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China) supplemented with complete protease
inhibitors (Shanghai Haoran Biotechnology Co., Ltd., Shanghai,
China) on ice. Aliquots containing identical amounts of protein
were fractionated by SDS-PAGE (Beyotime Institute of Biotechnology)
and then transferred onto polyvinylidene difluoride membranes (EMD
Millipore) following electrophoresis. After blocking in 5% (w/v)
non-fat milk for 1 h at room temperature, the membranes were
incubated at their respective appropriate dilutions of specific
primary antibodies overnight at 4°C. Following incubation with the
secondary antibodies for 2 h at 37°C, the signal was visualized
using horseradish peroxidase (HRP) substrate (EMD Millipore) and
analyzed using MD ImageQuant™ Software (G:Box; Syngene, Cambridge,
UK). The following rabbit anti-human primary antibodies were used
for the western blot analysis: Anti-IκB-α (polyclonal IgG;
dilution, 1:500; cat. no. sc-371; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA); anti-p-NF-κB-p65 (polyclonal IgG; dilution,
1:500; cat. no. sc-101749; Santa Cruz Biotechnology, Inc.);
anti-t-NF-κB-p65 (monoclonal IgG; dilution, 1:1,000; cat. no.
sc-372; Santa Cruz Biotechnology, Inc.); anti-N-cadherin
(polyclonal IgG; dilution, 1:800; cat. no. BS2224; Bioworld
Technology, Inc., St. Louis Park, MN, USA); anti-E-cadherin
(polyclonal IgG; dilution, 1:1,000; cat. no. BS1097; Bioworld
Technology, Inc.); anti-Vimentin (polyclonal IgG; dilution, 1:500;
cat. no. BS1855; Bioworld Technology, Inc.); anti-β-actin
(polyclonal IgG; dilution, 1:10,000; cat. no. AP0060; Bioworld
Technology, Inc.); and anti-histone (polyclonal IgG; dilution,
1:1,000; cat. no. BS1160; Bioworld Technology, Inc.).
HRP-conjugated goat anti-rabbit polyclonal IgG secondary antibodies
(dilution, 1:2,000; cat. no. L3012-2; Signalway Antibody Co., Ltd.,
Nangjing, China) were used.
Wound healing assay
SGC-7901 cells were allowed to grow to 50%
confluence and subsequently wounded by making a single scratch in
the monolayer using a pipette tip (0.1–10 µl; NICHIRYO Co., Ltd.,
Koshigaya, Japan). Following scratching, the medium was replaced in
order to remove floating cells and debris, and cells were incubated
at 37°C for 48 h to allow for growth and closure of the wound.
Inverted microscopic observations (magnification, ×100; TE300;
Nikon Corporation) were used to assess cell migration into the
scratched area. Images were captured at identical positions
relative to the wound at 0 and 48 h.
GC cell incubation with cytokines
To determine whether increased cytokine expression
in H. pylori-infected hucMSCs affected GC cell migration,
specific cytokines (IL-8, IL-6 and PDGF-B; R&D Systems, Inc.,
Minneapolis, MN, USA) were incubated with the SCG-7901 GC cell line
in cell culture inserts. Cell migration was assessed as already
described. Briefly, SGC-7901 cells (5×104 cells/200 µl)
were placed into the top chamber, and medium containing 10% FBS and
50 ng/ml uman cytokines (IL-6, IL-8 and PDGF) was placed into the
bottom chamber of the Transwell plates (8-µm pore size; Corning
Inc.). Following incubation at 37°C in 5% CO2 for 8 h,
the cells remaining on the upper surface of the membrane were
removed. Cells on the lower surface of the membrane were fixed and
stained with crystal violet. The migration ability of the cells was
determined by counting the cells under a microscope in at least 10
fields per assay.
hucMSC pre-treatment with pyrrolidine
dithiocarbamate (PDTC)
To further investigate whether NF-κB is involved in
hucMSC pro-inflammatory phenotype differentiation in response to
H. pylori infection and in H. pylori-induced hucMSC
cytokines expression, hucMSCs were cultured in L-DMEM medium
containing PDTC (10 µmol/ml; Jingmei BioTech Co., Ltd., Shanghai,
China), and were then co-cultured with the H. pylori strain
11673 (MOI, 100:1) at 37°C in a humidified atmosphere of 5%
CO2 for 30, 60, 120 or 180 min, or 24 h. SGC-7901 cells
were exposed to supernatant from hucMSCs, H. pylori-infected
hucMSCs, or H. pylori-infected PDTC-pre-treated hucMSCs for
48 h at 37°C.
Statistical analysis
Statistical analyses were conducted using SPSS
software version 16.0 (SPSS Inc., Chicago, IL, USA). Quantification
values are presented as the mean ± standard deviation of three or
more independent experiments, each performed in duplicate or
triplicate as indicated. Differences between groups were analyzed
using one-way analysis of variance. Differences between pyrrolidine
dithiocarbamate (PDTC) treatments were analyzed using a Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
H. pylori-infected hucMSCs induce an
EMT state in GC cells in vitro
In order to determine the effect exerted on GC cells
by H. pylori-infected hucMSCs, morphological alterations in
SGC-7901 GC cells cultured with H. pylori (11673; MOI,
100:1), or with the supernatants from hucMSCs or H.
pylori-infected hucMSCs, were observed. Infecting hucMSCs with
H. pylori significantly enhanced their ability to induce
SGC-7901 cells to acquire a fibroblastic phenotype (P=0.0079; data
not shown). Downregulation of E-cadherin, a universal epithelial
marker, is an early indicator of EMT (5). Western blot analysis of E-cadherin
expression levels revealed that E-cadherin protein levels were
decreased in SGC-7901 cells cultured with supernatants from H.
pylori-infected hucMSCs, hucMSCs or those treated with H.
pylori, compared with those cultured alone. H.
pylori-infected hucMSCs exerted a more marked effect on
E-cadherin expression than that of H. pylori or uninfected
hucMSCs (Fig. 1B). The observed
decrease in E-cadherin expression levels coincided with induction
of the expression of N-cadherin and vimentin (Fig. 1B).
Migration of SGC-7901 GC cells is
enhanced in the presence of H. pylori-infected hucMSCs
Dissemination of cancer cells during tumor
metastasis, a process which is enabled by EMT, requires enhancement
of cell migration ability. In light of the observation that H.
pylori-infected hucMSCs induce an EMT state in SGC-7901 cells,
it was hypothesized that H. pylori-infected hucMSCs may
promote SGC-7901 cell migration. To test this hypothesis, a
Transwell migration assay was performed (Fig. 1A, C and D). The basal migration rate
of SGC-7901 cells in the presence of the medium alone was identical
to that in the presence of H. pylori strains alone,
indicating that the presence of H. pylori alone was unable
to stimulate SGC-7901 cell migration (Fig. 1Cb and Db). hucMSCs alone were observed
to stimulate SGC-7901 cell migration (P=0.0255). However, H.
pylori-infected hucMSCs markedly promoted SGC-7901 cell
migration (Fig. 1Cd), compared with
SGC-7901 cells alone (P=0.003) or with hucMSCs (P=0.0062) (Fig. 1Da and Dc). The number of cells that
had migrated was subsequently quantified (Fig. 1D).
A wound-healing assay was performed to evaluate cell
migration ability. Following scratching, cells were allowed to
recover and their capacity to migrate and fill the area devoid of
cells was assessed. When SGC-7901 cells were co-cultured with H.
pylori or each type of supernatant, inverted microscopic
observations revealed that the size of the scratched area decreased
within 48 h. These observations also indicated that, although
SGC-7901 cells cultured under all four conditions migrated into the
scratched area, only those cultured with supernatants from H.
pylori-infected hucMSCs exhibited marked migratory behavior
(Fig. 1E). H. pylori-infected
hucMSCs more effectively enhanced SGC-7901 cell membrane
penetration and migration.
H. pylori infection upregulates the
expression of inflammatory cytokines in MSCs and a number of these
promote GC cell migration
To investigate the effect of H. pylori on
MSCs in vitro and identify the secreted cytokines in H.
pylori-infected MSCs, hucMSC cells were infected with H.
pylori 11673 strains as described. A Luminex assay was
conducted to determine the expression levels of
inflammatory-associated functional factors in the supernatants of
H. pylori-infected hucMSCs. The cytokines examined were
selected as they are pro-inflammatory factors (20–22) and
have previously been observed to be overexpressed in H.
pylori-infected hucMSC cells. The results of the present study
revealed that the expression levels of IL-8 (P=0.0095), PDGF-B
(P=0.0238), IL-6 (P=0.0128), GM-CSF (P=0.0082), MCP-1 (P=0.0449),
VEGF (P=0.0472), EGF (P=0.0360), IL-1β (P=0.0313) and TNF-α
(P=0.0367) were markedly increased in the supernatants of H.
pylori-infected hucMSCs (Fig. 2A and
B), while low levels of these cytokines were observed in
hucMSCs that were cultured alone (Fig. 2A
and B). To further confirm the increased expression of these
cytokines, an ELISA was performed to examine the levels of three of
these cytokines (IL-6, IL-8 and PDGF-B). It was revealed that,
consistent with the results of the Luminex assay, co-incubation
with H. pylori upregulated the expression of IL-6
(P=0.0059), IL-8 (P=0.023) and PDGF-B (P=0.0072) in hucMSCs
(Fig. 2C). To determine whether
increased cytokine expression in H. pylori-infected hucMSCs
affected GC cell migration, specific cytokines (IL-8, IL-6 and
PDGF-B) were incubated with the SCG-7901 GC cell line in cell
culture inserts for 8 h. The results revealed that the number of
migrated SGC-7901 cells was markedly increased following incubation
with each of these cytokines (IL-6, P=0.0163; IL-8, P=0.0087;
PDGF-B, P=0.0244), compared with SCG-7901 cells alone (Fig. 2D).
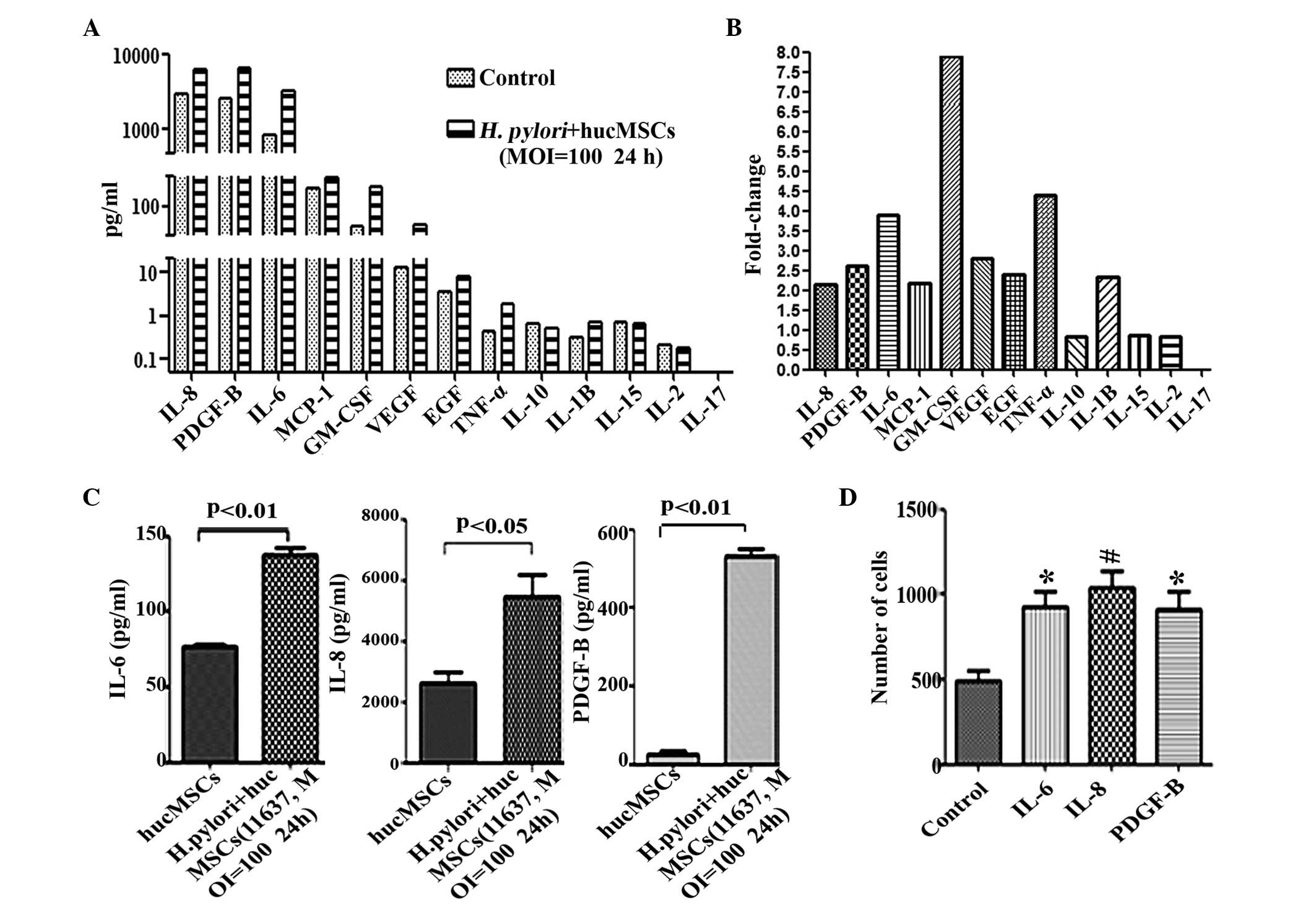 | Figure 2.Expression of inflammatory cytokines
in hucMSCs is upregulated following co-culture with H.
pylori, and the cytokines produced during co-culture promote
migration of SGC-7901 cells. (A) Luminex assay for the expression
of cytokines in the supernatants of hucMSCs and H.
pylori-infected hucMSCs. (B) The fold-changes in cytokine
expression in H. pylori-infected humMSCs relative to
uninfected cells were as follows: IL-8, 2.14; PDGF-B, 2.61; IL-6,
3.9; MCP-1, 2.17; GM-1, 8.0; VEGF, 2.8; EGF, 2.4; TNF-α, 4.4;
IL-1β, 2.35. Fold-change = concentration of cytokine in H.
pylori-infected humMSCs/uninfected humMSCs. (C) ELISA assay to
determine IL-6, IL-8 and PDGF-B levels in the supernatants of
hucMSCs and H. pylori-infected hucMSCs. (D) SGC-7901 cells
were stimulated by 50 ng/ml concentrations of purified cytokines
and migration assays were then performed. *P<0.05 and
#P<0.01 compared with migration medium alone. Values
are presented as the mean ± standard deviation. Data are
representative of three independent experiments. H. pylori,
Helicobacter pylori; hucMSCs, human umbilical cord
mesenchymal stem cells; IL, interleukin; PDGF, platelet-derived
growth factor; MCP, monocyte chemoattractant protein; GM-CSF,
granulocyte macrophage colony-stimulating factor; VEGF, vascular
endothelial growth factor; EGF, epidermal growth factor; TNF, tumor
necrosis factor; MOI, multiplicity of infection. |
H. pylori enhances the expression of
certain cytokines in MSCs through the NF-κB pathway
In order to explore the mechanisms responsible for
the promotion of cytokine secretion induced by H. pylori
infection of MSCs, the expression of NF-κB, one of several key
signaling transducers involved in inflammation and cancer (23), was determined in H.
pylori-infected MSCs. Western blotting revealed that the
expression levels of NF-κB-p65 were significantly higher in hucMSCs
that had been infected with H. pylori (Fig. 3A and B) compared with that of hucMSCs
cultured alone (P=0.0427). To further determine whether NF-κB had a
major role in the function of H. pylori-infected hucMSCs,
hucMSCs were pretreated with PDTC to in order to inhibit NF-κB
phosphorylation, and subsequently incubated with H. pylori.
It was revealed that induction of NF-κB phosphorylation by H.
pylori in hucMSCs was markedly inhibited by PDTC (Fig. 3C; P=0.0040). The activation of the
NF-κB pathway during H. pylori infection was confirmed by
western blotting, which indicated p65 subunit translocation from
the cytosol to the nucleus (Fig. 3D).
To determine the role of the NF-κB pathway in the production of the
cytokines responsible for SGC-7901 cell migration, IL-6, IL-8 and
PDGF-B expression levels were measured in the supernatants of
PDTC-pretreated H. pylori-infected hucMSCs. The
overexpression of cytokines observed in H. pylori-infected
hucMSCs, was abrogated in PDTC-pretreated hucMSC cells (IL-6,
P=0.041; IL-8, P=0.044; PDGF-D, P=0.031), as indicated in Fig. 3E.
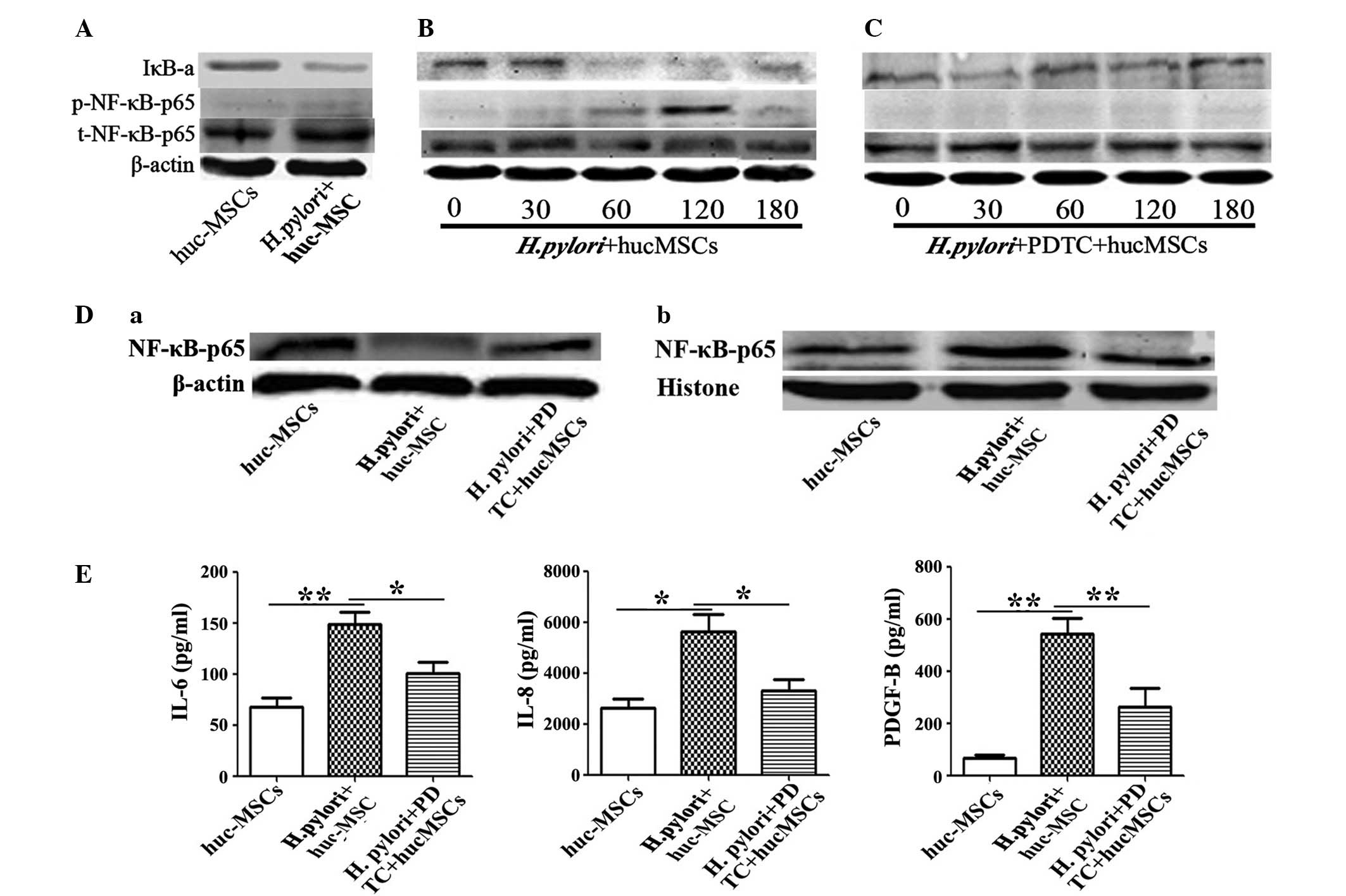 | Figure 3.hucMSCs infected with H.
pylori enhance SCG-7901 gastric cancer cell migration via NF-κB
activation. (A) H. pylori-induced NF-κB-p65 phosphorylation
and IκB-a change in hucMSCs for 24 h. (B and C) Time-course (0, 30,
60, 120, 180 min) of H. pylori induced NF-κB-p65
phosphorylation and IκB-a change in hucMSCs treated with H.
pylori in the presence or absence of PDTC (100nM). (D) Western
blot analysis of (a) cytoplasmic protein and (b) nucleoprotein
expression levels. (E) hucMSCs were pre-incubated with PDTC for 90
min, stimulated with H. pylori for 24 h and then the
concentration of cytokines in the cultured medium was determined by
ELISA. The NF-κB inhibitor PDTC was able to inhibit the expression
of numerous cytokines (IL-6, IL-8 and PDGF-B) in the hucMSCs
infected with H. pylori. Data are expressed as the mean ±
standard deviation; *P<0.05, **P<0.01; data are
representative of three independent experiments. H. pylori,
Helicobacter pylori; hucMSCs, human umbilical cord
mesenchymal stem cells, PDTC, pyrrolidine dithiocarbamate; IL,
interleukin; PDGF, platelet derived growth factor; IκB-a, nuclear
factor-κB polypeptide gene enhancer; NF-κB, nuclear factor-κB;
p-NF-κB, phosphorylated NF-κB; t-NF-κB, total NF-κB. |
PDTC-pretreated MSCs reverse the
migration ability of GC cells
Transwell migration and wound-healing assay
observations indicated that the enhanced migrations of SGC-7901
cells, facilitated by co-culture with H. pylori-infected
MSCs, was significantly reduced in the PDTC-pretreated group
(Fig. 4A and B, P=0.039; Fig. 4C, P=0.011). Furthermore, PDTC
pretreatment also reduced the ability of H. pylori-infected
MSC to induce EMT (defined as induction of vimentin and N-cadherin
expression and suppression of E-cadherin expression) in SCG-7901
cells in vitro (Fig. 4D).
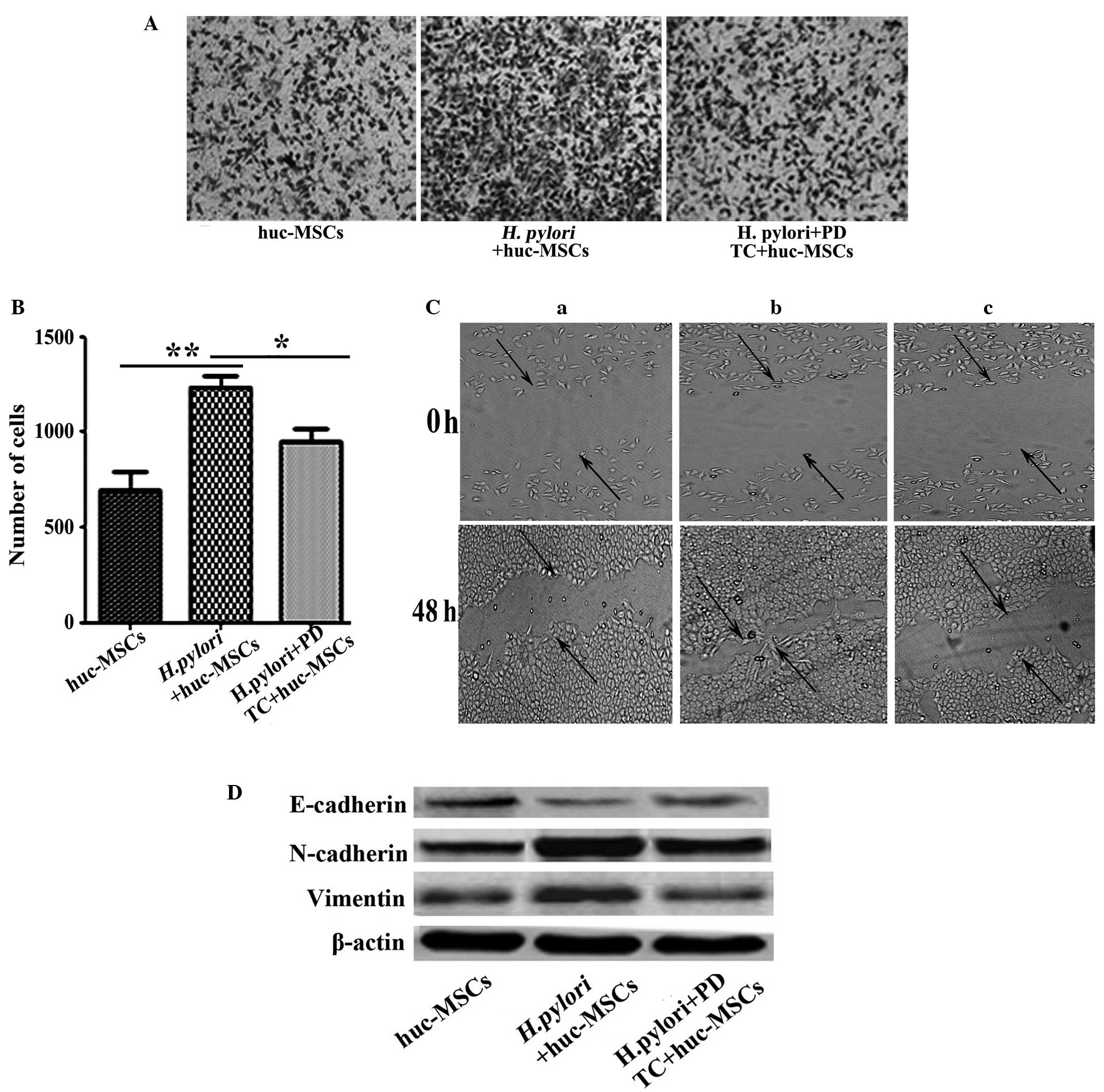 | Figure 4.Inhibition of NF-κB activation by
PDTC abrogates the effect of H. pylori-infected MSCs on
gastric cancer cells. (A) The ability of H. pylori-infected
MSCs to induce migration following pretreatment with PDTC was
evaluated using cell culture inserts; magnification, ×100. (B)
Histogram indicating the number of migrated SGC-7901 cells. Data
are expressed as the mean ± standard deviation; *P<0.05 and
**P<0.01. Data are representative of three independent
experiments. (C) Capacity of cells to migrate to fill a scratched
area devoid of cells was assessed in co-cultures of SCG-7901 cells
with supernatants from (a) hucMSCs, (b) H. pylori-infected
hucMSCs and (c) H. pylori-infected hucMSCs pretreated with
PDTC for 48 h, respectively. (D) Western blot analyses of
E-cadherin, N-cadherin and vimentin protein levels in SGC-7901
cells treated with the supernatants from hucMSCs, H.
pylori-infected hucMSCs and the infected hucMSCs pretreated
with PDTC, respectively. H. pylori, Helicobacter
pylori; hucMSCs, human umbilical cord mesenchymal stem cells,
PDTC, pyrrolidine dithiocarbamate; NF-κB, nuclear factor-κB. |
Discussion
MSCs are multipotent adult stem cells that have been
observed in multiple inflammation and cancer sites (11,12). The
tumor-homing properties of MSCs make them ideal candidates for use
as antitumor agent delivery vehicles (24). There has also been increasing interest
in understanding the role and fate of MSCs during tumor
progression. Korkaya et al (25) suggested that the stromal
microenvironment may be critical for regulating tumor development
in the ‘seed and soil’ hypothesis. Among the stromal cells, MSCs
communicate with cancer cells in order to induce tumor growth and
enhance metastatic potential. In addition, several studies have
indicated that MSCs may enhance tumor metastasis (26,27),
however the mechanism underlying the promotion of H. pylori
infection-associated GC cell migration has remained to be
elucidated. In the present study, hucMSCs were treated with H.
pylori to mimic the H. pylori infection-associated GC
microenvironment and thus determine whether H.
pylori-infected MSCs contribute to GC cell invasion and
metastasis. The results of the present study suggested that
short-term infection with H. pylori may induce MSCs to
acquire a ‘pro-inflammatory phenotype’ and become part of the tumor
microenvironment through the production of increased levels of
inflammatory cytokines.
Metastasis is a common clinical finding in numerous
types of human cancer, and the majority of patients with cancer
succumb to the disease due to metastases (28). EMT is critical in the occurrence of
tumor metastasis (4,29–31). EMT
is defined as a biological process, during which epithelial
cell-cell adhesion is reduced due to downregulation of adhesion
molecules, including E-cadherin. Additionally, cell morphology
becomes fibroblast-like due to the upregulation of vimentin and
N-cadherin (32). Studies have
indicated that improper EMT has been implicated as a factor
required for tumor progression through invasion and metastatic
spread (4,28), and that EMT additionally protects
tumor cells against apoptosis (32,33). In
the present study, it was observed that H. pylori-infected
MSCs induced EMT in GC cells and markedly increased GC cell
migration. To further explain this result, the effects of certain
cytokines (IL-6, IL-8 and PDGF-B), which were particularly highly
expressed by infected hucMSCs, on GC cell EMT, invasion and
metastasis were evaluated. The results of the present study
revealed that these cytokines were able to alter the migration and
EMT of GC cells. Taken together, these data suggest that H.
pylori-infected MSCs secreted higher levels of inflammatory
cytokines, which enhance GC cell invasion and metastasis abilities
by promoting the occurrence of EMT.
In order to explore the mechanism responsible for
the promoting role of H. pylori-infected MSCs in H.
pylori infection-associated GC cell migration, the expression
of the key signaling transducer for inflammation and cancer in
MSCs, NF-κB, was observed (34). The
results of the present study demonstrated that the levels of
p-NF-κB-p65 were markedly increased in H. pylori-infected
MSCs compared with those in the other experimental groups, and the
induction of NF-κB phosphorylation by H. pylori in MSCs was
markedly inhibited by PDTC. PDTC additionally inhibited the
upregulation of IL-6, IL-8 and PDGF-B in H. pylori-infected
MSCs. PDTC-pretreatment of hucMSCs reversed the ability of hucMSCs
to induce GC cell EMT, invasion and metastasis when infected with
H. pylori 11673 strains. These results further confirmed the
critical role of these cytokines in SGC-7901 cell migration and
their NF-κB-dependent secretion by MSCs in response to H.
pylori infection.
In conclusion, the results of the present study
demonstrated that the interaction of H. pylori with MSCs may
effectively induce the migration of GC cells due to the secretion
of a combination of cytokines, a number of which are NF-κB
dependent. These results also indicated that H.
pylori-infected MSCs promote GC cell migration by promoting the
occurrence of EMT. The present study provides evidence that H.
pylori-infected MSCs are able to activate the migratory
properties of GC cells, suggesting the existence of communication
between GC cells and H. pylori-infected MSCs in the gastric
mucosa via cytokine production. The results of the present study
may contribute to the development of viable strategies for the
design of novel targeted therapies, in order to prevent the
formation of distant metastases of H. pylori
infection-associated GC cells.
Acknowledgments
The present study was supported by the Major
Research Plan of the National Natural Science Foundation of China
(grant no. 91129718), the National Natural Science Foundation of
China (grant nos. 81071421, 81000181 and 81201660) and Anhui
Province's Natural Science Foundation (grant no. SBK201342044).
References
|
1
|
Liu GM, Zhou C, Xie C, Yang Z and Lv NH:
Recent advances in research of gastric cancer stem cells. World
Chinese Journal of Digestology. 7:574–579. 2012.(In Chinese).
|
|
2
|
Lee YY and Derakhshan MH: Environmental
and lifestyle risk factors of gastric cancer. Arch Iran Med.
16:358–365. 2013.PubMed/NCBI
|
|
3
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and
interleukin-8 in gastric cancer. World J Gastroenterol.
19:8192–8202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor MA, Parvani JG and Schiemann WP:
The pathophysiology of epithelial-mesenchymal transition induced by
transforming growth factor-beta in normal and malignant mammary
epithelial cells. J Mammary Gland Biol Neoplasia. 15:169–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nitta T, Mitsuhashi T, Hatanaka Y,
Miyamoto M, Oba K, Tsuchikawa T, Suzuki Y, Hatanaka KC, Hirano S
and Matsuno Y: Prognostic significance of epithelial-mesenchymal
transition-related markers in extrahepatic cholangiocarcinoma:
Comprehensive immunohistochemical study using a tissue microarray.
Br J Cancer. 111:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition-related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X and Xu X, Li J, Chen Z and Xu X: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells - basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma
D and Shimizu H: Mesenchymal stem cells are recruited into wounded
skin and contribute to wound repair by transdifferentiation into
multiple skin cell type. J Immunol. 180:2581–2587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: Defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glaire MA, El-Omar EM, Wang TC and
Worthley DL: The mesenchyme in malignancy: A partner in the
initiation, progression and dissemination of cancer. Pharmacol
Ther. 136:131–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taichman RS, Wang Z, Shiozawa Y, Jung Y,
Song J, Balduino A, Wang J, Patel LR, Havens AM, Kucia M, et al:
Prospective identification and skeletal localization of cells
capable of multilineage differentiation in vivo. Stem Cells
Dev. 19:1557–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Houghton J, Stoicov C, Nomura S, Rogers
AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR and Wang TC:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrand J, Lehours P, Schmid-Alliana A,
Mégraud F and Varon C: Helicobacter pylori infection of
gastrointestinal epithelial cells in vitro induces
mesenchymal stem cell migration through an NF-κB-dependent pathway.
PLoS One. 6:e290072011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F, Yang H, et al: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Wang M, Huang F, Yang T, Cai J,
Zhang X, Zhu W, Qian H and Xu W: H. pylori infection-induced
MSC differentiation into CAFs promotes epithelial-mesenchymal
transition in gastric epithelial cells. Int J Mol Med.
32:1465–1473. 2013.PubMed/NCBI
|
|
19
|
Yang T, Zhang X, Wang M, Zhang J, Huang F,
Cai J, Zhang Q, Mao F, Zhu W, Qian H and Xu W: Activation of
mesenchymal stem cells by macrophages prompts human gastric cancer
growth through NF-κB pathway. PLoS One. 9:e975692014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou H, Sheng L, Wang H, Xie H, Mu Y, Wang
T and Yan J: Anti-β2GPI/β2GPI stimulates activation of THP-1 cells
through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb Res.
132:742–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Cai J, Huang F, Zhu M, Zhang Q,
Yang T, Zhang X, Qiang H and Xu W: Pre-treatment of human umbilical
cord-derived mesenchymal stem cells with interleukin-6 abolishes
their growth-promoting effect on gastric cancer cells. Int J Mol
Med. 35:367–375. 2015.PubMed/NCBI
|
|
22
|
Okabe C, Borges RL, de Almeida DC, Fanelli
C, Barlette GP, Machado FG, Arias SC, Malheiros DM, Camara NO, Zatz
R and Fujihara CK: NF-κB activation mediates crystal translocation
and interstitial inflammation in adenine overload nephropathy. Am J
Physiol Renal Physiol. 305:F155–F163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Jiang W and Zuo Z: Pyrrolidine
dithiocarbamate attenuates surgery-induced neuroinflammation and
cognitive dysfunction possibly via inhibition of nuclear factor κB.
Neuroscience. 261:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan J, O'Donoghue K, de la Fuente J,
Roberts IA, Kumar S, Morgan JE and Fisk NM: Human fetal mesenchymal
stem cells as vehicles for gene delivery. Stem Cells. 23:93–102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korkaya H, Liu S and Wicha MS: Breast
cancer stem cells, cytokine networks, and the tumor
microenvironment. J Clin Invest. 121:3804–3809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shinagawa K, Kitadai Y, Tanaka M, Sumida
T, Kodama M and Higashi Y: Tanaka S, Yasui W and Chayama K:
Mesenchymal stem cells enhance growth and metastasis of colon
cancer. Int J Cancer. 127:2323–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu JM, Jun ES, Bae YC and Jung JS:
Mesenchymal stem cells derived from human adipose tissues favor
tumor cell growth in vivo. Stem Cells Dev. 17:463–473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grant CM and Kyprianou N: Epithelial
mesenchymal transition (EMT) in prostate growth and tumor
progression. Transl Androl Urol. 2:202–211. 2013.PubMed/NCBI
|
|
33
|
Christiansen JJ and Raiasekaran AK:
Reassessing epithelial to mesenchymal transition as prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tye H and Jenkins BJ: Tying the knot
between cytokine and toll-like receptor signaling in
gastrointestinal tract cancers. Cancer Sci. 104:1139–1145. 2013.
View Article : Google Scholar : PubMed/NCBI
|