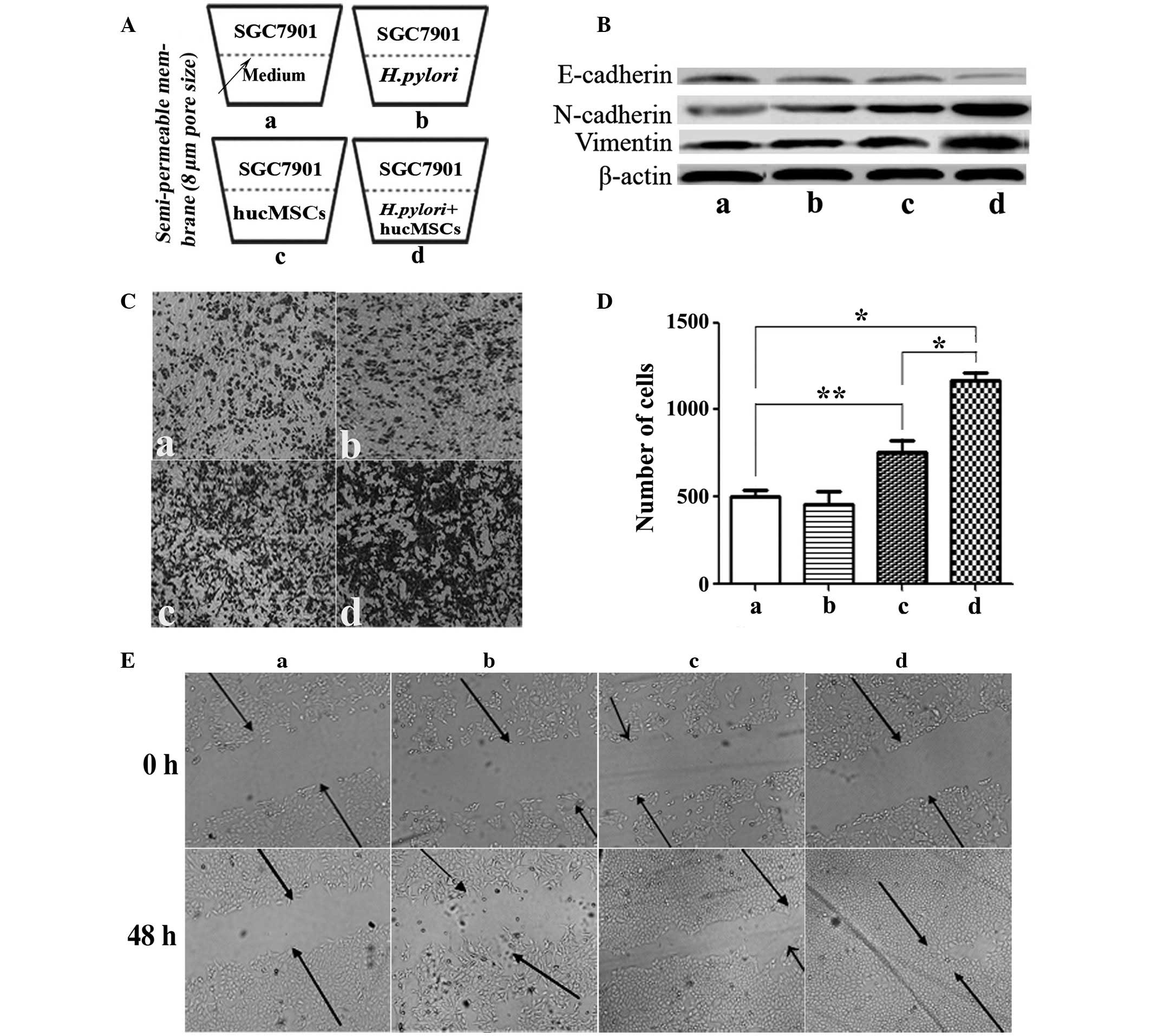
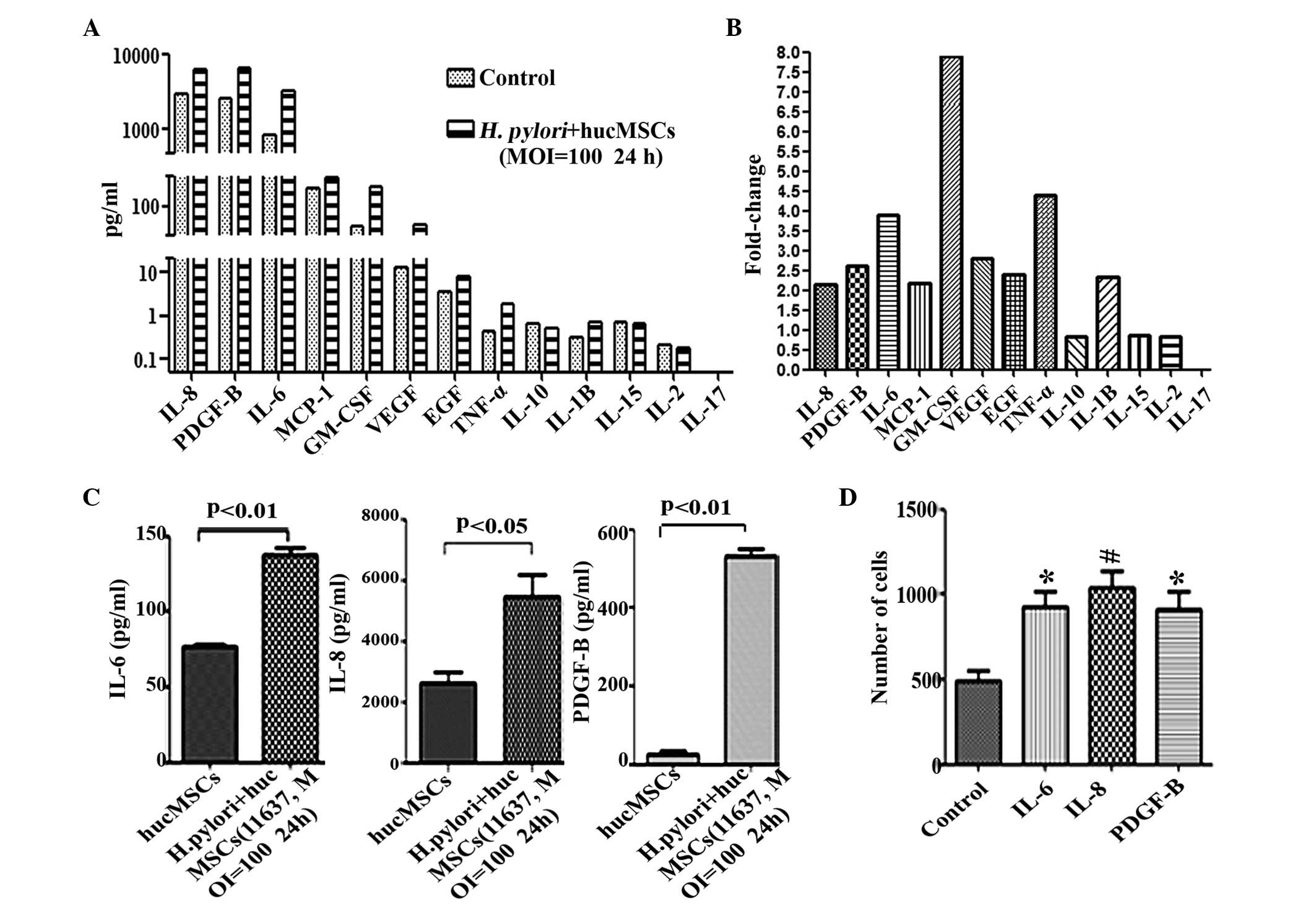
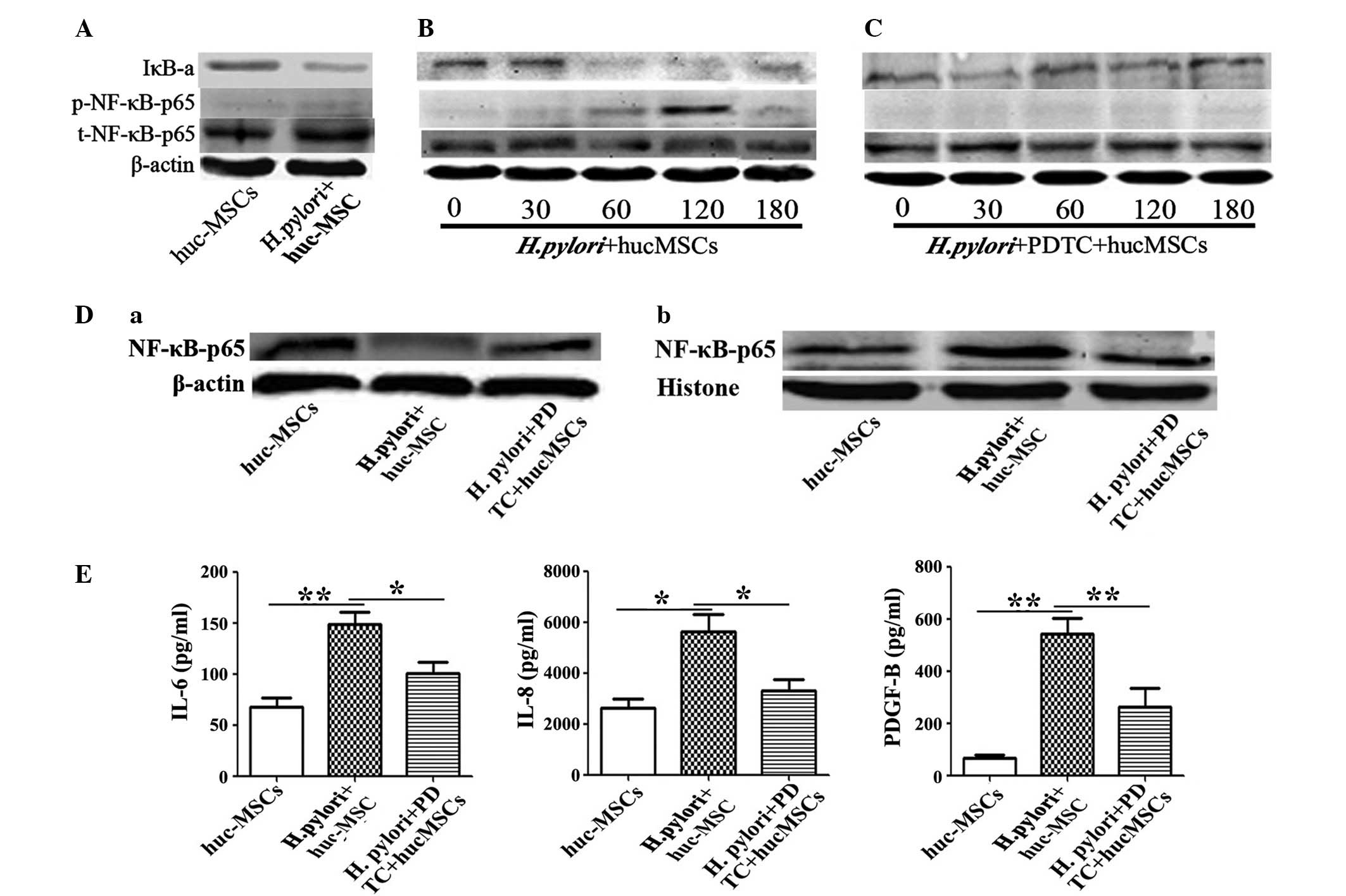
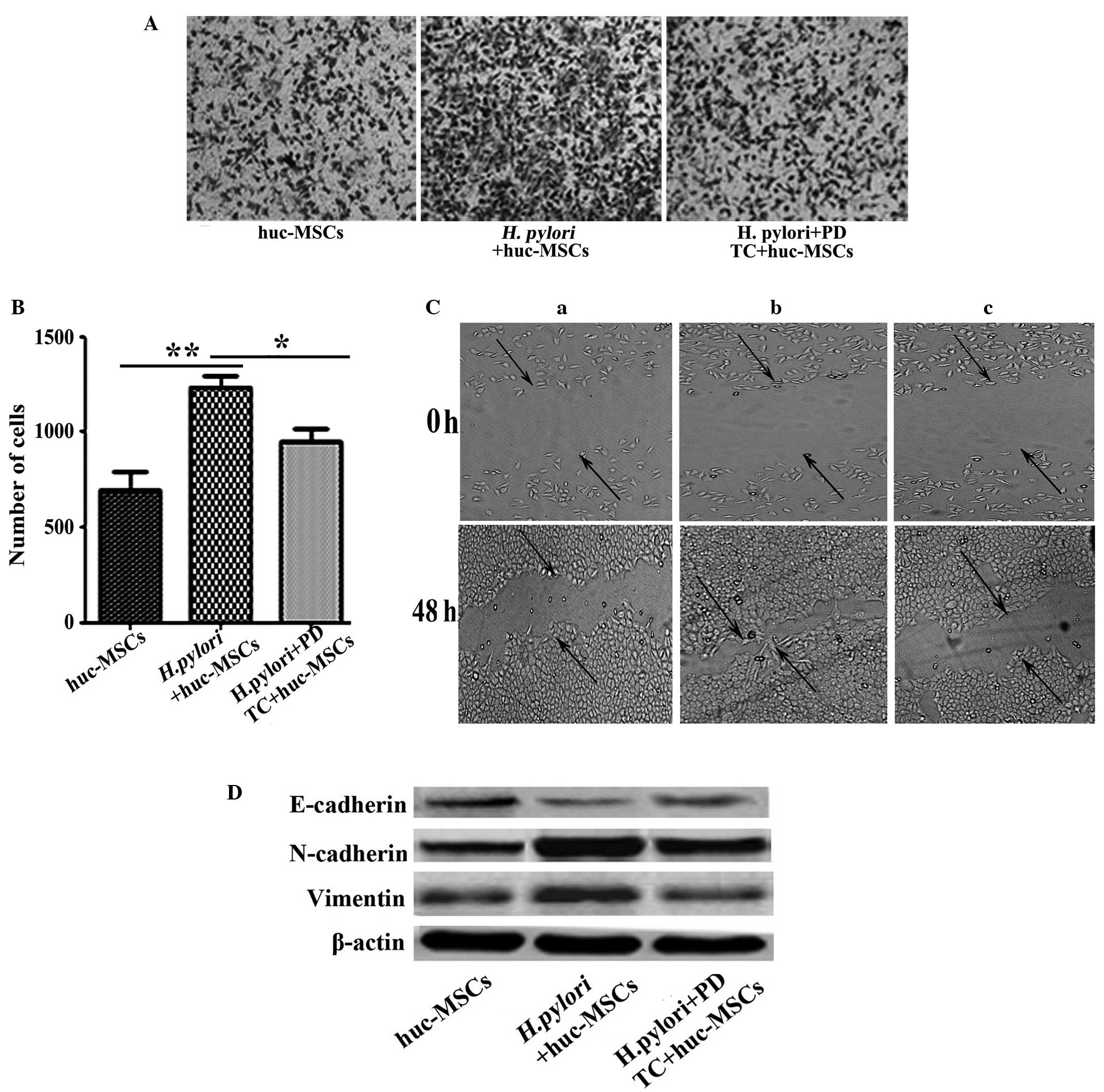
|
1
|
Liu GM, Zhou C, Xie C, Yang Z and Lv NH:
Recent advances in research of gastric cancer stem cells. World
Chinese Journal of Digestology. 7:574–579. 2012.(In Chinese).
|
|
2
|
Lee YY and Derakhshan MH: Environmental
and lifestyle risk factors of gastric cancer. Arch Iran Med.
16:358–365. 2013.PubMed/NCBI
|
|
3
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and
interleukin-8 in gastric cancer. World J Gastroenterol.
19:8192–8202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taylor MA, Parvani JG and Schiemann WP:
The pathophysiology of epithelial-mesenchymal transition induced by
transforming growth factor-beta in normal and malignant mammary
epithelial cells. J Mammary Gland Biol Neoplasia. 15:169–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nitta T, Mitsuhashi T, Hatanaka Y,
Miyamoto M, Oba K, Tsuchikawa T, Suzuki Y, Hatanaka KC, Hirano S
and Matsuno Y: Prognostic significance of epithelial-mesenchymal
transition-related markers in extrahepatic cholangiocarcinoma:
Comprehensive immunohistochemical study using a tissue microarray.
Br J Cancer. 111:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MA, Lee HS, Lee HE, Kim JH, Yang HK
and Kim WH: Prognostic importance of epithelial-mesenchymal
transition-related protein expression in gastric carcinoma.
Histopathology. 54:442–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao H, Xu W, Qian H, Zhu W, Yan Y, Zhou H,
Zhang X and Xu X, Li J, Chen Z and Xu X: Mesenchymal stem cell-like
cells derived from human gastric cancer tissues. Cancer Lett.
274:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells - basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma
D and Shimizu H: Mesenchymal stem cells are recruited into wounded
skin and contribute to wound repair by transdifferentiation into
multiple skin cell type. J Immunol. 180:2581–2587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spaeth E, Klopp A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: Defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quante M, Tu SP, Tomita H, Gonda T, Wang
SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al: Bone
marrow-derived myofibroblasts contribute to the mesenchymal stem
cell niche and promote tumor growth. Cancer Cell. 19:257–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glaire MA, El-Omar EM, Wang TC and
Worthley DL: The mesenchyme in malignancy: A partner in the
initiation, progression and dissemination of cancer. Pharmacol
Ther. 136:131–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taichman RS, Wang Z, Shiozawa Y, Jung Y,
Song J, Balduino A, Wang J, Patel LR, Havens AM, Kucia M, et al:
Prospective identification and skeletal localization of cells
capable of multilineage differentiation in vivo. Stem Cells
Dev. 19:1557–1570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Houghton J, Stoicov C, Nomura S, Rogers
AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR and Wang TC:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrand J, Lehours P, Schmid-Alliana A,
Mégraud F and Varon C: Helicobacter pylori infection of
gastrointestinal epithelial cells in vitro induces
mesenchymal stem cell migration through an NF-κB-dependent pathway.
PLoS One. 6:e290072011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F, Yang H, et al: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Wang M, Huang F, Yang T, Cai J,
Zhang X, Zhu W, Qian H and Xu W: H. pylori infection-induced
MSC differentiation into CAFs promotes epithelial-mesenchymal
transition in gastric epithelial cells. Int J Mol Med.
32:1465–1473. 2013.PubMed/NCBI
|
|
19
|
Yang T, Zhang X, Wang M, Zhang J, Huang F,
Cai J, Zhang Q, Mao F, Zhu W, Qian H and Xu W: Activation of
mesenchymal stem cells by macrophages prompts human gastric cancer
growth through NF-κB pathway. PLoS One. 9:e975692014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou H, Sheng L, Wang H, Xie H, Mu Y, Wang
T and Yan J: Anti-β2GPI/β2GPI stimulates activation of THP-1 cells
through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb Res.
132:742–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Cai J, Huang F, Zhu M, Zhang Q,
Yang T, Zhang X, Qiang H and Xu W: Pre-treatment of human umbilical
cord-derived mesenchymal stem cells with interleukin-6 abolishes
their growth-promoting effect on gastric cancer cells. Int J Mol
Med. 35:367–375. 2015.PubMed/NCBI
|
|
22
|
Okabe C, Borges RL, de Almeida DC, Fanelli
C, Barlette GP, Machado FG, Arias SC, Malheiros DM, Camara NO, Zatz
R and Fujihara CK: NF-κB activation mediates crystal translocation
and interstitial inflammation in adenine overload nephropathy. Am J
Physiol Renal Physiol. 305:F155–F163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Jiang W and Zuo Z: Pyrrolidine
dithiocarbamate attenuates surgery-induced neuroinflammation and
cognitive dysfunction possibly via inhibition of nuclear factor κB.
Neuroscience. 261:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan J, O'Donoghue K, de la Fuente J,
Roberts IA, Kumar S, Morgan JE and Fisk NM: Human fetal mesenchymal
stem cells as vehicles for gene delivery. Stem Cells. 23:93–102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korkaya H, Liu S and Wicha MS: Breast
cancer stem cells, cytokine networks, and the tumor
microenvironment. J Clin Invest. 121:3804–3809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shinagawa K, Kitadai Y, Tanaka M, Sumida
T, Kodama M and Higashi Y: Tanaka S, Yasui W and Chayama K:
Mesenchymal stem cells enhance growth and metastasis of colon
cancer. Int J Cancer. 127:2323–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu JM, Jun ES, Bae YC and Jung JS:
Mesenchymal stem cells derived from human adipose tissues favor
tumor cell growth in vivo. Stem Cells Dev. 17:463–473. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grant CM and Kyprianou N: Epithelial
mesenchymal transition (EMT) in prostate growth and tumor
progression. Transl Androl Urol. 2:202–211. 2013.PubMed/NCBI
|
|
33
|
Christiansen JJ and Raiasekaran AK:
Reassessing epithelial to mesenchymal transition as prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tye H and Jenkins BJ: Tying the knot
between cytokine and toll-like receptor signaling in
gastrointestinal tract cancers. Cancer Sci. 104:1139–1145. 2013.
View Article : Google Scholar : PubMed/NCBI
|