Introduction
Desmoplastic small round cell tumors (DSRCTs) are
rare and aggressive neoplasms that predominantly occur in young
adults, with ~90% of cases occurring in males (1). To date, <200 cases of DSRCTs have
been reported in the literature. Patients typically present with a
large intra-abdominal or pelvic mass with peritoneal and omental
spread of the tumor, lymph node involvement, and multiple
metastases to the liver, lungs and bone; however, spread to the
bone marrow or the central nervous system (CNS) is rare (2). Typical symptoms include abdominal
distension, abdominal pain and emesis. Characteristic histological
findings include nests of small round cells, which are positive for
epithelial (cytokeratins and epithelial membrane antigen),
mesenchymal (vimentin), neural [neuron-specific enolase and cluster
of differentiation (CD)56] and myogenic (desmin) markers, and
embedded in desmoplastic stroma. The definitive diagnosis of DSRCT
is based on the detection of the Ewing' sarcoma (EWS)/Wilm's tumor
protein 1 (WT1) fusion gene (3).
The P6 protocol, a high-dose alkylator-based
regimen, is the most common treatment for DSRCT; 90% of treated
patients exhibit a partial or complete response (3). However, despite the combination of
multi-agent chemotherapies, surgery and local irradiation
treatments available, the outcome of DSRCT remains extremely poor,
with a 5-year overall survival rate of ~15%, due to markedly high
rates of disease progression and relapse (3,4).
Generally, relapses present as a local recurrence and/or
hematogenous or lymphogenous metastasis (1,3,4). Until now, CNS recurrence had not been
reported. The present study reports the case of a 16-year-old male
with metastatic DSRCT who developed recurrence in the CNS following
aggressive multimodal treatment, including intensive chemotherapy
using frequent autologous stem cell support. Written informed
consent was obtained from the patient's family and the study was
approved by the ethics committee of the Graduate School of
Medicine, Kyoto University (Kyoto, Japan).
Case report
In May 2012, a 16-year-old male patient was admitted
to Otsu Red Cross Hospital (Otsu, Japan) with a 2 month history of
progressive abdominal pain, weight loss, dyschezia and
hematochezia. The patient's medical history was unremarkable.
Abdominal magnetic resonance imaging (MRI) and
18F-fluorodeoxyglucose positron emission
tomography-computed tomography (CT) revealed the presence of a
large tumor on the pelvic floor, multiple metastases to the liver,
bone and lymph nodes, and right kidney hydronephrosis due to
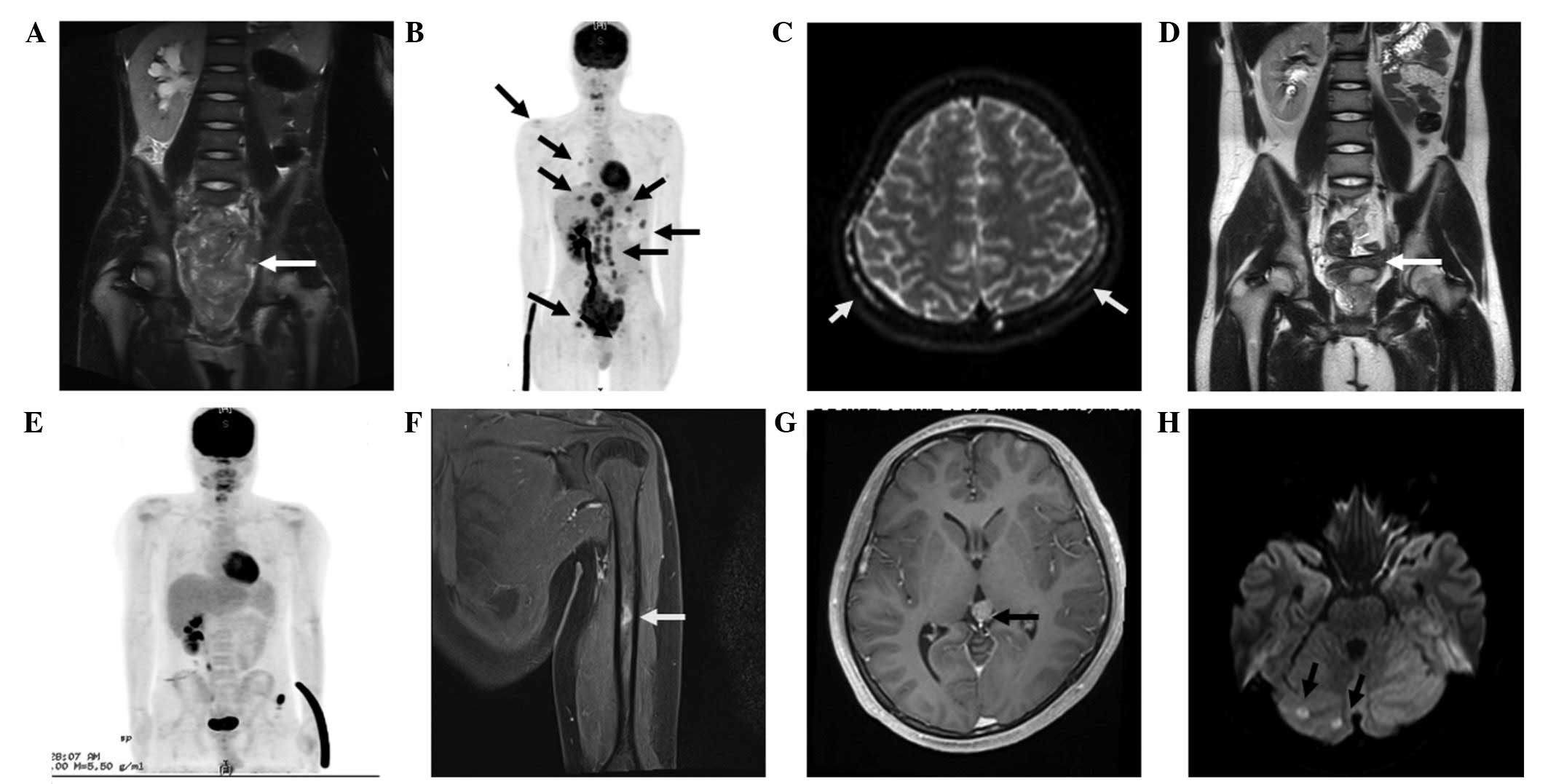
compression of the ureter (Fig.
1A–C). Right renal agenesis, an ipsilateral seminal vesicle
cyst and ejaculatory duct obstruction were also incidentally
detected, all of which are typical findings of Zinner syndrome
caused by the congenital loss of paramesonephric duct derivatives
(5). However, to date, the
association between DSRCT and Zinner syndrome has not been
investigated.
In June 2012, the patient was transferred to Kyoto
University Hospital (Kyoto, Japan), and underwent open biopsy of
the left groin lymph node metastasis. Histological observation of a
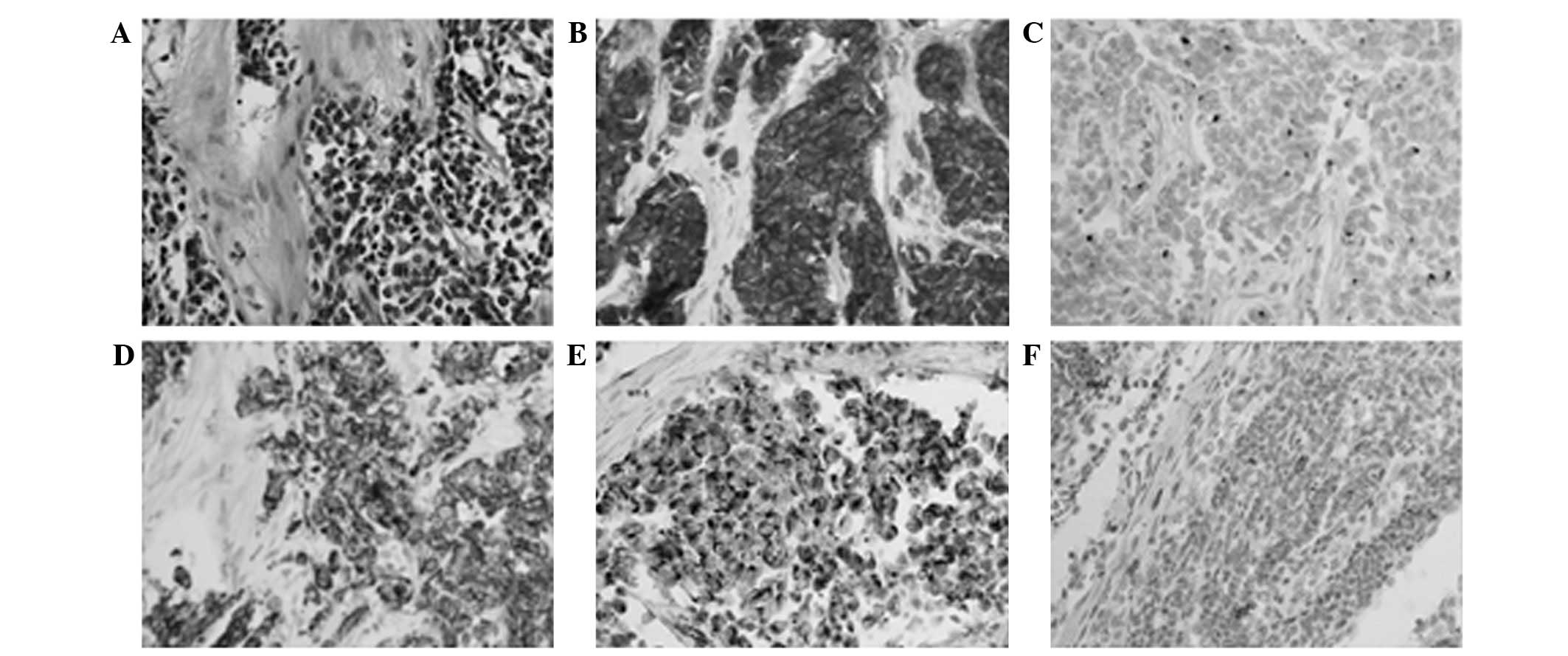
biopsy sample revealed groups of small, round, undifferentiated
cells embedded in a desmoplastic stroma (Fig. 2A). Immunohistochemistry of the
specimen was positive for neuron-specific enolase, desmin,
cytokeratin and WT1, focally positive for CD56, and negative for
CD99, myogenic differentiation 1 and S100 (Fig. 2B–F). The patient was diagnosed with
DSRCT based on detection of the EWS/WT1 fusion transcript by
reverse transcription (RT)-polymerase chain reaction (PCR).
Briefly, RT-PCR was performed using the PE7000 PCR detection system
(Perkin-Elmer, Inc., Waltham, MA, USA). The reaction system
contained 1 µl cDNA, 2 µl forward and reverse primers, 5 µl PCR
buffer, 3 µl MgCl2, 0.2 µl rTaq enzyme, 5 µl dNTPs (2
mM) and 31.8 µl dH2O. PCR was performed under the
following conditions: Initial denaturation step at 94°C for 2 min,
followed by 35 cycles of 94°C for 30 sec, 62°C for 30 sec and 72°C
for 1 min, with a final extension step at 72°C for 6 min. No
metastatic disease was detected by bone marrow aspiration or MRI of
the head.
The patient's clinical course is indicated in
Fig. 3. In June 2012, following
percutaneous nephrostomy and colostomy, the patient was initially
treated with eight courses (18–21 day cycles) of multi-agent
chemotherapy. A modified protocol of the P6 regimen (2) was used, as follows: Vincristine (2
mg/m2, day 1), doxorubicin (DOX; 37.5 mg/m2,
days 1 and 2; using pirarubicin instead of DOX for the fifth and
sevenths courses) and cyclophosphamide [Cy; 1.2 g/m2
(day 1) for the first course and 2.1 g/m2 (days 1 and 2)
for the third, fifth and seventh courses], alternating with
ifosfamide (IFO; 1.8 g/m2, days 1–5) and etoposide
(VP16; 100 mg/m2, days 1–5). Peripheral blood stem cells
(PBSCs), containing a total of 7.4, 4.5 and 5.0×106
cells/kg CD34+ cells, were harvested following
mobilization with granulocyte-stimulating factor after the second,
fourth and fifth courses of chemotherapy, respectively. To hasten
the hematological recovery, PBSCs, containing a total of 1.2, 1.0
and 1.1×106 cells/kg CD34+ cells, were
infused after the third, fifth and seventh courses of chemotherapy,
respectively. Following the completion of eight courses of
chemotherapy over 25 weeks, the primary tumor markedly regressed
and all metastatic lesions except one at the caudate lobe of the
liver disappeared (Fig. 1D and E). In
November 2012, the patient underwent a gross total resection of the
primary tumor on the pelvic floor, a subtotal resection of the
caudate lobe of the liver, while sparing the rectum, bladder and
ureter. Intraoperatively, multiple disseminated tumors were
identified on the peritoneum and diaphragm, which had not been
identified by preoperative imaging. Subsequently, radical resection
of the disseminated tumors on the peritoneum and diaphragm was also
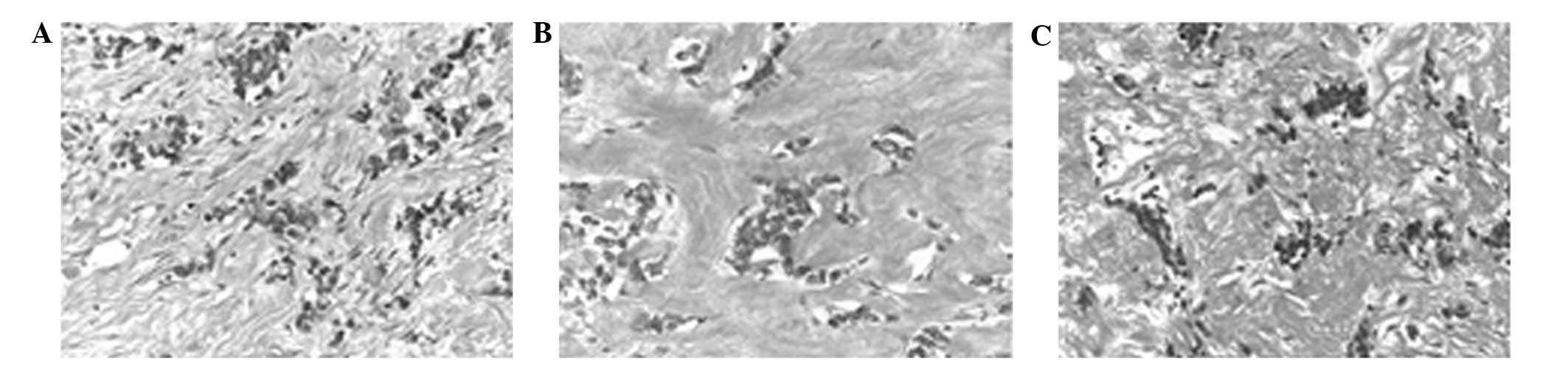
performed. Histological examination of the excised primary and
metastatic lesions revealed scattered viable cells embedded in a
dense desmoplastic stroma (Fig.
4A–C). Subsequently, in January 2013, the patient received one
28-day course of post-operative chemotherapy using topotecan (Topo;
0.75 mg/m2, days 1–5) and Cy (250 mg/m2, days
1–5), a combination that is considered to have potent antitumor
activity against DRSCT (6).
Thereafter, the patient was treated with two 28-day cycles of
high-dose chemotherapy using Topo (3 mg/m2, days 1–5)
and Cy (1 mg/m2, days 3–5), followed by the infusion of
57 and 48 ml PBSCs, containing 1.8 and 1.6×106
CD34+ cells/kg, respectively.
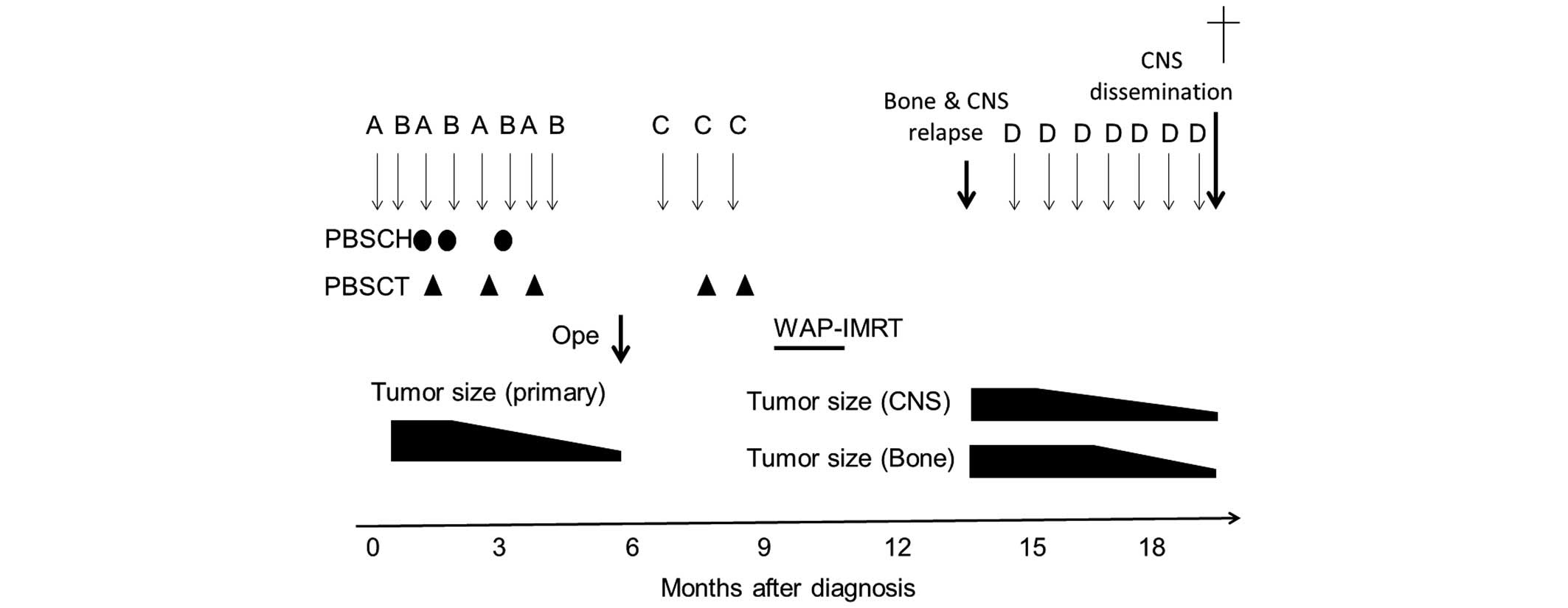 | Figure 3.Clinical course of the patient. The
patient received eight courses of chemotherapy, consisting of
vincristine, doxorubicin or pirarubicin, and cyclophosphamide (Cy)
(A), and ifosfamide and etoposide (B). Next, Ope was performed,
followed by three courses of post-operative chemotherapy,
consisting of topotecan and Cy (C), and WAP-IMRT. Following central
nervous system and bone relapse, the patient received seven courses
of chemotherapy consisting of irinotecan and temozolomide (D).
Circles and triangles indicate PBSCH and PBSCT, respectively. CNS,
central nervous system; PBSCH, peripheral blood stem cell harvests;
PBSCT, peripheral blood stem cell transplantation; Ope, gross total
resection of the primary and metastatic lesions; WAP-IMRT, whole
abdominopelvic intensity-modulated radiation therapy. |
There were no severe regimen-related toxicities and
no serious infections during the pre- or post-operative
chemotherapy treatment periods. Finally, in March 2013, the patient
received whole abdominopelvic intensity-modulated radiation therapy
at a dose of 30 Gy in 20 fractions for 28 days. The patient was
discharged without any active lesions 11 months after the initial
diagnosis.
Three months after discharge, the patient was
asymptomatic, however, MRI of the upper extremities demonstrated
bone metastases in the left humerus on follow-up (Fig. 1F). Furthermore, MRI of the head
demonstrated a mass in the pineal body and multiple cerebellar
lesions (Fig. 1G and H). No evidence
of active disease was revealed by abdominal and spinal MRI or by
cerebrospinal fluid (CSF) examination. The patient was treated with
irinotecan (CPT11; 35 mg/m2, days 1–5) and temozolomide
(TMZ; 120 mg/m2, days 1–5), without irradiation, with
the expectation that synergistic antitumor activity would be
exhibited and that TMZ could cross the blood-brain barrier.
Subsequent to four 28-day cycles of chemotherapy, a partial
response of the bone metastasis and pineal body was observed,
whereas the response of the cerebellar lesions was stable
disease.
In March 2014, the patient was readmitted to Kyoto
University Hospital due to a severe headache, which had lasted for
one week following seven 28-day cycles of chemotherapy.
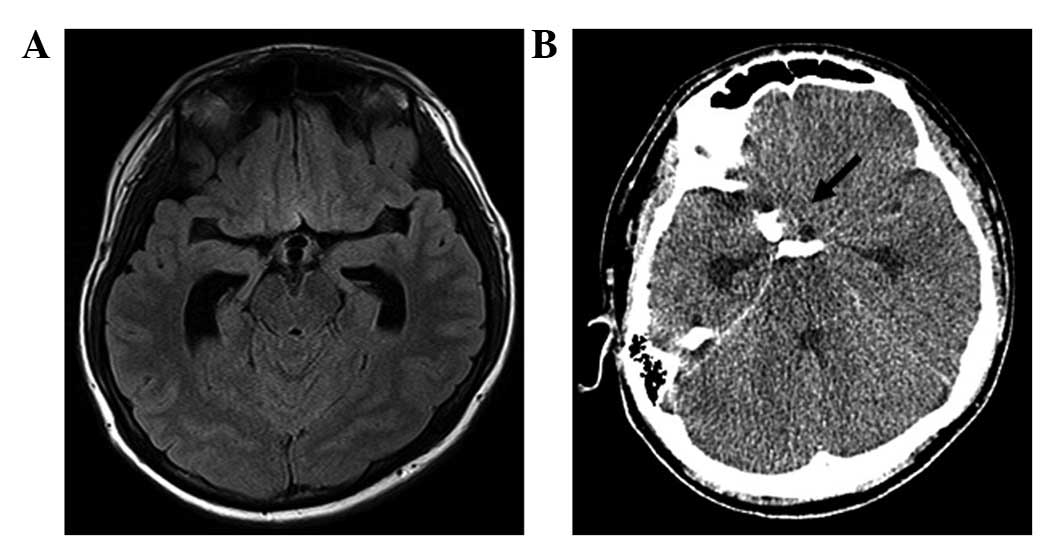
Fluid-attenuated inversion recovery MRI of the head demonstrated
dilatation of the lateral ventricles, although the intracranial
lesions had not increased in size (Fig.
5A). The CSF cell count was 20 cells/µl and CSF cytology was
positive for malignant, small, round cells, indicating CNS
dissemination of DSRCT. The CSF protein level was 34.9 mg/dl
(normal range, 10.0–40.0 mg/dl) and glucose was 22 mg/dl (normal
range, 40–75 mg/dl; serum glucose, 116 mg/dl, normal range, 78–110
mg/dl). Two days later, the patient suddenly exhibited a decreased
level of consciousness and a head CT revealed a subarachnoid
hemorrhage at the base of the brain (Fig.
5B). As a consequence, the patient succumbed due to progressive
CNS disease 1 year and 9 months after the initial diagnosis. An
autopsy was not performed, as permission could not be obtained from
the family.
Discussion
Gross tumor resection is strongly associated with
prolonging the overall survival of patients with DSRCT (4). Intensive chemotherapy, such as the P6
protocol, is initially effective for the majority cases of DSRCT
(2). However, severe bone marrow
suppression, even when granulocyte-stimulating factor is used,
requires the intervals between treatments to be lengthened after a
number of cycles. In a previous study, it was identified that the
regrowth of chemotherapy-resistant tumors eventually hindered gross
tumor resection, and that almost all patients undergoing incomplete
resection succumbed to the disease within 2 years (4).
The following chemotherapy regimen was planned for
the current patient in response to a large intra-abdominal DSRCT:
Increasing dose-intensity by decreasing the interval between
chemotherapy cycles, while maintaining an identical total dose
throughout. As a result, sustained antitumor activity was observed
following eight cycles of pre-operative chemotherapy using frequent
PBSC support at ~3-week intervals, and gross total resection of the
primary and intra-abdominal metastatic tumors was completed.
Furthermore, no active disease was observed at the site of the
abdominal or pelvic lesions at least 1 year and 9 months after the
initial diagnosis. Thus, interval-compressed chemotherapy, in
combination with surgery and local irradiation, is a promising
treatment strategy, at least for the control of localized
DSRCT.
There have been three reported cases of a primary
CNS tumor in DRSCT to date (7,8), but this
is the first report of DSRCT recurrence in the CNS (Table I). Although rare, reports of
neuroblastoma or EWS recurrence in the CNS are on the increase,
which may be associated with improvements in the outcome due to
aggressive multimodal therapy (9,10).
Notably, the present case exhibited multiple metastatic lesions in
the internal table of calvaria at the initial diagnosis, which
raises the possibility that the CNS involvement of DSRCT was
associated with tumor extension through the dura or skull into the
adjacent superficial brain parenchyma, as previously reported in
neuroblastoma and EWS (9,10). Thus, further successful advances in
treatments for DSRCT will require awareness of the potential for
CNS recurrence and the identification of risk factors for such an
unusual pattern of metastasis.
 | Table I.Summary of cases of desmoplastic small
round cell tumors with CNS involvement. |
Table I.
Summary of cases of desmoplastic small
round cell tumors with CNS involvement.
| Age,
years/gender | Disease status | CNS lesions | Surgery | Treatment | Outcome | Reference |
|---|
| 24/M | Primary | Posterior fossa | Partial
resection | PCNU, CDDP, VP16,
local irradiation, it-MTX | AWD (unknown) | 5 |
| 37/M | Primary | CPA resection | Partial
resection | CBDCA, TMZ, radiation
[WB and local (CPA, spine)] | DOD (24 months) | 6 |
| 39/M | Primary | CPA, spine | Partial
resection | CDDP, VP16, IFO,
resection radiation [WB and local (CPA, spine)] | AWD (27 months) | 6 |
| 16/M | Relapsed | Pineal body,
cerebellum | No | CPT11, TMZ | DOD (21 months) | Current case |
The three previously reported cases of a primary CNS
tumor in DSRCT followed aggressive courses, similar to that of
intra-abdominal DSRCT, despite an initial response to chemotherapy
regimes, such as IFO, VP16, TMZ and carboplatin, in combination
with whole-brain or spinal irradiation (Table I) (7,8). In the
current case, the combination of CPT11 and TMZ was selected as
salvage therapy for CNS recurrence of DSRCT, which has exerted an
good antitumor effect for refractory or relapsed neuroblastoma and
EWS (11,w). Indeed, the combination of CPT11 and TMZ was initially
effective against the CNS metastatic lesions; however, the patient
ultimately succumbed due to progressive CNS disease. Therefore,
additional studies are required to establish a novel chemotherapy,
in combination with craniospinal irradiation, to prevent or treat
DSRCT of the CNS.
References
|
1
|
Jordan AH and Pappo A: Management of
desmoplastic small round-cell tumors in children and young adults.
J Pediatr Hematol Oncol. 34(Suppl 2): S73–S75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gerald WL, Ladanyi M, de Alava E,
Cuatrecasas M, Kushner BH, LaQuaglia MP and Rosai J: Clinical,
pathologic, and molecular spectrum of tumors associated with
t(11;22)(p13;q12): Desmoplastic small round-cell tumor and its
variants. J Clin Oncol. 16:3028–3036. 1998.PubMed/NCBI
|
|
3
|
Kushner BH, LaQuaglia MP, Wollner N,
Meyers PA, Lindsley KL, Ghavimi F, Merchant TE, Boulad F, Cheung
NK, Bonilla MA, et al: Desmoplastic small round-cell tumor:
Prolonged progression-free survival with aggressive multimodality
therapy. J Clin Oncol. 14:1526–1531. 1996.PubMed/NCBI
|
|
4
|
Lal DR, Su WT, Wolden SL, Loh KC, Modak S
and LaQuaglia MP: Results of multimodal treatment for desmoplastic
small round cell tumors. J Pediatr Surg. 40:251–255. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Livingston L and Larson CR: Seminal
vesicle cysts with ipsilateral renal agenesis. AJR Am J Roentgenol.
175:177–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sayors RL III, Stine KC, Sullivan J,
Kepner JL, Wall DA, Bernstein ML, Harris MB, Hayashi R and Vietti
TJ: Pediatric Oncology Group: Cyclophosphamide plus topotecan in
children with recurrent or refractory solid tumors: A pediatric
oncology group phase II study. J Clin Oncol. 19:3463–3469.
2001.PubMed/NCBI
|
|
7
|
Tison V, Cerasoli S, Morigi F, Ladanyi M,
Gerald WL and Rosai J: Intracranial desmoplastic small-cell tumor.
Report of a case. Am J Surg Pathol. 20:112–117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neder L, Scheithauer BW, Turel KE, Arnesen
MA, Ketterling RP, Jin L, Moynihan TJ, Giannini C and Meyer FB:
Desmoplastic small round cell tumor of the central nervous system:
Report of two cases and review of the literature. Virchows Arch.
454:431–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matthay KK, Brisse H, Couanet D, Couturier
J, Bénard J, Mosseri V, Edeline V, Lumbroso J, Valteau-Couanet D
and Michon J: Central nervous system metastasis in neuroblastoma:
Radiologic, clinical and biologic features in 23 patients. Cancer.
98:155–165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo MF, Lin SM and Tu YK: Solitary
cerebellar metastasis from Ewing's sarcoma: Case report and review
of the literature. Childs Nerv Syst. 9:428–430. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagatell R, London WB, Wagner LM, Voss SD,
Stewart CF, Maris JM, Kretschmar C and Chon SL: Phase II study of
irinotecan and temozolomide in children with relapsed or refractory
neuroblastoma: A children's oncology group study. J Clin Oncol.
29:208–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagner LM, McAllister N, Goldsby RE,
Rausen AR, McNall-Knapp RY, McCaeville MB and Albritton K:
Temozolomide and intravenous irinotecan for treatment of advanced
Ewing sarcoma. Pediatr Blood Cancer. 48:132–139. 2007. View Article : Google Scholar : PubMed/NCBI
|